Abstract
Peptidase inhibitors (PIs) are defense proteins of plants which are active against gut peptidases of different insects. Sapindus mukorossi was identified as a source of bioactive PIs which could confer resistance against Bactrocera cucurbitae, a most devastating pest of several economically important crops. In the present study, a trypsin inhibitor was purified from mature dry seeds of S. mukorossi and characterized for its biochemical properties as well as its potential for bio control of B. cucurbitae. The purified fractions from RP- HPLC through SDS-PAGE gave an apparent molecular weight of ~29 kDa. S. mukorossi trypsin inhibitor (SMTI) was found to be a non-competitive inhibitor which was active over a broad range of temperature (10–100 °C) and pH (6–11). SMTI when incorporated in artificial diet inhibited the growth and development of B. cucurbitae larvae. Gene expression analysis of trypsin and chymotrypsin genes via qRT-PCR indicated that their mRNA expression was down-regulated while that of other genes namely, Catalase, Elastase, Superoxide Dismutase, Glutathione –S-transferase and Alkaline Phosphatase was up regulated. SMTI also showed deleterious effects against different bacterial strains. The results of this study indicated that S. mukorossi trypsin inhibitor has potential to be used as a bio control agent that can reduce the harm caused by melon fruit fly and other devastating pests.
Similar content being viewed by others
Introduction
The losses caused by insect pest attack on economically important crops have reached upto 20% in large cultivars and have become a matter of serious concern in food production1. The peptidase inhibitors (PIs) are a class of plant proteins that play an important role in natural defense mechanism provoked by herbivory2 and can be explored for lessen the destruction caused by pathogens and insects. Plant peptidase inhibitors (PPIs) are widely distributed in different plant families like leguminaceae, sapoteaceae and exist in various plant parts like flowers, leaves, stem including seeds, where these comprise upto 10% of total plant proteins3. Serine PIs are the most represented family of these plant PIs which include Kunitz and Bowman-Birk inhibitors having molecular mass ranging between 18 and 22 kDa. These typically inhibit chymotrypsin and trypsin peptidases and generally form two disulfide bridges with four cysteine residues4,5. Transgenic plants exhibiting PIs expression have been evaluated as an alternative approach for defense against insect pests as they are safe and environment friendly compared to chemical pesticides6. Therefore, there is a need to explore India’s large plant diversity for PIs which can be exploited for insect pest management.
Bactrocera cucurbitae is a polyphagous pest that attacks over 81 species of plants7. It is a significant economic pest of cucurbit crops, viz., Momordica charantia L. (Cucumis sativus L.), Cucumis anguria L., Trichosanthes anguina L., Luffa acutangula Roxb., Citrullus lanatus L., Cucurbita moschata Duchesne, Cucumis melo L. etc. Depending upon the season and cucurbit species, the extent of losses caused by melon fruit fly ranges from 30 to 100%8. Sapindus mukorossi is a deciduous tree belonging to the family Sapindaceae, also known as reetha, commonly found in North India. S. mukorossi has been extensively explored for its pharmacological action which include anti inflammatory, antimicrobial and hepato-protective activity. The ethanolic extracts of the plant have also been explored for insecticidal activity against Sitophilus oryzae and Pediculus humanus where the extracts caused significant mortality and repellent effect on the insects9. However, no studies have been carried out to evaluate the effect of the plant PIs from S. mukorossi against economically important agricultural insect pests. Therefore, in present study a trypsin inhibitor from S. mukorossi seeds was purified, characterized and further evaluated for its insecticidal effects against B. cucurbitae using bioassays, enzyme assays and gene expression analyses.
Results
Purification of SMTI
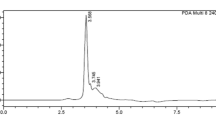
Trypsin inhibitor from S. mukorossi seeds was purified using ammonium sulphate precipitation followed by dialysis. It was found that 0–80% (F1) saturated protein fraction gave the maximum trypsin inhibitory activity compared to 20–80% (F2), 40–80% (F3), and 60–80% (F4) saturated fractions. SMTI was further purified using different chromatographic techniques. In the first step, DEAE cellulose chromatography separated the partially purified fractions into two peaks which showed maximum inhibitory activity for trypsin (Fig. 1A). With DEAE column, the trypsin inhibitor was purified to 3.46 fold with 66.85% yield and specific activity of 295 TIUmg−1 protein (Table 1). In the second step, peak showing the maximum trypsin inhibitory activity was subjected to trypsin sepharose 6B affinity chromatography and the eluted peak (Fig. 1B) exhibited highest trypsin inhibitory activity with specific activity 694 TIUmg−1 and 8.14 purification fold. The fractions/eluent from the affinity chromatography column was subjected to reverse phase HPLC which gave a single peak with a retention time of 2.738 min indicating the presence of single purified inhibitor (Fig. 2A). RP-HPLC gave 9.26 fold purified trypsin inhibitor with a yield of 10.47% and specific activity of 789 TIUmg−1 protein (Table 1).
(A) RP-HPLC chromatogram obtained after injection into C-18G column showing single peak at a retention time of 2.738 min (B) SDS-PAGE gel showing different protein fractions during each purification step. Lane- 1 benchmark prestained protein ladder (Invitrogen), lane-2 Crude extract, lane-3 ammonium sulphate precipitated fraction (0–80%), lane-4 partially purified fraction, lane-5 DEAE-cellulose fraction, lane-6 trypsin sepharose 6B fraction, lane-7 RP-HPLC purified fractions.
Characterization of SMTI
SDS PAGE
12% SDS-PAGE showed that SMTI exhibited a molecular mass of ~29 kDa, indicating that the inhibitor obtained from affinity chromatography and RP-HPLC column contains a single polypeptide chain (Fig. 2B).
Effect of temperature and pH
The inhibitory activity of SMTI increased with increase in temperature i.e. 10–30 °C, after that it declined but retained ~40% of the inhibitory activity at 100 °C after incubation of 30 min. Maximum trypsin inhibitory activity (83%) was observed at 30 °C (Fig. 3A). SMTI exhibits stability at broad pH range (pH 6.0–11.0). The maximum activity (~87%) was observed at pH 8.0. The SMTI was active in both acidic and basic range. It retained 56% of inhibitory activity at pH-11.0 (Fig. 3B).
(A) Temperature stability of SMTI after 30 min incubation at varying temperatures. (B) pH stability of SMTI after incubation at varying pH for 30 min at 25 °C. Data are mean ± Standard Deviation, two way ANOVA and Tukey’s HSD. Treatments with same letter indicate no significant difference p < 0.01. *And ** indicates significant at p < 0.01 and p < 0.001, respectively. F = F-ratio; HSD = Honestly Significance Difference.
