Abstract
The present study reported the preparation of BiVO4 by co-precipitation method. The as-prepared BiVO4 photocatalyst were deposited on rGO sheets to form BiVO4/rGO via the hydrothermal method. The crystalline structure, morphological, optical properties, and surface properties of the synthesized pure BiVO4 compared to BiVO4/rGO composite were studied using X-ray diffraction (XRD), scanning electronmicroscopy (SEM), photoluminescence (PL) spectrophotoscopy, UV–vis spectrophotometer with an integrating sphere, and N2 adsorption-desorption isotherm based on BET theory. The photocatalytic activity of the prepared samples were evaluated by the degradation of MB dye in aqueous medium under visible light irradiation. The result showed that the BiVO4/rGO composite exhibited greater photocatalytic efficiency compared to pure BiVO4 with the photocatalytic degradation efficiency remains stable up to fifth cycle. The improved activity of the BiVO4/rGO composite might be attributed to the high surface area available to adsorb more MB molecules, and efficient charge separation of BiVO4 through π electron on the rGO structure. According to experimental results, the possible photocatalytic mechanism of the BiVO4/rGO composite were determined and the active species hydroxyl radical were reported. Based on photocatalytic activity inhibition in the presence of both h+ (VB) and O2•− (CB) scavengers over the BiVO4 photocatalyst, it can be proposed that the hydroxyl radical generated during the photocatalytic degradation mechanism is mainly responsible by the main active species of h+ and O2•− at VB and CB positions, respectively.
Similar content being viewed by others
Introduction
Water resource pollution is of utmost concern by scientist globally due to the increasing demand of water for socioeconomic development and human health. As a result, the over use of water together with water pollution and climate change are the major reasons for water scarcity1. Among organic pollutant compound, the organicazo dyes and aromatic organics are normally used in all industries to color the products in order to make them attractive2. The removal of colors from wastewater is important owing to their negative effects on the environmental water quality even at low quantities of organic dyes. As a result of their stability in chemical structure, it is very difficult to biodegrade aromatic molecules naturally. Several conventional technologies have been used in recent years for organic contaminant treatment. Among them are some technologies such as adsorption, coagulation, membrane filtration and sedimentation, which can only change organic contaminants from the primary toxic pollutants to secondary pollutants in treatment process rather than degrade those substances completely3,4,5,6. In addition, they are not effective to meet certain criteria requirements or generate non-biodegradable organic pollutants appearing in effluents after a long period of time. Hence, alternative methods have been developed to solve this issue. Advanced oxidation process (AOPs) are alternative methods that have received much attention for the removal of organic contaminant such as pesticides, organic dyes and industrial organic wastes. For AOPs in wastewater treatment, the highly reactive hydroxyl radicals (OH•) are the most powerful oxidizing species in the oxidative reaction of organic pollutant7,8. Among AOPs, the heterogeneous photocatalysis based on semiconductor catalyst has established its efficiency in degrading organic compounds and mineralizing them to carbon dioxide, water, and other small fragments9. The basic principle of photocatalysis is a process in which the catalyst is excited by light and produces highly oxidizing free radicals such as OH•. Pollutants in wastewater are then adsorbed and degraded by these free radicals on the surface of catalyst until they transform into carbon dioxide, water, and non-toxic small fragments. The type of semiconductor catalyst plays an important role in the photocatalytic process such as TiO2, CeO2, ZnO, WO3, BiVO4 photocatalyst which are often applied in photocatalytic treatment10,11,12,13. Among these catalysts, bismuth vanadate (BiVO4) has recently attracted considerable attention due to its high photocatalytic activity under visible-light irradiation and its small band gap of ~2.4 eV14,15,16,17,18,19. Although, BiVO4 is widely used in the photocatalytic degradation of organic contaminants, the low photocatalytic activity of pure BiVO4 is unavoidable due to its poor adsorption performance and the difficulty in migration and separation of electron-hole pairs.
