Abstract
Current acute kidney injury (AKI) diagnostic criteria are restricted to the inpatient setting. We proposed a new AKI diagnostic algorithm for the outpatient setting and evaluate whether outpatient AKI (AKIOPT) modifies the disease course among patients with chronic kidney disease (CKD) enrolled in the national predialysis registry. AKIOPT was detected when a 50% increase in serum creatinine level or 35% decline in eGFR was observed in the 180-day period prior to enrollment in the predialysis care program. Outcomes were progression to end-stage renal disease (ESRD) and all-cause mortality. Association analyses were performed using multiple Cox regression and coarsened exact matching (CEM) analysis. Among 6,046 patients, 31.5% (1,905 patients) had developed AKIOPT within the 180-day period before enrollment. The adjusted hazard ratios of the 1-year and overall risk of ESRD among patients with preceding AKIOPT compared with those without AKIOPT were 2.61 (95% CI: 2.15–3.18) and 1.97 (1.72–2.26), respectively. For 1-year and overall risk of all-cause mortality, patients with AKIOPT had respectively a 141% (95% CI: 89–209%) and 84% (56–117%) higher risk than those without AKIOPT. This statistical inference remained robust in CEM analysis. We also discovered a complete reversal in the eGFR slope before and after the AKIOPT from −10.61 ± 0.32 to 0.25 ± 0.30 mL/min/1.73 m2 per year; however, the loss of kidney function is not recovered. The new AKIOPT diagnostic algorithm provides prognostic insight in patients with CKD.
Similar content being viewed by others
Introduction
By 2025, the International Society of Nephrology’s 0by25 initiative aims to eliminate all avoidable death by acute kidney injury (AKI) worldwide1. This goal seems to stem from advancements in medical big data and computing technology, which instantly allow for a large capacity of data collection and cloud storage. This capacity provides a solid foundation for real-time AKI monitoring and streamlined data management, particularly in hospital settings, to address unanswered questions regarding the nature and history of AKI2. However, research on health information technology reveals that the current burden of AKI may represent only part of a much larger problem in terms of the scale of community-acquired AKI (CA-AKI)3. Studies have demonstrated the mutually deteriorating interconnection between AKI and chronic kidney disease (CKD)4,5; thus, the clinical impacts of AKI are relatively complex and last in the long term, thereby characterizing the 0by25 initiative as overambitious.
The incidence of CA-AKI not requiring dialysis was first evaluated according to the criteria proposed by Hou et al. of hospital-acquired renal insufficiency using a database of 3.8 million individuals from Kaiser Permanente of Northern California, and it was estimated at 384.1 per 100,000 person-years between 1996 and 20036,7. In the United Kingdom, the incidence of CA-AKI was estimated to be 6.4% in the catchment area of Southeast Wales from 2011–2012 using creatinine criteria of the AKI Network classification8,9. In 2013, the first large-scale estimate of CA-AKI prevalence in the Chinese population was conducted at 44 hospitals of 22 provinces in four geographic regions of China with 2.2 million adult patients based on the 2012 KDIGO definition of AKI10,11. The detection rate of CA-AKI was 2.03%, and the most notable finding was that only 25% of CA-AKI cases were identified by supervising clinicians, indicating a critical health care gap in AKI1,10. In Taiwan, the estimated incidence of CA-AKI without preexisting CKD in a retrospective single-center cohort of 395,219 patients was 1.68% between 2010 and 2014 according to the Risk, Injury, Failure, Loss of Kidney Function, and End-Stage Kidney Disease (RIFLE) classification12,13. However, all of the aforementioned large epidemiologic studies were conducted in hospitalized populations.
To narrow this research gap, we evaluated the prognostic role of fluctuation in kidney function measured according to serum creatinine or estimated glomerular filtration rate (eGFR) in the outpatient setting throughout the 180-day period before the CKD patients were enrolled in a national pre-end-stage renal disease (pre-ESRD) care program. This phenotype of AKI was identified using our proposed diagnostic algorithm in an outpatient setting and was named outpatient AKI (AKIOPT). To avoid confusion, the term of CA-AKI is used for AKI that is speculated to occur outside the hospital according to the peak serum creatinine measured specifically in the hospital. By contrast, the diagnosis of AKIOPT is based on all available serum creatinine levels prior to the outpatient service no matter they were measured in the outpatient or inpatient setting.