Effect of metal ions, detergents and organic solvents
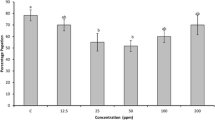
Trypsin inhibitory activity of SMTI increased with increase in concentration of Ca2+, Co2+, Mg2+, Zn2+, Ni2+ which ranged from 1 mM to 50 mM and for Cu2+ and Fe2+ increased from 1 mM to 25 mM but reduced at 50 mM. However it increased at 1 mM but reduced at 5 mM, 25 mM and 50 mM for Mn2+ (Fig. 4).
Metal ions stability of SMTI after 30 min incubation at room temperature. Data are mean ± Standard Deviation, two way ANOVA and Tukey’s HSD. Treatments with same letter indicate no significant difference p < 0.01. Ca2+(F = 6410.16**, HSD = 1.97), Co2+(F = 1479.83**, HSD = 3.7), Cu2+(F = 4472.91**, HSD = 4.35), Fe2+ (F = 1715.82**, HSD = 7.13), Mg2+ (F = 1713.92**, HSD = 3.57), Mn2+ (F = 999.78**, HSD = 9.02), Ni2+ (F = 1854.06**, HSD = 3.43) and Zn2+ (F = 1255.18**, HSD = 4.2). *And ** indicates significant at p < 0.01 and p < 0.001, respectively. F = F-ratio; HSD = Honestly Significance Difference.
Trypsin inhibitory activity of SMTI decreased upon addition of Tween-80. It was also decreased in the presence of other detergents like Triton-X, CTAB and Urea. But, there was a slight increase in the presence of SDS upto 5 mM there after it was reduced (Fig. 5).
Detergent stability of SMTI after 30 min incubation at room temperature. Data are mean ± Standard Deviation, two way ANOVA and Tukey’s HSD. Treatments with same letter indicate no significant difference p < 0.01. Triton X (F = 151.47, HSD = 10.84), CTAB (F = 102.94**, HSD = 13.45), Urea (F = 15.8, HSD = 26.53), SDS (F = 218.9**, HSD = 22.48), Tween 80 (F = 50.85, HSD = 23.12). *And ** indicates significant at p < 0.01 and p < 0.001, respectively. F = F-ratio; HSD = Honestly Significance Difference.
SMTI activity increased slightly at 10%, 20%, 30% and 40% concentrations of acetone, benzene, chloroform, DMSO, hexane and petroleum ether (Fig. 6). No significant effect of ethyl acetate, acetonitrile and methanol was noticed on its activity.
Solvents stability of SMTI after 30 min incubation at room temperature. Data are mean ± Standard Deviation, two way ANOVA and Tukey’s HSD. Treatments with same letter indicate no significant difference p < 0.01. Acetone (F = 531.86**, HSD = 6.06), Acetonitrile (F = 29.03**, HSD = 21.74), Benzene (F = 1210.05**, HSD = 4.19), Chloroform (F = 507.74**, HSD = 6.81), DMSO (F = 354.81**, HSD = 8.31), Ethanol (F = 25.17**, HSD = 24.78), Ethyl-acetate (F = 3.1, HSD = 17.29), Hexane (F = 971.39**, HSD = 4.64), Methanol (F = 86.37**, HSD = 10.81), Petroleum Ether (F = 1484.5**, HSD = 3.58). *And ** indicates significant at p < 0.01 and p < 0.001, respectively. F = F-ratio; HSD = Honestly Significance Difference.
IC50, IC90, Casein hydrolysis test and Kinetic Study
The IC50 and IC90 values of SMTI obtained from linear regression equation (Y = 17.60 × 3.907) were 3.07 ± 0.08 µM and 5.34 ± 0.14 µM, respectively (Fig. 7A). The inhibition kinetics of SMTI was evaluated using Line weaver Burk plot. In the presence of inhibitor there was a decrease in Vmax but there was no significant change in Km which suggested that the mode of inhibition of trypsin by SMTI was non-competitive (Fig. 8). The Ki value obtained for SMTI was 15.73 ± 0.16 µM.
(A) Effect of SMTI against bovine trypsin. The IC50 of SMTI on trypsin was 3.07 ± 0.08 µM and IC90 was 5.34 ± 0.14 µM. (B) Casein hydrolysis test: Zone of inhibition caused by different concentrations of SMTI in wells 1, 2, 3, 4. Well 1 = 25 µg/ml, Well 2 = 50 µg/ml, Well 3 = 75 µg/ml, Well 4 = 100 µg/ml, C = Control (Without inhibitor).
Inhibition kinetics of SMTI: Kinetic analysis of trypsin using SMTI as an enzyme inhibitor and BApNA as a substrate. The mechanism of inhibition was evaluated using Line weaver Burk plot (or double reciprocal lot), in which the inverse of the initial rate was plotted against the inverse of the substrate concentrations representing the inhibitory effect of the SMTI in presence of its different concentrations from 1.0 μM to 5.0 μM on trypsin.
The hydrolysis zone around the wells decreased with increase in concentration of SMTI as compared to control (Fig. 7B) indicating trypsin inhibition by SMTI (Table 2). The least hydrolysis zone was observed at highest concentration i.e. at 100 µg/ml in well 4.
Larval Growth Index (LGI) and Total Growth Index (TGI)
Growth indices results revealed that the LGI and TGI of second instar larvae of B. cucurbitae was significantly (F6,35 = 186.89; 416.32 P < 0.001) reduced when fed with different concentrations of SMTI incorporated in artificial diet. At 625 µg/ml, LGI and TGI were markedly reduced by 90.14% and 92.89% respectively as compared to the control (Fig. 9).
Larval Growth Index (LGI) and Total Growth Index (TGI) of B. cucurbitae in days when second instar larvae were fed on different concentrations SMTI. Data are mean ± Standard Deviation, two ways ANOVA and Tukey’s HSD (six biological replicates). Treatments with same letter indicate no significant difference p < 0.01. LGI (F = 186.89**, HSD = 0.98), TGI (F = 416.32**, HSD = 0.28). *And ** indicates significant at p < 0.01 and p < 0.001, respectively. F = F-ratio; HSD = Honestly Significance Difference.
Enzyme assay
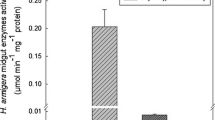
Inhibitory effects of SMTI at LC50 concentration (6.98 µg/ml) (Fig. S1) were observed for both trypsin and chymotrypsin in gut extracts. The activity of trypsin and chymotrypsin decreased significantly in all the time intervals i.e. at 24, 48 and 72 h when second instar larvae (64–72 h) were fed with LC50 concentration of SMTI. The decrease in activity of both enzymes was found to increase with increase in treatment interval (Fig. 10).