Many researchers have modified BiVO4 photocatalysts by metal doping and coupling with other semiconductors in order to enhance charge separation as well as increase the photocatalytic activity. Some research works have been found to combine photocatalyst with reduced graphene oxide (rGO) for the photocatalytic degradation of organic dyes20,21,22,23,24,25,26,27,28,29. Since the rGO has shown high performance in many applications due to its excellent charge separation ability between the intrinsic delocalized π–π electron, the rGO could promote electron transport between the composite BiVO/rGO photocatalyst and the organic pollutant molecules30,31. Because of its high surface area, rGO can be applied as a supporting material, not only to significantly increase the surface area of the system, but also to increase the number of surface active sites. As a result, BiVO4/rGO composite particles are able to disperse and stabilize on a very high specific surface area of rGO for potential applications in photocatalysis32,33.
Therefore, the aim of this study is to synthesize a multifunctional material of BiVO4/rGO composite, combined with the photocatalytic activity of BiVO4 coupled with the adsorption and trapping abilities produced from rGO. The synthesized multifunctional material was also applied in the removal of methylene blue (MB). In addition, the analytical techniques including XRD, SEM, PL, DRS, and BET of BiVO4 compared to BiVO4/rGO composite were analyzed and discussed. Finally, the main radicals participating in the photocatalytic process was conducted by a trapping experiment and the photocatalytic mechanism of BiVO4 compared to BiVO4/rGO composite was further discussed.
Experimental
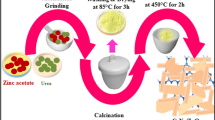
Preparation of BiVO4 photocatalyst by co-precipitation method
Firstly, BiVO4 powders was prepared by dissolving bismuth (III) nitrate, and ammonium vanadate in 3 M nitric acid under continuous stirring. The resulting dispersions were separated by centrifugation, washed with deionized water until the pH became neutral, dried at 70 °C for 24 h, and calcined at 550 °C for 4 h.
Preparation of BiVO4/rGO composite by hydrothermal method
Reduced graphene oxide (rGO) powders was synthesized from natural graphite powder by chemical oxidation using the synthesis methods from our previous work34. Firstly, the as-prepared rGO powders was added into the mixed solution of bismuth (III) nitrate, ammonium vanadate in 3 M nitric acid under continuous stirring until a homogeneous mixture was obtained. The mixture was then transferred into a 100 mL Teflon-lined stainless autoclave and the experiment was conducted at 160 °C for 12 h. After heat treatment, the precipitate was separated by centrifugation and re-suspension was done in DI water. The final stage of BiVO4/rGO composite was obtained after drying at 70 °C for 24 hrs.
Characterization
The crystal structure and phase composition of BiVO4 compared with composite materials were studied by X-ray diffraction (XRD, Philips X’Pert MPD) using Cu K-alpha radiation. The morphology of the prepared samples were determined using scanning electron microscopy(SEM, JSM-6335F, JEOL) and transmission electron microscopy (TEM,JSM-2010, JEOL). The photoluminescence (PL) spectrophotometer excited with 350 nm was applied in order to analyze the emission wavelength of 500–650 nm (Fluoromax-4 Horiba JobinYvon). The Brunauer-Emmett-Teller (BET) method was used to estimate the surface properties from N2 adsorption-desorption isotherm (Adtosorb 1 MP, Quantachrome). The UV–vis spectrophotometer (DRS, Shimadzu, UV–3101PC) with an integrating sphere attachment for diffuse reflectance analysis were used to study the reflectance spectra and further calculate the optical band gap from absorbance data using a Tauc plot of the Kubelka-Munk function35,36.
Photocatalytic activity
Photocatalytic properties of BiVO4 in comparison with BiVO4/rGO composites were tested over the degradation of methylene blue (MB) aqueous solution (3 ppm, 100 mL) with 0.02 g of photocatalyst. The photocatalytic system were irradiated with halogen lamps (Essential MO, Philips, Thailand) with power of 54 W and light intensity of 145 μW/cm2.The mixed suspension between photocatalyst powder and MB solution were stirred for 30 min without light irradiationto ensure that the MB molecules was adsorbed on the catalyst surface. The change in the MB concentration after visible irradiations for 120 min was analyzed from the decrease in absorbance intensity at the wavelength of 664 nm using UV-6100 double beam spectrophotometer (Shanghai Mapada Instruments Co., Ltd).