Results
The study cohort was composed of a total of 6,046 patients enrolled in the pre-ESRD program, contributing to a total of 13,467.68 person-years of follow-up. The median age at enrollment in the pre-ESRD program was 67. 4 years (IQR: 56.9–76.5 years). The median follow-up times for outcomes of ESRD requiring dialysis and all-cause mortality were 1.68 (IQR: 0.80–3.01) and 1.69 (IQR: 0.81–3.03) years, respectively. Overall, 68.5% (4,141 patients) of the study population did not meet the diagnostic threshold of AKIOPT, whereas the remaining 31.5% (1,905 patients) had developed AKIOPT. Among patients with CKD who had a history of AKIOPT, 80.7% (n = 1573) had stable AKIOPT and nearly 20% (n = 368) had deteriorating AKIOPT (Table 1). Both maximum and minimum serum creatinine level were measured in the inpatient setting for 5% of the study population.
Compared with patients without AKIOPT, those with AKIOPT episodes tended to be older, female, nonsmokers, less educated, and have lower BMI and etiologies related to systemic diseases (e.g., diabetes, hypertension, and CVD) (Table 1). In addition to medication use for comorbidities that commonly accompany AKIOPT, exposure to nephrotoxic agents such as NSIADs and radiocontrast was more prevalent among CKD patients with AKIOPT. Patients with a history of AKIOPT were also more likely to receive hypouricemic and antigout therapy than were those without AKIOPT (Table 1). At the time of enrollment in the pre-ESRD program, patients with deteriorating CA-AKI had the lowest median eGFR (9.5 vs. 30.8 mL/min/1.73 m2 in patients without AKIOPT and 22.9 mL/min/1.73 m2 in patients with stable AKIOPT). The median difference and percent change between the maximum and minimum serum creatinine levels were 0.30 mg/dL (IQR: 0.16–0.60) and 17.2% (IQR: 9.8–27.0) and 1.63 mg/dL (IQR: 0.98–3.00) and 77.1% (IQR: 54.9–122.0), respectively, for patients without and with AKIOPT. Kidney function markers, including serum creatinine, blood urea nitrogen, and urine protein to creatinine ratio, demonstrated a significant increasing trend across the AKIOPT subgroups (No AKIOPT, stable AKIOPT, and deteriorating AKIOPT). For hemoglobin and serum albumin, corresponding decreasing trends were observed (Table 1).
In multiple Cox regression analyses, the fully adjusted hazard ratios (aHRs) of the 1-year and overall risk of ESRD among patients with AKIOPT were 2.61 (95% CI: 2.15–3.18) and 1.97 (95% CI: 1.72–2.26), respectively, compared with those without a history of AKIOPT (Table 2, Model 4, ESRD). Among patients with deteriorating AKIOPT, the 1-year and overall risk of ESRD increased by 272% (95% CI: 175%–402%) and 152% (95% CI: 98%–221%), respectively. The corresponding risk differences were 115% (95% CI: 75–164%) and 77% (95% 53–104%) among patients with stable AKIOPT (Table 2, Model 4, ESRD). For 1-year and overall risk of all-cause mortality, patients with AKIOPT had respectively a 141% (95% CI: 89%–209%) and 84% (95% CI: 56%–117%) higher risk than those without AKIOPT (Table 2, Model 4, all-cause mortality). Patients with stable and deteriorating AKIOPT had hazard ratios for 1-year and overall all-cause mortality of 2.41 (95% CI: 1.86–3.13) and 1.79 (95% CI: 1.50–2.13), and 2.41 (95% CI: 1.57–3.70) and 2.07 (95% CI: 1.52–2.81), respectively (Table 2, Model 4, all-cause mortality). The growth piecewise linear mixed modeling revealed a complete reversal in the eGFR slope before and after the AKIOPT event from −10.61 ± 0.32 to 0.25 ± 0.30 mL/min/1.73 m2 per year (Fig. 1. However, the loss of kidney function could not be recovered after a 2-year follow-up. Among patients with diabetes and renin-angiotensin system (RAS) inhibitors, the post- AKIOPT slope remained negative at −0.55 ± −0.39 and −0.39 ± 0.40 mL/min/1.73 m2 per year, respectively (Fig. 2, Table 3, and Supplementary Fig. S1 and Table S1).
eGFR slope (red line) with the light red shaded area representing 95% confidence intervals before and after the AKIOPT event, modeled using the growth piecewise linear mixed model by incorporating random effects. Blue and orange points represent eGFR measurements before and after enrollment into pre-ESRD program.