Effect of LC50 (6.98 µg/ml) of SMTI on Trypsin and Chymotrypsin enzymes of second instar larvae of B. cucurbitae at 24 h, 48 h and 72 h. Data are mean ± Standard Deviation, two way ANOVA and Tukey’s HSD (six biological replicates). Treatments with same letter indicate no significant difference p < 0.01. *And ** indicates significant at p < 0.01 and p < 0.001, respectively. F = F-ratio; HSD = Honestly Significance Difference.
Gene expression analysis
The change in expression level of various genes in (64–72 h) larvae (2nd instar) of melon fruit fly fed on LC50 concentration (6.98 μg/ml) of SMTI was investigated using qRT-PCR. There was a considerable down regulation of genes involved in digestion (trypsin and chymotrypsin) after 24, 48 and 72 h of incorporating SMTI in the diet. At 72 h, the resultant fold change in mRNA level of trypsin was 0.28 and that of chymotrypsin was 0.22. On the other hand, the mRNA level of esterase in the presence of SMTI was elevated by 2.71 fold as compared to control at 72 h. The mRNA level of SOD, AP, catalase and GST was also up regulated in second instar larvae due to the effect of SMTI in comparison to control (Fig. 11).
Effect of SMTI on different genes of second instar larvae of B. cucurbitae fed on artificial diet supplemented with and without SMTI (**indicates without SMTI). Data are mean ± Standard Deviation, two way ANOVA and Tukey’s HSD. Treatments with same letter indicate no significant difference p < 0.01. *And ** indicates significant at p < 0.01 and p < 0.001, respectively.
Antibacterial activity
SMTI was shown to have antibacterial activity against Bacillus thuringiensis, Escherichia coli and Pseudomonas aeruginosa (Table 3). However, SMTI was found to have no inhibitory effect on Mycobacterium smegmatis (Fig. S2).
Discussion
Plants are constantly exposed to stress due to changing environmental conditions. In addition, pathogens and predators continuously attack them. To overcome these adverse conditions plants produce toxic substances to reduce insect damage10. PIs are among these compounds which have attracted significant attention because of their role in plant defense and possible applications in transgenic plants to enhance resistance to pathogens and other insect pests. In view of the insect control potential of PIs, we purified trypsin inhibitor from S. mukorossi seeds and assessed its insecticidal effect on B. cucurbitae larvae along with its antibacterial effect.
A trypsin inhibitor from S. mukorossi seeds (SMTI) was purified with a recovery of 10.47% and purification fold of 9.26 which was greater than the trypsin inhibitor purified from Inga vera seeds11 (yield 7.14%; purification fold 1.36) and Lonchocarpus sericeus seeds10 (yield 0.88%; purification fold 8.26). SDS-PAGE depicted a molecular mass of ~29 kDa, similar to that of other trypsin inhibitors12,13.
Serine peptidase inhibitors are resistant to extreme pH and temperature and this uncommon stability is due to non-covalent interactions and disulfide bonds in the inhibitor molecule14. The SMTI showed stability over wide temperature and pH range which indicates that, SMTI possesses high intrinsic stability in its native state. These characteristic were also noted in other trypsin inhibitors15,16,17. A broad range of temperature and pH were studied to check the stability of peptidase inhibitor with respect to seasonal and daily temperature variation. Also there is so much of variation in pH of soil and different plants/plant parts around different demographic areas. SMTI inhibitory activity was also influenced by different metal ions (Mn2+ Co2+, Ni2+, Fe3+, Cu2+, Zn2+, Ca2+, Mg2+), solvents (DMSO, benzene, petroleum-ether acetone, hexane, and chloroform) and detergents (SDS, CTAB, Triton X-100, Urea, Tween-80). The activity of peptidase inhibitor purified from the seeds of Albizia amara was affected in the presence of different metal ions (increased in presence of Cu2+ and decreased in presence of Ni2+, Fe3+, Ba2+, Mn2+ and Mg2+) and detergents (enhanced in presence of Triton X-100 and diminished in presence of Tween-20, SDS and Tween – 80)18. However, the activity of trypsin inhibitor purified from D. biflorus declined in presence of Cu2+ and Ni2+ but DMSO enhanced the chymotrypsin inhibitory activity while no effect was observed on trypsin inhibitory activity19. The binding of different metal ions might cause a conformational change in a protein (peptidase inhibitor) which may or may not favour its binding to protein (trypsin), thus resulting in inhibition or no inhibition20,21. Metal ions play a vital role in protecting the structural stability of PIs that is essential for different biological actions and is susceptible to oxidative/reductional damage22,23. Bacha et al.24 noted that the cumulative impact of PIs and detergents enhances membrane protein solubilization and other physiological components as well as reduces undesirable proteolysis. In addition, solvents are commonly used with PIs for purification, solubilization, and stabilization18. Solvents and detergents increase the solubility of the protein and lower the risk of microbial contamination during preparations/downstream processing, therefore they are tested in the present study25.
With regards to mechanism of action, SMTI showed a non-competitive type of inhibition similar to that reported in Sapindus trifoliatus seeds12. Vogel et al.26 noted that most inhibitors adopt a inhibition kinetics of non-competitive type, although a few trypsin inhibitors have exhibited a competitive form of inhibition27,28,29. The low value of Ki depicts strong affinity of SMTI to trypsin.
The decrease in growth rate of B. cucurbitae larvae indicated by decline in percentage pupation and percentage emergence suggests a deterrent effect of the SMTI on the development of the 2nd instar larvae (64–72 h) of melon fruit fly. Kaur and Sohal30, perceived that different concentrations of the pea peptidase inhibitor, when added in artificial diet of second instar larvae (64–72 h) of B. cucurbitae, decreased the larval growth and total development period. Results analogous to present findings were also observed by Kaur et al.31 in B. cucurbitae where they found that soybean trypsin-chymotrypsin inhibitor (Bowman–Birk Inhibitor, SBBI) prolonged the larval and pupal period of second instar larvae (64–72 h) of B. cucurbitae. Punithavalli and Jebamalaimary32 too had reported prolonged development of sugarcane borer Chilo infuscatellus fed with PI extracted from Erianthus arundinaceus. The significant delay observed in larval and total development period in the present study might be due to the deficiency of essential amino acids required for the normal growth and inhibition of trypsin which is a major digestive peptidase33. Machado et al.16 had tested Acacia polyphylla seeds (AcKI) containing proteinaceous inhibitors (Kunitz-type inhibitor) against Anagasta kuehniella (polyphagous pest) and observed reduction in development, survival and enzymatic activity of A. kuehniella larvae.