Results and Discussion
As shown in Fig. 1, the XRD patterns of BiVO4, and BiVO4/rGO shows that all the diffraction peaks corresponded to the monoclinic phase of BiVO4 (JCPDS 14-0688)37. In addition, the typical diffraction peak of rGO near 10.8°38 were not observed in the XRD pattern of BiVO4/rGO composite due to the fact that the addition of rGO in composite sample could yield the stacking disorder of rGO owing to the intercalating of BiVO4 into stacked rGO layers, which is in agreement with the literature report of Khalid et al.39. Also, the introduction of rGO to BiVO4 crystal structure might lead to decreasing crystallinity, and result in the broader peaks of BiVO4 in composite sample.
The morphologies of the prepared samples in Fig. 2(a–c) were separately analyzed by the scanning electron microscope at x1000 magnification. It was found that the BiVO4 in Fig. 2(a) constituted the surface roughness of individual spherical particles, which can be obtained in the size ranges of Σ5 μm. In Fig. 2(b), the rGO presented the corrugated structure of mixed-morphology of rGO sheets. In case of BiVO4/rGO composite, BiVO4 spherical-like particles were uniformly incorporated with rGO sheet, which firmly adhered BiVO4 spherical particles outside the surface, as shown in Fig. 2(c). In addition, the smaller particle size with high surface areas of BiVO4 was found in the BiVO4/rGOcomposite coupled system.The TEM image of rGO sheet in Fig. 2(d) reveals the local wrinkled structure with a thin layer, whereas the TEM images of BiVO4/rGO exhibits a BiVO4 particles attached on a wrinkled surface of rGO.
Photoluminescence spectroscopy (PL) has been carried out in order to examine the recombination efficiency of photo-induced electrons and holes in photocatalyst. Generally, high emission PL intensity means the rapid charge recombination rate, while a photocatalyst with low PL intensity refers to a low rate of electron/hole pairs. Figure 3(a) shows the PL spectra of BiVO4 compared to BiVO4/rGO, the PL emission intensity of the latter was the slightly lower intensity corresponding to the lower recombination rate. This improved the separation of electron/hole pairs, and subsequently suppressed the recombination process of BiVO4, which is promising for enhancing the photocatalytic activity.
Some part of oxygen-containing functional groups on the surface of rGO disappeared during the phase transformation process (in hydrothermal process), leaving unpaired π electrons on rGO sheets. Thus, rGO can help trapping electron transfering to form π–π electrons, coupling within the aromatic region on rGO surface (see Fig. 3(b)). The results are similar to those reported by Wang et al.40 and Yang et al.41 for the enhanced photocatalytic activity of TiO2 combined with reduced graphene oxide (rGO).
Band gap determination in Fig. 4 can be obtained from Tauc’s plot as a function of photon energy (eV) vs adsorption multiplied with photon energy. The extrapolation of the straight line in a certain region means that the band gap values was estimated to be Σ2.80 eV and Σ2.60 eV for BiVO4 and BiVO4/rGO, respectively. The decrease in band-gap possibly linked to the interaction of unpaired π electrons of rGO with free electrons on the BiVO4’s surface, playing a significant role in enhancing photocatalytic activity42,43.
The surface properties of rGO from our previous work34 revealed that the specific surface area of rGO was 1,323.39 m²/g, while the average pore volume and pore diameter are 0.68 cm³/g and 2.06 nm, respectively (See Table 1).The nitrogen adsorption-desorption isotherm of the composite materials compared with BiVO4 is revealed in Fig. 5. Both samples presented a typical type IV isotherm characteristic and showed hysteresis loops at the P/P0 ranges of 0–1.0, which demonstrated the characteristics of mesoporous materials44,45 corresponding to a pore diameter of 8.59 to 17.98 nm for BiVO4/rGO and BiVO4, respectively. From the hysteresis loops, the adsorbed quantity was found to increase when the rGO was added, leading to an enhanced specific surface area of the composite (228.39 m2/g) compared to that in pure BiVO4 (16.24 m2/g). Thus, the increase in the strength value of the specific surface due to combination of BiVO4 with rGO porous material does not only inhibit the electron–hole recombination, but rGO is also beneficial for adsorption to enrich the pollutants around the BiVO4 catalyst surface.
