Abstract
Exploiting photocatalysts with characteristics of low cost, high reactivity and easy recovery offer great potentials for complete elimination of toxic chemicals and environmental remediation. In this work, Au/TiO2 network-like nanofibers were fabricated using a facile electrospinning technique followed by calcinations in air. Photocatalytic tests indicate that the Au/TiO2 network-like nanofibers possess an excellent photodegradation rate of rhodamine B (RB) under UV, visible and natural light radiation. The enhanced photocatalytic activity can be attributed to the plasmonic resonance absorption of Au nanoparticles, and photogenerated electrons and holes are effectively separated by the Au/TiO2 heterojunction structures. Furthermore, the three-dimensional network structure can provide a large number of active sites for RB degradation.
Similar content being viewed by others
Introduction
Because of the heavy use of fossil fuels, environmental pollutions, such as air and water pollution, have caused wide public concerns. Photocatalysis, as a “environment-friendly” technology, shows tremendous potentials for complete degradation of toxic contaminant and is identified as one of the most efficient and preiswert means to solve the issue of environmental pollution1,2. As a photocatalyst, titanium dioxide (TiO2: 3.2 eV for anatase and 3.0 eV for rutile) has the characteristics of inexpensive, nontoxic, environmental friendly and high-efficiency and it has been extensively studied and applied for the elimination of environment pollutants3,4,5. However, TiO2 has many drawbacks, such as very wide band gap, fast recombination rate of photogenerated electrons and holes, which hinder the utilization and commercialization of TiO26,7. The hole-migration occurs typically through charge transferring from adjacent position, and electrons usually travel faster in the conduction band8,9. If electrons and holes are not recombined at once, they can move to the surface of the particle and at there react with chemical species. Therefore, how to improve the separation of photoinduced electron-hole pairs in TiO2 is an important issue for exploiting the TiO2-based photocatalysts for further applications. Furthermore, due to the wide band gap, TiO2 is only activated under UV light. Much attempts have been devoted to exploring TiO2 as visible-light-active photocatalysts for effectively utilizing solar energy, which included doping with metal or non-metal elements10,11, introducing defects12, and coupling with metallic or semiconducting nanoparticles13,14,15.
Recently, noble metals have been testified to do good job in prohibiting electron-hole recombination due to the fact that noble metals would facilitate the separation of photogenerated electron–hole pairs and promote interfacial charge transfer16,17,18,19,20. In addition, noble metal nanoparticles have the ability to improve the visible-light absorption of TiO221,22,23,24,25. In particular, gold nanoparticles embedded in a TiO2 film (Au-TiO2) has attracted much attention due to that Au is a virulent precious metal with prominent catalytic activity26,27.
Currently, photocatalysts are normally in particle form which are suffering from aggregating. To achieve a high degree of utilization of photocatalyst, dispersing catalysts onto support materials with large surface area are often employed. Electrospinning is a cost-efficient and simple means, it is capable of fabricating network-like three-dimensional (3D) nanofibers that have been studied in many fields because of high specific surface area and mesoporous structure28,29,30. The nanofibers are fabricated by electrospinning that can be successfully prepared as network-like three-dimensional (3D) nanostructures with good stability characteristics31. What’s more, because of their particular properties compared with other nanostructure, network-like nanofibers are always attractive. The nanofibers have good thermal stability and excellent charge carrier mobility, which are especially useful to improve the catalytic performance of materials32,33,34. Furthermore, the network-like 3D nanofibers can work as supporters for Au nanoparticles which avoids the problem of aggregating35.
For these concerns above mentioned, in this work, we fabricated nanofibers composed of Au NPs and TiO2 by electrospinning method. The prepared nanofibers presented a macropores 3D network-like structure. The elementary composition and morphology of Au/TiO2 nanocomposite were investigated by X-ray diffraction (XRD) and transmission electron microscopy (TEM). The as-prepared 3D network-like nanofibers showed good photocatalytic performance in the degrading RB under visible and UV light because of the efficient charge separation between the formed heterojunction between Au and TiO2 NPs and high efficient utilization of photocatalysts. Besides, because of the long length of the nanofibers, the photocatalysts can be simply recycled by centrifuging, washing and drying with little damage of the photocatalytic performance.