CEM analysis revealed that the effects of AKIOPT on the progression to ESRD gradually attenuated in subsequent years (e.g., aHR [1.44, 95% CI: 1.10–1.77] for 1-year mortality to aHR [1.10, 95% CI: 0.99–1.41] for 5-year mortality) following pre-ESRD enrollment; however, its effects on all-cause mortality were stable, ranging from an aHR of 1.7 to 1.9 throughout the follow-up period (Fig. 3). Supplementary Table S2 indicates that the matched variables in CEM between patients with and without AKIOPT were well balanced. In the multiple logistic regression of risk markers associated with the risk of developing AKIOPT, we found female gender, advanced CKD stage, diabetes, CVD, and the utilization of NSAIDs, contrast, and diuretics were significantly associated with AKIOPT (Fig. 4).
Discussion
The history of acute change in kidney function prior to pre-ERSD enrollment is prognostically critical in risk assessment and management in patients with CKD. In the present study, patients with AKIOPT were associated with a higher risk of progression to ESRD and all-cause mortality than were those without a history of AKIOPT. The risk was particularly high among patients with the deteriorating type of AKIOPT. We also found that the loss of kidney function before and during the AKIOPT event could not be completely recovered even with meticulous multidisciplinary care. The study results not only provide insight into how AKIOPT modifies the course of CKD but also emphasize the unmet need for the development of a universal screening-based diagnostic workflow to detect AKIOPT.
The first systematic evaluation of CA-AKI conducted by Kaufman in 1991 established the basic concept of CA-AKI detection14. The main diagnostic scheme is to screen admitted patients for impaired kidney function and then track the history, or the baseline serum creatinine level (the lowest reference serum creatinine level) if available, within the 12 months prior to admission or prospective serum creatinine during the entire hospital course14. Before the RIFLE criteria for AKI was proposed in 2004, all research had defined CA-AKI passively in the inpatient setting using Kaufman’s approach6,15,16,17,18. For instance, Obialo et al. defined CA-AKI as serum creatinine levels of >2.0 mg/dL at admission without a history of kidney disease17. Similarly, Hsu et al. defined the incidence of CA-AKI in a large community-based population according to all available inpatient serum creatinine measurements and then selected reference and index serum creatinine level to estimate the incidence of AKI using diagnostic criteria proposed by Hou et al.6,7. After 2010, researchers began to use RIFLE-based criteria to define CA-AKI in the inpatient setting8,19,20, except for Talabani et al., who used a fixed cohort covering the outpatient setting21. Collectively, a clear trend was observed and indicated that CA-AKI is more prevalent than HA-AKI and has a much lower mortality rate22. However, it remains unclear why compared with patients with HA-AKI, patients with CA-AKI tend to be classified in the highest AKI severity group but have a much lower risk of mortality22. This observation questions the feasibility of applying RIFLE-based inpatient AKI criteria for patients from the community.
The diagnostic algorithm we proposed changes the paradigm for AKI diagnosis and extends the scope of clinical AKI into the outpatient setting. Based on longitudinal clinical data, we verified our proposed definition; a 50% fluctuation in serum creatinine in the 180-day period prior to the pre-ESRD enrollment significantly modified the course of CKD. More importantly, we discovered that complete recovery of kidney function after AKIOPT is unlikely in patients with CKD. The change in eGFR slope from −10.61 back to 0.25 mL/min/1.73 m2 represents the acute and reversible nature of kidney injury as the accelerated rate in deterioration of kidney function can be recovered; however, loss of kidney function could not be completely regained as the post-AKI eGFR slope was not steep upward enough to recover to the baseline kidney function before the event of AKIOPT (Fig. 1). This finding is in concordance with prior evidence indicating that HA-AKI defined using RIFLE-based criteria increases the risk of de novo CKD and accelerates CKD progression in critically ill patients23,24,25 and admitted patients26,27 and that a dose–response relationship exists between AKI stage and CKD progression28. However, these inferences are limited to inpatient settings and underestimate the true effect of AKI, particularly CA-AKI, which has yet to be adequately defined in the literature.