The photocatalytic performance of the pure BiVO4 and BiVO4/rGO were evaluated by the degradation of model dyes (MB). The degree of MB dye photocatalytic degradation (Ct/C0) was obtained by calculating the change in concentration from the variation of absorbance at the specific wavelength of 664 nm.
In order to study the equilibrium contact time, effect of contact time on MB adsorption in dark on BiVO4 and GO were studied by varying adsorption times from 10 to 60 min, and the results are illustrated in the Figure below. It was found that the removal of MB rises rapidly along with the contact time and attains the equilibrium after 30 min. The adsorption study was continued further for 60 min but no significant increase was observed in MB adsorption after 30 min contact time. Therefore, 30 min was considered as an equilibrium contact time for dark adsorption (light off) for both of BiVO4 and rGO samples (see Fig. 6). Hence, in this study, the remaining experiments for BiVO4/GO were carried out and stirred for 30 min under dark to allow adsorption/desorption equilibrium of MB on the catalysts.
After dark adsorption for 30 min, the photocatalytic degradation efficiency of MB dye reached 10, and 40% for BiVO4 and BiVO4/rGO, respectively, which is related to the increase in specific surface area obtained from BET results (see Fig. 7(a)). After visible light being on for 120 min, the degradation efficiencyof MB dye was negligible when no photocatalysts were added (about 4%). In case of single phase rGO, the photocatalytic degradation efficiencyof MB reached 90% by adsorption in the dark, but did not further degrade under light irradiation. For pure BiVO4, the visible-light photocatalytic performance of MB reached 60% after 120 min. Meanwhile, the degradation efficiency reached 95% when the photocatalysts were replaced by BiVO4/rGO.
For the sake of comparison, the mechanical mixed sample of BiVO4 and rGO has been prepared and its photocatalytic degradation experiment has been carried out. In a typical process, the as-prepared BiVO4 and the rGO were mechanically mixed in the agate mortar. After photocatalytic reaction, the photocatalytic degradation efficiency of the mechanical mixed sample was determined and found to be lower than that of BiVO4/rGO prepared by wet chemical process. This might be the result from the decrease of specific surface area of BiVO4/rGO via mechanical mixing due to the aggregation of BiVO4 particles and also the agglomeration of graphene oxide layers. As shown in Table 1, the specific surface area values measured for mechanical mixed sample amount to 183.36 m2/g, which is lower than the BiVO4/rGO composite prepared by hydrothermal method (228.39 m2/g). In addition, large specific surface area could provide more active sites for the adsorption of pollutants causing further degradation under light illumination.
In order to compare the speed of the photocatalyst under light irradiation, the apparent rate constants (k) were obtained from slopes of the graphs by plotting ln (Ct/C0) versus t. The pseudo-first-order rate constants can be calculated using the following equation43:
where C0 and Ct are the initial and remaining concentrations of MB at the different irradiated time (t), respectively. The corresponding pseudo-first-order kinetic plots are shown in Fig. 7(b). The photocatalytic degradation rate constant (k) were 0.0144 and 0.0067 min–1for BiVO4/rGO and BiVO4, respectively, as listed in Table 2.
Moreover, the reusability of BiVO4/rGO composite was tested by recovering photocatalyst for multiple cycles, as shown in Fig. 7(c). The result shows that photocatalytic degradation efficiency of MB over BiVO4/rGO composite does not significantly differ (Σ5–10%) up to 5th cycle, indicating that the prepared photocatalyst in this study was stable and can be reused up to fifth cycle.