The activity of trypsin and chymotrypsin decreased at LC50 concentration of the SMTI. A previous study reported inhibition in trypsin and chymotrypsin activity in B. cucurbitae larvae by the action of peptidase inhibitor extracted from peas30. Silva et al.34 observed that Adenanthera pavonina trypsin inhibitor (ApTI) inhibited trypsin and chymotrypsin by 87% and 63%, respectively. The result in this study also revealed that at higher concentrations, the activity of trypsin is more affected by SMTI as compared to that of chymotrypsin suggesting trypsin is an important enzyme in the larval gut. Ferreira et al.35 found that recombinant PI from Bauhinia bauhinioides (kallikrein inhibitor) inhibited the midgut peptidases of Nasutitermes corniger workers and soldiers, thus reducing their digestive capacity and affecting the growth of the insect. Saikhedkar et al.36 reported reduction in expression of HaTry7 (H. armigera Trypsin 7) and HaChy4 (H. armigera Chymotrypsin 4) gene when H. armigera larvae were fed on bicyclic peptide derived from plant Pin-II type PI. Inhibitory activities of peptidase inhibitors from chick pea and black gram against trypsin-like enzymes have also been reported in the larvae of H. armigera and S. litura, respectively37,38. The down regulation of protein digestion in B. cucurbitae larvae could be because of the inhibitory action of PIs on insect’s midgut peptidases39. The inhibition of digestive peptidase indicates interaction of PIs with the active site of the enzyme, interfering with its digestive function33. These findings suggest the interference of SMTI with insect’s metabolism.
This study also evaluated the mRNA expression level of different genes present in melon fruit fly larvae when fed on SMTI incorporated diet. In a previous study it was documented that the herbivorous insects regulate their digestive functions according to diet so that they can evade the inhibitory effect of dietary peptidase inhibitor40. The mRNA level of trypsin and chymotrypsin was down-regulated in second instar larvae of melon fruit fly fed on artificial diet incorporated with SMTI. The primary enzymes associated with digestive mechanisms in dipterans are trypsin and chymotrypsin41. The inhibition of these enzymes leads to unavailability of essential amino acids causing physiological stress42. The mRNA expression level for Alkaline phophatase (AP) increased after SMTI consumption by the larvae, resulting in weight loss and reduced reproductive strength of the insect43. AP has been postulated to play a significant role in binding toxins and mediating their insertion into the membrane thus helping in their absorption in the insect44,45,46. SMTI also changed the mRNA expression level of some genes associated with detoxification and antioxidant pathways. The intake of SMTI incorporated artificial diet led to enhanced mRNA expression level of esterases and GST. Insect’s detoxification enzymes are mainly recognized for their role in defending against harmful compounds and maintaining the body’s normal physiological functions47,48. The mRNA expression level of both catalase and SOD was also up regulated which suggested metabolic stress in the insect. Catalase and SOD are crucial enzymes that assist an organism to deal with the body’s metabolic stress. SOD helps to maintain natural physiological stability by developing the first line of defense toward different toxic chemicals including plant compounds49. Catalase eliminates the hazardous hydrogen peroxide generated by the SOD action50.
Borate et al.51 revealed that anti-microbial activity is also possessed by certain PIs, suggesting that such PIs might be part of proteins related to defensive strategy that offer some protection against fungal and bacterial infections. Previous study52 had reported that Abelmoschus moschatus trypsin inhibitor from seeds displayed strong antibacterial activity towards E. coli, Streptococcus pneumoniae, Staphylococcus aureus, Proteus vulgaris, Bacillus subtilis, Bacillus cereus and moderate activity towards Pseudomonas syringae, Klebsiella pneumoniae, Streptococcus pyogenes and Pseudomonas aeruginosa. However, it also affected different fungal species, Candida tropicalis, Candida albicans, Candida glabrata, Asperigillus flavus and Asperigillus niger. Nabi et al.17 reported that serine PIs purified from the Sophora japonica seeds exhibited anti bacterial activity against Bacillus subtilis, Staphylococcus aureus and Streptococcus pneumonia whereas it didn’t show any inhibitory activity against E. coli, Klebsiella pneumonia, Saccharomyces cerevisiae and Candida albicans.
Material and Methods
Purification of trypsin inhibitor from S. mukorossi seeds
The seeds of S. mukorossi belonging to the Sapoteaceae family were obtained from Palampur, Himachal Pradesh, India. The endosperms of mature dry seeds of S. mukorossi were collected by removing the hard cover using pestle and mortar. To remove any bacterial or fungal contamination, the endosperms were washed with 0.01% mercury chloride and then with double distilled water. The treated endosperms were air dried and fine-ground flour was obtained by crushing them using liquid nitrogen. This flour was then defatted twice by stirring in acetone and hexane (1:1) mixture for 3 h at 4 °C. The defatted seed flour was then air dried and solubilised in 0.01 M sodium phosphate buffer (pH 7.6) by constant stirring for 3 h at 4 °C. Subsequently, the seed suspension was centrifuged at 5000 rpm for 20 min at 4 °C and the pellet was discarded. The supernatant obtained was termed as Crude extract (CE). The ammonium sulphate precipitation of the crude extract was done using different saturation ranges: 0–80% (F1), 20–80% (F2), 40–80% (F3), and 60–80% (F4). The precipitate from each saturation range was centrifuged at 10,000 rpm at 4 °C for 30 min. Each precipitate was then re-suspended in sodium phosphate buffer of 0.01 M (pH 7.6) and dialyzed on a 12 kDa cut off pore membrane for 24 h against the same buffer. The dialyzed sample with maximum trypsin inhibitory activity was subjected to DEAE-Cellulose column (1.5 × 16 cm) chromatography pre equilibrated with sodium phosphate buffer (0.01 M, pH 7.6). To remove the impurities from the column, it was washed with the same buffer and further eluted at a flow rate of 0.5 ml/min with increasing concentrations of NaCl (0.1–0.5 M) dissolved in sodium phosphate buffer (0.01 M, pH 7.6). Trypsin inhibitory activity of the collected fractions (1 ml/fraction) was checked and the fractions with maximum activity were pooled. The collected sample was dialyzed overnight at 4 °C with sodium phosphate buffer (0.01 M, pH 7.6) and further concentrated using amicon filters (Millipore).