In the present study, when we applied the KDIGO AKI criteria, only 2,758 patients exhibited consecutive serum creatinine measurements within a 7-day time period. Among them, 699 patients had AKI, and 16.3% of the AKI patients exhibited peak serum creatinine levels in inpatient settings (Data not shown). Therefore, it is not feasible to use RIFLE-based diagnostic criteria to detect AKI in the community. Indeed, the difference between AKIOPT and rapid progressive CKD (when annual eGFR declining rate >5 mL/min/1.73 m2) may be marginal as the two phenotypes shared common risk factors such as nephrotoxic agents, dehydration, or obstructive uropathy29,30. It is therefore difficult to differentiate AKIOPT from so called rapid progression of CKD, particularly at the onset of these events. Furthermore, if triggering factors of acute kidney insults are not promptly identified and managed, the reversibility of the AKI and recovery of kidney function will then develop into an irreversible kidney injury leading to the phenotype of rapid progression of CKD with an annual eGFR declining rate persistently >5 mL/min/1.73 m2. However, we found a significantly slower progression (from approximately −10 mL/min/1.73 m2 per year to no progression) after AKI events, which contradicts traditional notions of persistent chronicity. This observation signifies that the phenotype of AKIOPT identified by our proposed criteria is clearly different from that of rapid progression of CKD. The mechanisms underlying the AKI-CKD continuum have been extensively explored in animal models5. Maladaptive repair, infiltration of inflammatory cells, stimulation of fibrocysts and myofibrocysts, and tubulointersitital fibrosis have been linked to the development of de novo CKD and CKD progression after AKI31. These injurious molecular pathways are triggered in intrarenal microenvironments rich in damage-associated molecular patterns that are sustained by mutually aggravating mechanisms such as hypoxia, reactive oxygen species, or inflammation32,33. These mechanistic insights provide conceptual coherence between laboratory and epidemiological findings that supports the causality of AKI-to-CKD progression.
The increased risk of progression to ESRD gradually decreased within 5 years following the pre-ESRD enrollment with the highest risk appearing in the first year (Fig. 3). This finding can be explained by the sudden drop of eGFR before the AKIOPT event and the slow increase of eGFR after the AKIOPT event (Fig. 1). The significant loss of kidney function before AKIOPT event may put patients in advanced CKD stage, which increases their risk of progression to ESRD in the first two years following the event of AKIOPT because regain of kidney function is unlikely in the first two years. Therefore, the first year following AKIOPT is a critical period for clinicians to halt the accelerated progression before patients suffered from persistent uremic symptoms. If patients’ dialysis-free status can be maintained in the first two years following AKIOPT, the risk of progression to ESRD would be gradually faded due to better preserved kidney function during the event of AKIOPT or the more pronounced recovery in kidney function after the event of AKIOPT. Our findings regarding the fully adjusted cross-sectional associations between selected clinical factors such as history of CVD and exposures to NSAID or contrast prior to the event of AKIOPT provides useful information on risk markers for development of AKIOPT in real-world practice (Fig. 4). However, more research must be conducted to discover new risk factors or effective prevention for AKIOPT.