The proposed mechanism for photocatalytic activity of MB dye over BiVO4/rGO composites is shown in Fig. 8(a). Under visible-light irradiation with an appropriate excitation energy, the electrons of BiVO4 are excited from the valence band (VB) to the conduction band (CB), thereby, forming the photogenerated electron−hole pairs. The excited electrons in CB of BiVO4 can migrate to rGO, and generate OH• via the reduction of O2 to yield superoxide radical (O2•−) and hydroxyl radical (OH•), which subsequently degrade the MB dye molecules. Meanwhile, the hydroxyl ion (OH−) adsorbed on the surface can be reduced by the photogenerated holes at VB of BiVO4 to give OH• and further react with the target products. Therefore, the BiVO4/rGO composites can enhance the photocatalytic activity of BiVO4 through the enhanced lifetime of photogenerated electrons/holes and specific surface area.
In order to determine the active species generated by hydroxyl radical (OH•) during the photocatalytic process, the terephthalic acid (TA) nonfluorescent substance was introduced as the trapping substance to yield a long lived highly fluorescent 2-hydroxyterephthalic acid (TAOH)46. Fluorescence spectra of a TAOH solution generated by BiVO4/rGO is represented in Fig. 8(b). The emission intensity at 425 nm (excited by 315 nm) increases with the increased irradiation time from 60 to 120 min, which corresponded to the higher photodegradation efficiency over irradiation time. Also, the oxidation of TA to TAOH confirms the OH• generation during photocatalysis as well as indicate the successful transfer of charge carrier and separation.
In order to prove the photocatalytic mechanism that the photocatalytic reactions happen based on the hydroxyl radical generated via VB or CB, the active species trapping experiment was conducted. In the typical process as reported in literatures47,48,49, p-benzoquinone and ammonium oxalate with concentration of 3 ppm were added to the photocatalytic reaction as O2•− (CB) and h+ (VB) scavengers, respectively. Photocatalytic degradation of MB in the presence of different scavengers over the BiVO4 photocatalyst was presented in Fig. 8(c). The results showed that the addition of h+ (VB) and O2•− (CB) scavengers inhibited the photocatalytic degradation of MB. It can be concluded that the position of photocatalytic mechanism as well as the OH· generation could be occurred via both of h+ at the CB level and O2•− at the VB position.
Conclusions
This study aimed to prepare the monoclinic spherical-shaped BiVO4 combined with rGO sheets via co-precipitation using the hydrothermal method. The pseudo-first-order rate constant of BiVO4/rGO was about two times higher than that of BiVO4. The enhancement in visible photocatalytic activity originated from the injection of excited electrons from the CB of BiVO4. The π–π electron coupling between the aromatic regions on rGO surface, increased the separation efficiency of photogenerated electron–hole pairs and further generate OH•. Thus, rGO in composites do not only help increasing the MB concentration near the surface active site of BiVO4 due to its high specific surface area, but also significantly promoting photogenerated charge separation. In addition, the recycling of the BiVO4/rGO photocatalyst became possible and can be effectively separated for 5 cycles. The studies of detailed mechanism using terepthalic acid, p-benzoquinone, and ammonium oxalate scavengers confirmed that the hydroxyl radicals are mainly responsible for photocatalytic activity, which produced from the h+ (VB) and O2•− (CB) in photocatalytic degradation of MB. The hydrothermal synthesis of pure BiVO4 are recommended for future research as different synthesis routes can cause the different morphology, particle size and crystallization resulting to different photocatalytic activities.
References
Houas, A. Photocatalytic degradation pathway of methylene blue in water. Applied Catalysis B: Environmental 31, 145–57 (2001).
Arami, M., Limaee, N., Mahmoodi, N. & Tabrizi, N. Equilibrium and kinetics studies for the adsorption of direct and acid dyes from aqueous solution by soy meal hull. Journal of Hazardous Materials 135, 171–179 (2006).
Yang, W., Wu, D. & Fu, R. Effect of surface chemistry on the adsorption of basic dyes on carbon aerogels. Colloids and Surfaces A: Physicochemical and Engineering Aspects 312, 118–124 (2008).