The concentrated sample was further purified using CNBr-activated Trypsin-Sepharose affinity column (Sigma Aldrich, Germany). The column was eluted with KCl (2 mM, pH 2.0) for further studies. The fractions possessing maximum trypsin inhibitory activity were pooled and designated as S. mukorossi trypsin inhibitor (SMTI). The same SMTI was analyzed using a Reverse Phase-High Performance Liquid Chromatography (Shimadzu RP-HPLC system, LC-60AD) column by employing binary elution system (0–60% acetonitrile and 0.1% aqueous trifluoroacetic acid) at a flow rate of 3 ml/min for 15 min at 30 °C. The elutions were monitored at 220 nm for the detection of protein.
Protein concentration and Trypsin inhibition assay
Protein quantification was done by the Bradford method using bovine serum albumin (BSA) as a standard53. A previously described method was used to determine the inhibition in residual hydrolytic activity of bovine trypsin against BApNA (N α- benzoyl-DL-arginine-p-nitroanilide)54. SMTI was incubated with 20 μL trypsin (1 mg/ml in 0.05 M Tris-HCl, pH-8.2) at 37 °C for 10 min. Then, 100 μL of BApNA (prepared in 0.1 mM Dimethyl sulfoxide) was added to the reaction mixture and the change in absorbance was recorded continuously at 405 nm at an interval time of 1 min in a microplate reader (Model 680XR Plate reader (Bio-Rad Lab. Ltd)). One trypsin inhibitor activity unit (TIU) was defined as reduction of 0.01 units of absorbance at 410 nm.
SDS-PAGE
The homogeneity of SMTI was analyzed on a 12% resolving gel by SDS-PAGE following Laemmli’s method55. The molecular weight of the purified SMTI was determined by comparing with the corresponding relative movement of pre stained benchmark protein ladder (Invitrogen).
Optimal pH, optimal temperature and stability
Optimum pH for SMTI was determined by peptidase inhibitor assay at a range of pH varying from 6 to 11. Substrate casein (1%) was prepared in different buffers of different pH which included phosphate buffers (pH 6–8), Tris – HCl (pH 8–9) and carbonate – bicarbonate buffer (pH 9–11). The stability of SMTI over a range of pH was checked after incubating SMTI in buffers of different pH (6–11) for 30 min, at 4 °C. After incubation, 1 ml of the sample was assayed for trypsin inhibitory activity.
The thermal stability of SMTI was evaluated according to Dias et al.56. SMTI (1 mg ml−1) was dissolved in 1x PBS buffer, pH 7.5, and 100 μL aliquots were incubated in a water bath at different temperatures (10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 °C) for 30 min. Before testing the residual trypsin inhibitory activity, the samples were cooled to room temperature (25 ± 2 °C).
Effect of metal ions, detergents and organic solvents on peptidase inhibitor activity
Effect of various metal ions (Ca2+, Co2+, Cu2+, Fe3+, Zn2+, Mg2+, Ni2+, Mn2+) on activity of SMTI was evaluated using 1 mM, 5 mM, 25 mM and 50 mM final concentrations in the reaction mixture. The SMTI equilibrated with different concentrations of above listed metal ions were incubated for 30 min at 25 °C, and then assayed for trypsin inhibitor activity.
Effect of various non-ionic and ionic surfactants such as Triton X-100, SDS, Tween-80, CTAB, and Urea on SMTI activity was determined by incubating SMTI for 30 min with each surfactant at different concentrations 0.1%, 0.5%, 1.0% and 2.0%. Effect of various solvents namely acetone, acetonitrile, benzene, chloroform, DMSO, ethanol, ethylacetate, hexane, methanol and petroleum ether on SMTI activity was determined by incubating SMTI with different concentrations (10%, 20%, 30% and 40%) of these solvents for 30 min at 25 °C.
IC50, IC90, Kinetic parameters and Casein hydrolysis test
The SMTI concentration which reduces 50% and 90% of the trypsin activity (IC50 and IC90) was determined using linear regression as described in previous section50 using different SMTI concentrations (0.5 µM to 5.0 µM). The kinetic measurements of SMTI were done following the method of Dias et al.56. The different concentrations of SMTI (0.5 µM to 5 µM) were incubated with 20 µl of trypsin (1 mg/ml in 0.05 M Tris HCl, pH 8.2) for 10 min at 37 °C. The reaction was initiated by adding 100 μl of different concentrations (0.5 mM to 5 mM) of BApNA. The absorbance change was continuously monitored in a microplate reader (Model 680XR Plate reader (Bio-Rad Lab. Ltd)) for 10 min at 405 nm at an interval time of 1 min. A Line weaver Burk plot was plotted (using Graphpad Prism 8) to calculate Km and Vmax whereas Ki was calculated using Dixon plot by the intersection of the lines at the x-axis, corresponding to the substrate concentrations (0.5–5.0 µM). The inhibitory activity of SMTI was also examined by simple casein hydrolysis test using agar plates. The agar plates were prepared by mixing the separately autoclaved casein (2%) and bacteriological agar (2%). About 30 µl of trypsin (100 µg/ml) was mixed with each concentration of SMTI (0–100 µg/ml) and the solution was poured into each well made in casein agar plates. The well without SMTI was considered as control and the assays were done in triplicate under controlled conditions.
Insect assays
Laboratory Rearing of Melon Fruit Fly
The adults of melon fruit fly were reared on artificial and natural diet in insect culture room /B.O.D. of the laboratory under controlled conditions of temperature (25 ± 2 °C), relative humidity (70–80%) and photoperiod (10 L:14D).
Larval Growth Index (LGI) and Total Growth Index (TGI)
The effect of purified SMTI on second (64–72 h old) instar larvae of melon fruit fly was studied following the method of Srivastava57. Melon fruit fly adults deposited eggs on pumpkin pieces placed in wire mesh boxes. The pumpkin pieces (Charged) were then transferred to controlled conditions for egg hatching. Larvae were collected at 64–72 h stage in culture vials containing SMTI incorporated artificial diet (0.2 µg/ml, 1 µg/ml, 5 µg/ml, 25 µg/ml, 125 µg/ml, 625 µg/ml and control (water)). Evaluation of different parameters viz. larval period, total development period, percent pupation and percent adult emergence was done daily. There were six replicates for each concentration with 15 larvae in each replication. Larval Growth Index (LGI) and Total Growth Index (TGI) were calculated by using formula given as58.
LGI = Percent Pupation/Larval Period (in days).
TGI = Percentage Emergence/Total Development Period (in days).