This study has several limitations. First, the Health and Welfare Data Science Center (HWDC) of Taiwan did not release the biochemical data through the Health Insurance Medical Information Cloud Inquiry System until 2017; therefore, patients’ serum creatinine measurements outside of our hospital were unavailable. Information bias could not be completely excluded; however, a high retention rate among patients in our hospital, which is the largest tertiary medical center in central Taiwan, should have effectively minimized the risk of misclassification. Second, we could not completely exclude the possibility of residual confounding and over-adjustment for variables that could be in the causal pathway. For example, detailed information on environmental factors such as diet, exposure to nephrotoxicants, and physical activity was not available. Third, the study population that were drawn from a pre-ESRD program poses a limitation in terms of generalizability. However, our proposed diagnostic cutoffs for the percent change of serum creatinine and eGFR approximated the 75th percentile of the overall distribution, which improves generalizability of the proposed AKIOPT algorithm to patients with normal kidney function as within-day variability of serum creatinine above 30% is rarely observed in general population (submitted data) (Supplementary Fig. S2).
In conclusion, we validated an AKIOPT algorithm in the CKD population by demonstrating that this classification could accurately predict the risks of progression to ESRD and all-cause mortality. Our study also revealed that the use of conventional RIFLE-based AKI criteria significantly underestimates the role of AKI in the general population. Despite the full recovery of eGFR declining slope after AKI event, the loss of kidney function is not likely recovered, which strengths the causal link between AKI and CKD progression.
Methods
Study population
In 2002, Taiwan’s National Health Insurance launched the Project of Integrated Care of CKD, initially targeting patients with an eGFR lower than 60 mL/min/1.73 m2; since 2007, the program has used a multidisciplinary approach to focus on CKD stages 3b–534. This pre-end-stage renal disease (ESRD) program utilizes a multidisciplinary approach (involving nephrologists, renal nursing specialists, pharmacists, healthcare educators and dieticians) to design individualized care plans for a wide range of CKD patients. China Medical University Hospital (CMUH) joined pre-ESRD program in 2003 and has been consecutively enrolling patients who were willing to participate this care program and had a diagnosis of CKD based either on the working diagnoses of nephrologists or in accordance with the criteria outlined in the National Kidney Foundation (NKF)/KDOQI Guidelines35. Up-to-date, the CMUH pre-ESRD program includes more than 11 000 participants with an overall retention rate of 90%. Patients in CKD stage 3b, 4 and 5 were, respectively, followed up at 12, 8 and 4 weeks, or when necessary. Biochemical markers of renal injury including serum creatinine, eGFR, and the spot urine protein–creatinine ratio (PCR) were measured at intervals of no more than 12 weeks36. All patients enrolled in the program were followed up until the initiation of maintenance dialysis for ESRD, loss to follow-up, death, or December 31, 2015. ESRD status was verified through active contact and review of electronic medical records (EMRs). Complete mortality data were obtained from the National Death Registry.
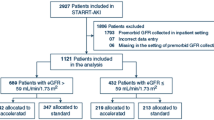
In this study, 6,046 patients aged 20–90 years who remained dialysis-naïve and had records for at least two eGFR measurements before pre-ESRD program enrollment were selected from among 10,277 program participants (the selection process is detailed in Supplementary Fig. S3). The index date was defined as the day of the first AKI event based on our proposed diagnostic criteria of the AKIOPT. All methods in this study were performed in accordance with the relevant guidelines/regulations. The study was approved by the Big Data Center of China Medical University Hospital and the Research Ethical Committee/Institutional Review Board of China Medical University Hospital (CMUH105-REC3-068) and the need to obtain informed consent for the present study was waived by the Research Ethical Committee of China Medical University Hospital.
Criteria for outpatient acute kidney injury
We tracked all serum creatinine measurements of the patients up to 180 days before pre-ESRD enrollment. Serum creatinine was measured using the Jaffe rate method (kinetic alkaline picrate) at CMUH Central Laboratory using a Beckman UniCel DxC 800 immunoassay system (Beckman Coulter Inc., Brea, CA, USA). Calculations of eGFR were performed using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation37. An AKIOPT event was defined as a fluctuation of >50% in serum creatinine or >35% in eGFR in the 180-day period preceding pre-ERSD enrollment. The 180-day time frame was chosen based on prior evidence and represents the potentially longer duration of kidney vulnerability to nephrotoxins in elder patients or patients with CKD, particularly nonsteroidal anti-inflammatory drugs (NSAID)38,39. Baseline serum creatinine was defined as the best (lowest) serum creatinine within 180 days prior to the pre-ESRD enrollment. Fluctuations in serum creatinine were calculated as the difference between the maximum and minimum values of serum creatinine divided by the minimum value of serum creatinine. Fluctuations in eGFR were calculated as the difference between the maximum and minimum values of eGFR divided by the maximum value of eGFR. We further classified the AKI episode as deteriorating or stable based on the difference between the last S serum creatinine value in the 180-day period and baseline serum creatinine at the time of pre-ESRD enrollment. If the difference was positive and greater than 0.3 mg/dL, then the AKIOPT was defined as deteriorating AKI, whereas if the difference was less than 0.3 mg/dL, it was categorized as stable AKIOPT. This cutoff was selected empirically based on KDIGO serum creatinine criteria for Stage 1 AKI11. An alternative definition using 0 mg/dL as the cut-off was also evaluated (Supplementary Table S3). Detailed information of other variables such as sociodemographic characteristics was provided in Supplementary text.