Mathivanan, M. & Elumalai, S. S. Moringa oleifera: A cost effective coagulant for dye degradation. Rasayan Journal of Chemistry 10, 1097–1103 (2017).
Buscio, V., Brosillon, S., Mendret, J., Crespi, M. & Gutiérrez-Bouzán, C. Photocatalytic membrane reactor for the removal of C.I. disperse red 73. Materials 8, 3633–3647 (2015).
Ito, T., Adachi, Y., Yamanashi, Y. & Shimada, Y. Long-term natural remediation process in textile dye-polluted river sediment driven by bacterial community changes. Water Research 100, 458–465 (2016).
Thiruvenkatachari, R., Vigneswaran, S. & Moon, S. A review on UV/TiO2 photocatalytic oxidation process. Korean Journal of Chemical Engineering 25, 64–72 (2008).
Mishra, N. S. et al. A review on advanced oxidation processes for effective water treatment, Current World. Environment 12, 470–490 (2017).
Nickheslat, A., Amin, M. M., Izanloo, H., Fatehizadeh, A. & Mousav, S. M., Phenol photocatalytic degradation by advanced oxidation process under ultraviolet radiation using titanium dioxideม Journal of Environmental and Public Health 2013 1–9 (2013)
Lee, S. & Park, S. TiO2 photocatalyst for water treatment applications. Journal of Industrial and Engineering Chemistry 19, 1761–1769 (2013).
Channei, D., Inceesungvorn, B., Wetchakun, N., Ukritnukun, S. & Nattestad, A. Photocatalytic degradation of methyl orange by CeO2 and Fe–doped CeO2 films under visible light irradiation Scientific reports 4, 5757 (1–7) (2004)
Ong, C. B., Ng, L. Y. & Mohammad, A. W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renewable and Sustainable Energy Reviews 81, 536–551 (2018).
Dong, P., Hou, G., Xi, X., Shao, R. & Dong, F. WO3-based photocatalysts: morphology control, activity enhancement and multifunctional applications. Environmental Science: Nano 4, 539–557 (2017).
Malathi, A., Madhavan, J., Ashokkumar, M. & Arunachalam, P. A review on BiVO4 photocatalyst: Activity enhancement methods for solar photocatalytic applications. Applied Catalysis A: General 555, 47–74 (2018).
Hlophe, P. V., Mahlalela, L. C. & Dlamini, L. N. A composite of platelet-like orientated BiVO4 fused with MIL-125(Ti): Synthesis and characterization. Scientific Reports 9(1–14), 10044 (2019).
Li, H., Yu, H., Quan, X., Chen, S. & Zhao, H. Improved photocatalytic performance of heterojunction by controlling the contact facet: High electron transfer capacity between TiO2 and the {110} facet of BiVO4 caused by suitable energy band alignment. Advanced Functional Materials 25, 3074–3080 (2015).
Kim, Y. et al. Hydrogen evolution: Hybrid Z‐scheme using photosystem I and BiVO4 for hydrogen production. Advanced Functional Materials 25, 2345–2345 (2015).
Wetchakun, N. et al. BiVO4/CeO2 nanocomposites with high visible-light-induced photocatalytic activity. ACS Applied Materials & Interfaces 4, 3718–3723 (2012).
Liu, H., Hou, H., Gao, F., Yao, X. & Yang, W. Tailored fabrication of thoroughly mesoporous BiVO4 nanofibers and their visible-light photocatalytic activities. ACS Applied Materials & Interfaces 8, 1929–1936 (2016).
Sharma, M., Behl, K., Nigam, S. & Joshi, M. TiO2-RGO nanocomposite for photocatalysis and environmental applications: A green synthesis approach. Vacuum 156, 434–439 (2018).
Lv, S., Wan, J., Shen, Y. & Hu, Z. Preparation of superlong TiO2 nanotubes and reduced reduced graphene oxide composite photocatalysts with enhanced photocatalytic performance under visible light irradiation. Journal of Materials Science: Materials in Electronics 28, 14769–14776 (2017).