Enzyme assay
LC50 was calculated for carrying out the enzyme assays. The second instar larvae of B. cucurbitae were fed with LC50 concentration of SMTI incorporated in artificial diet. The trypsin and chymotrypsin was extracted and isolated by the method described by Christellar et al.59,60. Actively feeding larvae were removed in distilled water and the larval homogenate was prepared in 0.15 M NaCl followed by centrifugation at 13500 rpm for 10 min at 4 °C. The supernatant was collected and stored at −20 °C for further analysis. For assay about 50 μl of extract was added to 50 μl of buffer in 96-well Elisa plate and incubated for 10 min at 37 °C. The reaction was started by adding 100 μl of 1 mM of BApNA, mixed in Dimethyl Sulfoxide (DMSO). The reaction was monitored at 405 nm using a microplate reader (Model 680XR Plate reader (Bio-Rad Lab. Ltd)) and residual trypsin inhibitory activity was calculated. Blanks were run in each case by replacing extract with distilled water.
Gene expression analysis
The relative expression of different enzymes related genes in the second instar larvae of melon fruit fly was measured using quantitative RT-PCR. To extract the total RNA using Trizol method (Invitrogen), the 2nd instar larvae of melon fruit fly fed with LC50 concentration of SMTI were collected at 24 h, 48 h and 72 h intervals. Agarose gel electrophoresis (1%) and Nanodrop spectrophotometer were used to check the quality and concentration of total RNA. The iScript cDNA synthesis kit of Biorad was used to synthesize cDNA from 1.0 μg of total RNA. The cDNA was diluted (10 fold) for further use and stored at −20 °C. The mRNA sequences of various genes, used to design primers of genes of interest were obtained from NCBI (Table 4) and actin was used as an internal reference gene. Power SYBR Green PCR Master Mix (Applied Biosystems) with Step One Real Time PCR System (Applied Biosystems) was used to perform the qRT-PCR for different genes. To determine the relative gene expression, 2−ΔΔCT method61 was used to calculate the threshold values (Ct).
Antibacterial activity
The purified SMTI’s antibacterial activity was evaluated using disc diffusion assay against different bacterial strains (B. thuringiensis, E. coli, P. aeruginosa and M. smegmatis). Different concentrations of SMTI were soaked in the sterile paper discs (6 mm diameter) and dried in air under sterile conditions. About 100 μL bacterial suspension was spread on LB (Luria Bertani) agar plates after growing the bacterial strains for 24 h. SMTI soaked disks were placed on agar plates and incubated at 37 °C overnight. The discs soaked with different antibiotics (Rifampin-100 µg, Ampicillin-100 µg, Carbenicillin-100 µg, and Kanamycin-50 µg) and sterile distilled water were used as positive and negative control, respectively. The minimum inhibitory concentration (MIC) for different bacterial strains was measured by comparing the zone of inhibition produced by SMTI concentration to that of positive control.
Statistical analysis
All analyses were reported as means with the standard error. For each set of results, analysis of variance (ANOVA) was applied, followed by the Dunnett or Tukey test.
Ethical approval and consent to participate
This article does not contain any studies involving human participants or animals performed by any of the authors.
Conclusion
Our findings suggest that SMTI has potential to impair the growth and development of B. cucurbitae larvae by altering its physiological and biochemical processes and can be explored for the development of resistant plants by plant breeders. It is also important to point out that delaying or interrupting the insect life cycle, such as that of B. cucurbitae, is an important strategy to mitigate their population without directly killing them, thereby helping in reducing the economic losses caused by phytophagous insect pests. In addition present findings contribute to a better understanding of the anti microbial and insecticidal potential of plant peptidase inhibitors and especially suggest that S. mukorossi is a potential source of bioactive proteins. Therefore, SMTI can be employed as an important tool for pest control as it has potential to protect plants against insect pests as well as bacterial pathogens.
Data availability
All data generated or analyzed during this study are included in this published article and its additional files.
References
Ferry, N., Edwards, M. G., Gatehouse, J. A. & Gatehouse, A. M. R. Plant-insect interactions: molecular approaches to insect resistance. Curr. Opin. Biotechnol. 15, 155–161 (2004).
Macedo, M. L. R., Sa, C. M., Freire, M. G. M. & Parra, J. R. P. A kunitz-type inhibitor of coleopteran proteases, isolated from Adenanthera pavonina L. seeds and its effects on Callosobruchus maculatus. J. Agric. Food Chem. 52, 2533–2540 (2004).
Richardson, M. Seed storage proteins: the enzyme inhibitors. In Methods in Plant Biochemistry, Amino Acids, Proteins and Nucleic Acids; Rogers, J. L. Ed.; Academic Press: New York 5, 259–305 (1991).
Ee, K. Y., Zhao, J., Rehman, A. & Agboola, S. O. Purifications and characterizations of a kunitz-type trypsin inhibitor from Acacia victoriae Bentham seeds. J. Agric. Food. Chem. 57, 7022–7029 (2009).
Oliveira, C. F. R. et al. Purification and biochemical properties of a Kunitz-type trypsin inhibitor from Entada acaciifolia (Benth.) seeds. Process Biochem. 47, 929–934 (2012).
Schuler, T. H., Poppy, G. M., Kerry, B. R. & Denholm, I. Environmental risk assessment of a transgene products using honey bee (Apis mellifera) larvae. Trends Biotechnol. 16, 68–175 (1998).
Dhillon et al. The melon fruit fly, Bactrocera cucurbitae: A review of its biology and management. J. Insect Sci. 5, 16 (2005).
Praveen, H. M., Nandeesh, M., Chandra Mouli, M. R., Rao, G. V. G. & Vijaysegaran, S. Management of melon fly, Bactrocera cucurbitae (Coquillett) infesting gherkin : an area wide control programme adopted in peninsular India. J. Hortl. Sci. 7(1), 68–74 (2012).
Suhagia, B. N., Rathod, I. S. & Sindhu, S. Sapindus Mukorossi (Areetha): An Overview. IJPSR. 2(8), 1905–1913 (2011).
Filho, L. C. A., Tabosa, P. M. S., Hissa, D. C., Vasconcelosa, I. M. & Carvalhob, A. F. U. First insights into insecticidal activity against Aedes aegypti and partial biochemical characterization of a novel low molecular mass chymotrypsin-trypsin inhibitor purified from Lonchocarpus sericeus seeds. Pest Manag. Sci. 74, 1362–1373 (2018).
Bezerra, C. S. et al. Exploiting the biological roles of the trypsin inhibitor from Inga vera seeds: a multifunctional Kunitz inhibitor. Process Biochem. 51, 792–803 (2016).
Gandreddi, V. D. S., Kappala, V. R., Zaveri, K. & Patnala, K. Evaluating the role of a trypsin inhibitor from soap nut (Sapindus trifoliatus L. Var. Emarginatus) seeds against larval gut proteases, its purification and characterization. BMC Biochemistry. 16, 23, https://doi.org/10.1186/s12858-015-0052-7 (2015).