Statistical analyses
Continuous variables are expressed as medians and interquartile ranges (IQRs) and were compared using the nonparametric Kruskal–Wallis test, whereas categorical variables are expressed as a frequency (percentage) and were compared using the chi-square test. Associations between AKI status (with and without AKIOPT, stable AKIOPT, and deteriorating AKIOPT) and the 1-year and overall risks of ESRD requiring dialysis and all-cause mortality were estimated using a multivariable Cox regression analysis. Multivariable Cox regression models were initially adjusted for sociodemographic and lifestyle variables, including age, sex, education (<9, 9–12, 12–16 or >16 years), smoking status (never, former or current) and alcohol consumption (never, former or current), followed by adjustments for comorbidities including diabetes mellitus, hypertension, CVD, primary etiologies of CKD, baseline medications (details provided in Table 1) and the baseline serum creatinine defined by the best (lowest) serum creatinine identified within the diagnostic window of AKIOPT. For outcomes of progression to ESRD, we performed competing risk analysis in accordance with the methods outlined by Fine and Gray to minimize potential bias introduced by a competing risk of death40. We also applied coarsened exact matching (CEM) analysis with matching criteria of age, sex, baseline eGFR, diabetes, hypertension, and CVD to specifically adjust for imbalances in baseline kidney function between patients with and without AKIOPT41.
To compare the eGFR progression change before and after the index episode AKIOPT, we further identified a total of 1,106 patients who had undergone at least three serum creatinine measurements within a 2-year interval before and after the index AKIOPT event. We applied the growth piecewise linear mixed model by incorporating random effects for correlated eGFR measurements on the same patient to understand the effect of AKIOPT events on CKD progression using the following equation42:
where δij = 1 for the period before the AKIOPT event and δij = 0 for the period after the AKIOPT event.
Lastly, we used a multivariable logistic regression model to investigate the risk markers, such as demographic and selected clinical factors, for developing AKIOPT. All statistical analyses were performed in SAS (version 9.4, SAS Institute Inc., Cary, NC, USA) and R (version 3.2.3, R Foundation for Statistical Computing, Vienna, Austria). The 2-sided statistical significance level was set at α = 0.05.
Ethical approval
The study was approved by the Research Ethical Committee/Institutional Review Board of China Medical University Hospital (CMUH105-REC3-068).
Data availability
The data that support the findings of this study are available on request from the corresponding author, CCK. The data are not publicly available due to them containing information that could compromise research participant privacy.
References
Mehta, R. L. et al. International Society of Nephrology’s 0 by 25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 385, 2616–2643, https://doi.org/10.1016/S0140-6736(15)60126-X (2015).
Wilson, F. P. et al. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet 385, 1966–1974, https://doi.org/10.1016/S0140-6736(15)60266-5 (2015).
Sawhney, S. & Fraser, S. D. Epidemiology of AKI: Utilizing Large Databases to Determine the Burden of AKI. Advances in chronic kidney disease 24, 194–204, https://doi.org/10.1053/j.ackd.2017.05.001 (2017).
Chawla, L. S. & Kimmel, P. L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney international 82, 516–524, https://doi.org/10.1038/ki.2012.208 (2012).