Li, X. et al. Reduced graphene oxide enhanced amine-functionalized titanium metal organic framework for visible-light-driven photocatalytic oxidation of gaseous pollutants. Applied Catalysis B: Environmental 36, 501–508 (2018).
Liu, X. & Cai, L. A novel double Z-scheme BiOBr-RGO-polyaniline photocatalyst: Study on the excellent photocatalytic performance and photocatalytic mechanism. Applied Surface Science 483, 875–887 (2019).
Wang, Q. et al. Enhanced photocatalytic degradation and antibacterial performance by RGO/CN/BiOI composites under LED light. Applied Surface Science 497(497), 143753 (2019).
Ghouri, Z. K., Elsaid, K., Abdala, A., Al-Meer, S. & Barakat, N. A. M. Surfactant/organic solvent free single-step engineering of hybrid graphene-Pt/TiO2 nanostructure: Efficient photocatalytic system for the treatment of wastewater coming from textile industries. Scientific Reports 9, 1–10 (2018).
Xu, Y., Li, Y., Wang, P., Wang, X. & Yu, H. Highly efficient dual cocatalyst-modified TiO2 photocatalyst: RGO as electron-transfer mediator and MoSx as H2-evolution active site. Applied Surface Science 430, 176–183 (2018).
Wang, X., Zhao, X., Zhang, D., Li, G. & Li, H. Microwave irradiation induced UIO-66-NH2 anchored on graphene with high activity for photocatalytic reduction of CO2. Applied Catalysis B: Environmental 228, 47–53 (2018).
Yu, H., Xiao, P., Tian, J., Wang, F. & Yu, J. Phenylamine-Functionalized rGO/TiO2 photocatalysts: Spatially separated adsorption sites and tunable photocatalytic selectivity. ACS Applied Materials & Interfaces 8, 29470–29477 (2016).
Lonkar, S. P., Pillai, V. V. & Alhassan, S. M. Facile and scalable production of heterostructured ZnS-ZnO/Graphene nano-photocatalysts for environmental remediation. Scientific Reports 7, 1–14 (2018).
Liu, B., Zhenhua, W., Zhou, S. & He, J. Synthesis and characterization of a novel BiVO4/SiO2 nanocomposites. Materials Letters 160, 218–221 (2015).
Fang, D. et al. BiVO4-rGO with a novel structure on steel fabric used as high-performance photocatalysts. Scientific Reports 7, 1–9 (2017).
Channei, D., Nakaruk, A., Khanitchaidecha, W., Jannoey, P. & Phanichphant, S. Adsorption and photocatalytic processes of Mesoporous SiO2-Coated Monoclinic BiVO4. Frontiers in chemistry 6, 1–7 (2018).
Strobel, R., Metz, H. J. & Pratsinis, S. E. Brilliant yellow, transparent pure, and SiO2-coated BiVO4 nanoparticles made in flames. Chemistry of Materials 20, 6346–6351 (2008).
Channei, D., Nakaruk, A. & Phanichphant, S. Controlled oxidative ageing time of graphite/graphite oxide to reduced graphene oxide in aqueous media. Journal of the Australian Ceramic Society 54, 91–96 (2018).
Murphy, A. B. Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemical water-splitting. Solar Energy Materials & Solar Cells 91, 1326–1337 (2007).
Muniz, E. C. et al. Synthesis and characterization of mesoporous TiO2 nanostructured films prepared by a modified sol–gel method for application in dye solar cells. Ceramics International 37, 1017–1024 (2011).
Ma, Y., Jiang, H., Zhang, X., Xing, J. & Guan, Y. Synthesis of hierarchical m-BiVO4 particles via hydro-solvothermal method and their photocatalytic properties. Ceramics International 40, 16485–16493 (2014).
Sampath, S. et al. Direct exfoliation of graphite to graphene in aqueous Mmedia with diazaperopyrenium dications. Advanced Materials 25, 2740–2745 (2013).
Khalid, N. R., Ahmed, E., Hong, Z., Sana, L. & Ahmed, M. Enhanced photocatalytic activity of grapheme-TiO2 composite under visible light irradiation. Current Applied Physics 13, 659–663 (2012).