Hernandez, C. S., Gallardo, N. M., Rangel, A. G., Rodriguez, S. V. & Frier, J. D. Trypsin and a-amylase inhibitors are differentially induced in leaves of amaranth (Amaranthus hypochondriacus) in response to biotic and abiotic stress. Physiologia Plantarum. 122, 254–264, https://doi.org/10.1111/j.0031-9317.2004.00398.x (2004).
Joshi, R. S., Mishra, M., Suresh, C. G., Gupta, V. S. & Giri, A. P. Complementation of intramolecular interactions for structural–functional stability of plant serine proteinase inhibitors. Biochim. Biophys. Acta. 1830, 5087–5094 (2013).
Macedo, M. L. R. et al. A trypsin inhibitor from Peltophorum dubium seeds active against pest proteases and its effect on the survival of Anagasta kuehniella (Lepidoptera: Pyralidae). Biochimica et Biophysica Acta. 1621, 170–182, https://doi.org/10.1016/S0304-4165(03)00055-2 (2003).
Machado, S. W. et al. Purification of a Kunitz-type Inhibitor from Acacia polyphylla DC Seeds: Characterization and Insecticidal Properties against Anagasta kuehniella Zeller (Lepidoptera: Pyralidae). J. Agric. Food Chem. 61, 2469–2478, https://doi.org/10.1021/jf3049565 (2013).
Nabi, M. et al. Physio-chemical Characterization and Anti-microbial Activity of Serine Protease Inhibitors Purified from the Sophora japonica Seeds. Pakistan Journal of Biological Sci. 21(9), 432–440, https://doi.org/10.3923/pjbs.2018.432.440 (2018).
Dabhade, A. R., Mokashe, N. U. & Patil, U. K. Purification: characterization and antimicrobial activity of nontoxic trypsin inhibitor from Albizia amara Boiv. Process Biochem. 51, 659–674 (2016).
Kuhar, K. et al. A Bowman–Birk protease inhibitor with antifeedant and antifungal activity from Dolichos biflorus. Acta Physiol Plant 35, 1887–1903, https://doi.org/10.1007/s11738-013-1227-8 (2013).
Chanphai, P. & Tajmir-Riahi, H. A. Trypsin and trypsin inhibitor bind milk beta-lactoglobulin: Protein–protein interactions and morphology. International Journal of Biological Macromolecules 96, 754–758 (2017).
Hebia, C., Bekale, L., Chanphai, P., Agbebavi, J. & Tajmir-Riahi, H. A. Trypsin inhibitor complexes with human and bovine serum albumins: TEM and spectroscopic analysis. Journal of Photochemistry and Photobiology B: Biology 130, 254–259 (2014).
Flora, S. J. S., Mittal, M. & Mehta, A. Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J. Med. Res. 128, 501–523 (2008).
Puntambekar, A. & Dake, M. Protease inhibitor from white Cranberry Beans (Phaseolus Vulgaris): isolation, purification and characterization. Int. J. Pharmacy and Pharmaceutical Sci. 9(9) https://doi.org/10.22159/ijpps.2017v9i9.20472 (2017).
Bacha, A. B., Jemel, I., Moubayed, N. M. S. & Abdelmalek, I. B. Purification and characterization of a newly serine protease inhibitor from Rhamnus frangula with potential for use as therapeutic drug. 3 Biotech. 7, 148, https://doi.org/10.1007/s13205-017-0764-z (2017).
Klibanov, A. M. Improving enzymes by using them in Organic solvents. Nature. 409, 241–246, https://doi.org/10.1038/35051719 (2001).
Vogel, R., Trautschold, I. & Werle, E. Natural Proteinase Inhibitors. Academic Press, New York (1968).
Oliveira, C. F. R. et al. Characterization of a Kunitz trypsin inhibitor from Enterolobium timbouva with activity against Candida species. International Journal of Biological Macromolecules. 119, 645–653 (2018).
Patthy, A. T., Molnár, P., Porrogi, R. & Naude, L. Graf, Isolation and characterization of a protease inhibitor from Acacia karroo with a common combining loop and overlapping binding sites for chymotrypsin and trypsin. Arch. Biochem. Biophys. 565, 9–16 (2015).
Rufino, F. P. et al. Inhibitory effects of a Kunitz-type inhibitor from Pithecellobium dumosum (Benth) seeds against insect-pests’ digestive proteinases. Plant Physiol. Biochem. 63, 70–76 (2013).
Kaur, A. P. & Sohal, S. K. Pea protease inhibitor inhibits protease activity and development of Bactrocera cucurbitae. Journal of Asia-Pacific Ento. 19, 1183–1189 (2016).
Kaur, H., Kaur, A., Kaur, A. P., Rup, P. J. & Sohal, S. K. Assessment of soybean inhibitor as a biopesticide against melon fruit fly, Bactrocera cucurbitae (Coquillett). J Plant Dis Prot., https://doi.org/10.1007/s41348-017-0108-6 (2017).
Punithavalli, M. & Jebamalaimary, A. Inhibitory activities of proteinase inhibitors on developmental characteristics of sugarcane Chilo infuscatellus (Snellen). Phytoparasitica. 47, 43–53, https://doi.org/10.1007/s12600-018-00710-1 (2019).
Salzman, K. Z. & Zeng, R. Insect response to plant defensive protease inhibitors. Annu Rev Entomol. 7(60), 233–52, https://doi.org/10.1146/annurev-ento-010814-020816 (2015).
Silva, D. S., Oliveira, C. F. R., Parra, J. R. P., Marangoni, S. & Macedo, M. L. R. Short and long-term anti nutritional effect of the trypsin inhibitor ApTI for biological control of sugarcane borer. J. Insect Physiol. 61, 1–7 (2014).
Ferreira R.S., et al. Effects of two protease inhibitors from Bauhinia bauhinoides with different specificity towards gut enzymes of Nasutitermes corniger and its survival Chemosphere, https://doi.org/10.1016/j.chemosphere.2019.01.108 (2019).
Saikhedkara, N. S. et al. Phyto-inspired cyclic peptides derived from plant Pin-II type protease inhibitor reactive centre loops for crop protection from insect pests. BBA - General Subjects 1863, 1254–1262, https://doi.org/10.1016/j.bbagen.2019.05.003 (2019).
Prasad, E. R., Dutta-Gupta, A. & Padmasree, K. Purification and characterization of a Bowman–Birk proteinase inhibitor from the seeds of black gram (Vigna mungo). Phytochemistry. 71, 363–372 (2010).