Chawla, L. S., Eggers, P. W., Star, R. A. & Kimmel, P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. The New England journal of medicine 371, 58–66, https://doi.org/10.1056/NEJMra1214243 (2014).
Hsu, C. Y. et al. Community-based incidence of acute renal failure. Kidney international 72, 208–212, https://doi.org/10.1038/sj.ki.5002297 (2007).
Hou, S. H., Bushinsky, D. A., Wish, J. B., Cohen, J. J. & Harrington, J. T. Hospital-acquired renal insufficiency: a prospective study. The American journal of medicine 74, 243–248 (1983).
Wonnacott, A., Meran, S., Amphlett, B., Talabani, B. & Phillips, A. Epidemiology and outcomes in community-acquired versus hospital-acquired AKI. Clinical journal of the American Society of Nephrology: CJASN 9, 1007–1014, https://doi.org/10.2215/CJN.07920713 (2014).
Mehta, R. L. et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical care 11, R31, https://doi.org/10.1186/cc5713 (2007).
Yang, L. et al. Acute kidney injury in China: a cross-sectional survey. Lancet 386, 1465–1471, https://doi.org/10.1016/S0140-6736(15)00344-X (2015).
(KDIGO), K. D. I. G. O. Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl., 1–138 (2012).
Hsu, C. N. et al. Incidence, Outcomes, and Risk Factors of Community-Acquired and Hospital-Acquired Acute Kidney Injury: A Retrospective Cohort Study. Medicine 95, e3674, https://doi.org/10.1097/MD.0000000000003674 (2016).
Bellomo, R. et al. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical care 8, R204–212, 10.1186/cc2872 (2004).
Kaufman, J., Dhakal, M., Patel, B. & Hamburger, R. Community-acquired acute renal failure. American journal of kidney diseases: the official journal of the National Kidney Foundation 17, 191–198 (1991).
Liano, F. & Pascual, J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney international 50, 811–818 (1996).
Feest, T. G., Round, A. & Hamad, S. Incidence of severe acute renal failure in adults: results of a community based study. Bmj 306, 481–483 (1993).
Obialo, C. I., Okonofua, E. C., Tayade, A. S. & Riley, L. J. Epidemiology of de novo acute renal failure in hospitalized African Americans: comparing community-acquired vs hospital-acquired disease. Archives of internal medicine 160, 1309–1313 (2000).
Wang, Y., Cui, Z. & Fan, M. Hospital-acquired and community-acquired acute renal failure in hospitalized Chinese: a ten-year review. Renal failure 29, 163–168, https://doi.org/10.1080/08860220601095918 (2007).
Schissler, M. M. et al. Characteristics and outcomes in community-acquired versus hospital-acquired acute kidney injury. Nephrology 18, 183–187, https://doi.org/10.1111/nep.12036 (2013).
Der Mesropian, P. J. et al. Long-term outcomes of community-acquired versus hospital-acquired acute kidney injury: a retrospective analysis. Clinical nephrology 81, 174–184, https://doi.org/10.5414/CN108153 (2014).
Talabani, B. et al. Epidemiology and outcome of community-acquired acute kidney injury. Nephrology 19, 282–287, https://doi.org/10.1111/nep.12221 (2014).
Mesropian, P. D. et al. Community-acquired acute kidney injury: A challenge and opportunity for primary care in kidney health. Nephrology 21, 729–735, https://doi.org/10.1111/nep.12751 (2016).
Rimes-Stigare, C. et al. Evolution of chronic renal impairment and long-term mortality after de novo acute kidney injury in the critically ill; a Swedish multi-centre cohort study. Critical care 19, 221, https://doi.org/10.1186/s13054-015-0920-y (2015).
Gammelager, H. et al. Five-year risk of end-stage renal disease among intensive care patients surviving dialysis-requiring acute kidney injury: a nationwide cohort study. Critical care 17, R145, https://doi.org/10.1186/cc12824 (2013).
Lai, C. F. et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Critical care 16, R123, https://doi.org/10.1186/cc11419 (2012).
Pannu, N., James, M., Hemmelgarn, B. & Klarenbach, S., Alberta Kidney Disease, N. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clinical journal of the American Society of Nephrology: CJASN 8, 194–202, https://doi.org/10.2215/CJN.06480612 (2013).