Wang, H. et al. Facile prepared ball-like TiO2 at RGO composites for oxytetracycline removal under solar and visible lights. Water Research 160, 197–205 (2019).
Yang, W. D., Li, Y. R. & Lee, Y. C. Synthesis of r-RGO/TiO2 composites via the UV assisted photocatalytic reduction of graphene oxide. Applied Surface Science 380, 249–256 (2016).
Khannam, S. K. D. M., Sharma, S. & Dolui, S. A reduced graphene oxide incorporated TiO2 photoanode for high efficiency quasi solid state dye sensitized solar cells based on a poly-vinyl alcohol gel electrolyte. RSC Advances 6, 55406–55414 (2016).
Li, L., Yu, L., Lin, Z. & Yang, G. Reduced TiO2-reduced graphene oxide heterostructure as broad spectrum-driven efficient water-splitting photocatalysts. ACS Applied Materials & Interfaces 8, 8536–8545 (2016).
Sotomayor, F. J., Cychosz, K. A. & Thommes, M. Characterization of micro/mesoporous materials by physisorption: Concepts and case studies. Accounts of Materials & Surface Research 3, 34–50 (2018).
Xue, C. et al. Fluoride doped SrTiO3/TiO2 nanotube arrays with a double layer walled structure for enhanced photocatalytic properties and bioactivity. RSC Advances 7, 49759–49768 (2017).
Ishibashi, K. I., Fujishima, A., Watanabe, T. & Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochemistry Communications 2, 207–210 (2000).
Rodríguez, E. M., Márquez, G., Tena, M., Álvarez, P. M. & Beltrán, F. J. Determination of main species involved in the first steps of TiO2 photocatalytic degradation of organics with the use of scavengers: The case of ofloxacin. Applied Catalysis B: Environmental 178, 44–53 (2015).
Zhang, Z. et al. Facile one-step synthesis of TiO2/Ag/SnO2 ternary heterostructures with enhanced visible light photocatalytic activity. Scientific Reports 8(1–11), 10532 (2018).
Qi. Zhang, N. et al. Advanced Fabrication of Chemically Bonded Graphene/TiO2 Continuous Fibers with Enhanced Broadband Photocatalytic Properties and Involved Mechanisms Exploration. Scientific Reports 6(38066), 1–15 (2016).
Acknowledgements
This work was financially supported by “The Thailand Research Fund (TRF) and Office of the Higher Education Commission (CHE) under grant number MRG6280017”. This research work was partially supported by Chiang Mai University.
Author information
Authors and Affiliations
Contributions
D. Channei wrote the main manuscript text, worked on material characterisation and prepared all figures. S. Phanichphant initiated and A. Nakaruk reviewed the manuscript. K. Chansaenpak replied the comments of peer reviewers and worked on material characterisation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Phanichphant, S., Nakaruk, A., Chansaenpak, K. et al. Evaluating the photocatalytic efficiency of the BiVO4/rGO photocatalyst. Sci Rep 9, 16091 (2019). https://doi.org/10.1038/s41598-019-52589-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52589-5
- Springer Nature Limited
This article is cited by
-
High-performance photocatalytic reduction of Cr(VI) using a retrievable Fe-doped WO3/SiO2 heterostructure
Discover Nano (2024)
-
Enhanced photocatalytic performance of a rGO-Ca2Fe2O5 nanocomposite for photodegradation of emergent pollutants
npj Clean Water (2024)
-
Development of Nb-Doped BiFeO3 via Hydrothermal Method for Photocatalytic Degradation of Rhodamine B (RhB) Dye
JOM (2024)
-
Development of magnetically separable MoS2/NiFe2O4 heterostructure for improved photocatalytic efficiency of malachite green (MG) degradation
Journal of Materials Science: Materials in Electronics (2024)
-
Enhanced photocatalytic activity of V3O7 / V2O5 – reduced graphene oxide nanocomposite towards methylene blue dye degradation
Environmental Science and Pollution Research (2024)