Srinivasan, A., Chougule, N. P., Giri, A. P., Gatehouse, J. A. & Gupta, V. S. Pod borer (Helicoverpa armigera Hubn.) does not show specific adaptations in gut proteinases to dietary Cicer arietinum Kunitz proteinase inhibitor. J. Insect Physiol. 51, 1268–1276 (2005).
Pandey, P. K., Singh, D., Singh, S., Khan, M. Y. & Jamal, F. A non host Peptidase Inhibitor of ∼14 kDa from Butea monosperma (Lam.) Taub. Seeds Affects Negatively the Growth and Developmental Physiology of Helicoverpa armigera. Biochemistry Research International 361821, 11, https://doi.org/10.1155/2014/361821 (2014).
Schluter, U. et al. Recombinant protease inhibitors for herbivore pest control: a multitrophic perspective. J. Exp. Bot. 61(15), 4169–4183 (2010).
Gomes, C. E. M. et al. Effect of trypsin inhibitor from Crotolaria pallida seeds on Callosobruchus maculatus (cowpea weevil) and Ceratitis capitata (fruit fly). Plant Physiol Biochem. 43, 1095–1102 (2005).
Chen, H., Gonzales-Vigil, E., Wilkerson, C. G. & Howe, G. A. Stability of Plant Defense Proteins in the Gut of Insect Herbivores. Plant Physiology. 143, 1954–1967 (2007).
Fan, S. G. & Wu, G. J. Characteristics of plant proteinase inhibitors and their applications in combating phytophagous insects. Bot. Bull. Acad. Sin. 46, 273–292 (2005).
Terra, W. R. & Ferreira, C. Insect digestive enzymes: properties, compartmentalization and function. Comparative Biochemistry and Physiology Part B: Comparative Biochem. 109, 1–62 (1994).
Yi, S. X. & Adams, T. S. Age and diapauses related acid and alkaline phosphatase activities in the intestine and malpighian tubules of the Colorado potato beetle, Leptinotarsa decemlineata (Say). Archives of Insect Biochemistry and Physiology. 46, 152–163 (2001).
Arenas, I., Bravo, A., Soberon, M. & Gomes, I. Role of Alkaline Phosphatase from Manducta sexta in the mechanism of action of Bacillus thuringiensis Cry1Ab toxin. The. J. Bio. Chm. 285(17), 12497–12503, https://doi.org/10.1074/jbc.M109.085266 (2010).
Taskin, V., Taskin, B. G., Kucukakyuz, K. & Kence, M. Determination of esterase enzyme polymorphism in housefly (Musca domestica L.) populations in Turkey. Turkish Journal of Zoology 35, 869–877 (2011).
Salinas, A. E. & Wong, M. G. Glutathione S-transferases- A review. Current Medicinal Chemistry 6, 279–309 (1999).
Bowler, C., Montagu, M. V. & Irize, D. Superoxide dismutase and stress tolerance. Annual Review of Plant Physiology and Plant Molecular Biology 43, 83–116 (1992).
McCord, J. M. & Fridovich, I. Superoxide dismutase. Journal of Biological Chemistry. 244, 6049–6055 (1969).
Borate, A., Khambhapati, A., Udgire, M., Paul, D. & Mathur, S. Preliminary Phytochemical Studies and Evaluation of Antibacterial Activity of Psoralea corylifolia Seed Extract. American Journal of Phytomedicine and Clinical Therapeutics. 2(1), 95–101 (2014).
Dokka, M. K. & Davuluri, S. P. Antimicrobial activity of a trypsin inhibitor from the seeds of Abelmoschus moschatus. L. Int. J. Curr.Microbiol. App. Sci. 3(5), 184–199 (2014).
Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Vasudev, A. & Sohal, S. K. Partially purified Glycine max proteinase inhibitors: potential bioactive compounds against tobacco cutworm, Spodoptera litura (Fabricius, 1775) (Lepidoptera: Noctuidae). Turk. J. Zoo. 40, 379–387 (2016).
Laemmli, U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 227, 680–685 (1970).
Dias, L. P. et al. A trypsin inhibitor purified from Cassia leiandra seeds has insecticidal activity against Aedes aegypti. Process Biochemistry. 57, 228–238 (2017).
Srivastava, B. G. A chemically defined diet for Dacus cucurbitae (Coquillett) larvae under aseptic conditions. Entomol. News Lett. 5, 24 (1975).
Kumar, A., Sood, S., Mehta, V., Nadda, G. & Shanker, A. Biology of Thysanoplusia orichalcea (Fab.) in relation to host preference and suitability for insect culture and bioefficacy. Indian Journal of Applied Entomology 18, 16–21 (2004).
Christeller, J. T., Laing, W. A., Markwick, N. P. & Burgess, E. P. J. Midgut protease activities in twelve phytophagous lepidopteran larvae: dietary and protease inhibitor interactions. Insect Biochem. Mol. Biol. 22, 735–746 (1992).
Christeller, J. T., Laing, W. A., Shaw, B. D. & Burgess, E. P. J. Characterization and partial purification of the black field cricket, Telleogryllus commodus (Walker): elastase is a major component. Insect Biochem. 20, 157–164 (1990).
Livak, K. J. & Schmittgen, T. D. Analysis of Relative Gene Expression Data Using Real Time Quantitative PCR and the 22DDCT Method. Methods 25, 402–408, https://doi.org/10.1006/meth.2001.1262 (2001).
Acknowledgements
The grant received from UGC New Delhi under the scheme “University with Potential for Excellence (UPE)” for conducting the research work is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: Samiksha and S.K.S. Performed the experiments: Samiksha, D.S. Analysed the data: Samiksha, S.K.S., D.S. and A.K.K. Wrote the manuscript: Samiksha. Revised the manuscript: Samiksha, D.S. and S.K.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samiksha, Singh, D., Kesavan, A.K. et al. Exploration of anti-insect potential of trypsin inhibitor purified from seeds of Sapindus mukorossi against Bactrocera cucurbitae. Sci Rep 9, 17025 (2019). https://doi.org/10.1038/s41598-019-53495-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53495-6
- Springer Nature Limited
This article is cited by
-
The effects of maternal care on the developmental transcriptome and metatranscriptome of a wild bee
Communications Biology (2023)
-
Protease Inhibitors: An Induced Plant Defense Mechanism Against Herbivores
Journal of Plant Growth Regulation (2023)
-
Peptidase inhibitor from Mucuna pruriens seeds inhibits the growth and development of Zeugodacus cucurbitae larvae
Phytoparasitica (2021)