Heung, M. et al. Acute Kidney Injury Recovery Pattern and Subsequent Risk of CKD: An Analysis of Veterans Health Administration Data. American journal of kidney diseases: the official journal of the National Kidney Foundation 67, 742–752, https://doi.org/10.1053/j.ajkd.2015.10.019 (2016).
Chawla, L. S., Amdur, R. L., Amodeo, S., Kimmel, P. L. & Palant, C. E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney international 79, 1361–1369, https://doi.org/10.1038/ki.2011.42 (2011).
Go, A. S. et al. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC nephrology 19, 146, https://doi.org/10.1186/s12882-018-0942-1 (2018).
Perazella, M. A. Renal vulnerability to drug toxicity. Clinical journal of the American Society of Nephrology: CJASN 4, 1275–1283, https://doi.org/10.2215/CJN.02050309 (2009).
Varrier, M., Forni, L. G. & Ostermann, M. Long-term sequelae from acute kidney injury: potential mechanisms for the observed poor renal outcomes. Critical care 19, 102, https://doi.org/10.1186/s13054-015-0805-0 (2015).
Anders, H. J. & Ryu, M. Renal microenvironments and macrophage phenotypes determine progression or resolution of renal inflammation and fibrosis. Kidney international 80, 915–925, https://doi.org/10.1038/ki.2011.217 (2011).
Hultstrom, M., Becirovic-Agic, M. & Jonsson, S. Comparison of acute kidney injury of different etiology reveals in-common mechanisms of tissue damage. Physiological genomics 50, 127–141, https://doi.org/10.1152/physiolgenomics.00037.2017 (2018).
Lin, C. M., Yang, M. C., Hwang, S. J. & Sung, J. M. Progression of stages 3b-5 chronic kidney disease–preliminary results of Taiwan national pre-ESRD disease management program in Southern Taiwan. Journal of the Formosan Medical Association = Taiwan yi zhi 112, 773–782, https://doi.org/10.1016/j.jfma.2013.10.021 (2013).
Foundation, N. K. KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. Am J Kidney Dis 48, S1–S322 (2006).
Tsai, C. W. et al. Uric acid predicts adverse outcomes in chronic kidney disease: A novel insight from trajectory analyses. Nephrology Dialysis Transplantation, In press (2017).
Levey, A. S. et al. A new equation to estimate glomerular filtration rate. Annals of internal medicine 150, 604–612 (2009).
Forni, L. G. et al. Renal recovery after acute kidney injury. Intensive Care Med 43, 855–866, https://doi.org/10.1007/s00134-017-4809-x (2017).
Perazella, M. A. & Markowitz, G. S. Drug-induced acute interstitial nephritis. Nature reviews. Nephrology 6, 461–470, https://doi.org/10.1038/nrneph.2010.71 (2010).
Fine, J. P. & Gray, R. J. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 94, 496–509, https://doi.org/10.2307/2670170 (1999).
King, G. & Nielsen, R. Why Propensity Scores Should Not Be Used for Matching. Political Analysis (2019).
Naumova, E. N., Must, A. & Laird, N. M. Tutorial in Biostatistics: Evaluating the impact of ‘critical periods’ in longitudinal studies of growth using piecewise mixed effects models. Int J Epidemiol 30, 1332–1341 (2001).
Acknowledgements
This study was supported by the Ministry of Science and Technology of Taiwan (grant number: 106-2314-B-039-041- MY3 and 108-2314-B-039 -038 -MY3) and the China Medical University Hospital (grant number: CRS-106-018).
Author information
Authors and Affiliations
Contributions
H.C.Y. and C.C.K. designed the study; C.C.K. and H.C.H. analyzed the data and made the figures; I.W.T. and H.Y.C. drafted and revised the paper; all authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yeh, HC., Ting, IW., Huang, HC. et al. Acute Kidney Injury in the Outpatient Setting Associates with Risk of End-Stage Renal Disease and Death in Patients with CKD. Sci Rep 9, 17658 (2019). https://doi.org/10.1038/s41598-019-54227-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54227-6
- Springer Nature Limited