Experimental Section
Chemicals and materials
All chemical reagents used in this study were analytical grade without further purification. Tetrabutyl titanate (C16H36O4Ti) and acid glacial (C2H4O2) were purchased from Chengdu Kelon Chemical Reagent Factory. Polyvinylpyrrolidone (PVP) was purchased from Shanghai Macklin Biochemical Co., Ltd. Ethanol (CH3CH2OH) was purchased from Chongqing Sichuan East Chemical Co., Ltd. Gold chloride solution (HAuCl4) and sodium citrate (C6H5Na3O7·2H2O) were purchased from Shanghai Aladdin biochemical technology Co., Ltd.
Preparation of Au nanoparticles
The synthetic method is that 2 mL of 50 mM HAuCl4 and 98 mL of deionized water were added in flask. The flask was heated in oil bath at 120 °C until the solution boiling. Then, 10 mL of 38.3 mM sodium citrate was added in flask quickly. Finally, keeping heating for 20 min under magnetic stirring, gold nanoparticles were synthesized. In the whole experiment process, the mouth of flask covered a layer of plastic wrap for reducing the evaporation of the water.
Preparation of Au/TiO2 nanofibers
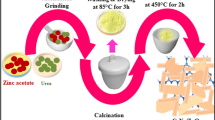
In this paper, Au/TiO2 nanofibers were prepared by using electrostatic spinning. The electrostatic spinning set-up was composed by an injector, a spinneret (a 15-gauge stainless steel needle), a high voltage power supply (18 kV), and a grounded aluminum foil used as collector. The precursor solution was consisted of tetrabutyl titanate (1 mL), acetic acid (1.5 mL), ethanol (5 mL) and polyvinylpyrrolidone (PVP) solution (10 mL). The as-prepared precursor solution was stirred at room temperature for about 12 h vigorously. Subsequently, the as-synthesized Au NPs solution with the amount of 5 μL, 10 μL, and 15 μL were added to the precursor solution, respectively. The precursor solution was stirred for 30 min at room temperature after adding Au. Then this mixture was put in a plastic syringe with a 15-gauge spinneret. The whole device was carried out under an electric voltage of 18 kV and was controlled the distance between the tip of the needle and the collector was 15 cm. The injection rate was set at 1.0 mL/h during electrospinning. The obtained fibers were then transferred into the muffle furnace for annealing at 500 °C for 12 h in air with a heating rate of 5 °C/min to produce Au/nanofibers. The as-prepared Au/TiO2 nanofiber with different Au NPs contents were marked as Au(x)/TiO2, where X represented the volume of milliliters of the Au solution that was added to the precursors solution. For comparison, pure TiO2 nanofibers were also made by the same process without adding Au NPs to the precursor.
Characterization
X-ray diffraction (XRD) analysis for the crystal structures of the samples was executed by X-ray diffractometer (Rigaku D/MAX2500PC) with Cu Kα radiation. The morphologies and size of the samples were tested by scanning electron microscopy (SEM, TESCAN MIRA) and transmission electron microscopy (TEM, FEI TECNAI G2 F20). UV-vis diffuse reflectance spectra of the samples were measured by a UV3600 spectrophotometer (Shimadzu, Japan) and BaSO4 was used as a reference.
Photocatalytic test
The photocatalytic performance of these Au/TiO2 nanofibers were measured by detecting the quantities of the RB degradation in water. An internal 350 W Mercury lamp was adopted as UV light source and a 300 W Xe lamp was equipped with an optical filter as visible light (λ > 420 nm). To cool the lamps, a circulating water system was used. Firstly, the initial concentration of the 50 mL RB solution was 10 mg L−1 and solid catalyst (0.02 g) was added and the solution was stirred without UV-Vis light for 30 min to obtain a good dispersion for establishing adsorption-desorption balance between the catalyst surface and the organic molecules. At given intervals of irradiation (time interval was 5 min in UV irradiation and 30 min in visible light), the reaction solutions (3 ml) as samples were extracted and centrifuged. Then the filtrates were analyzed by a spectrophotometer to calculated the decreased amount in the concentration of RB solution.
Results and Discussion
Structure and morphology
For revealing the crystalline and phase structures of the prepared nanocomposites, XRD patterns were measured and shown in Fig. 1. Specifically, the peaks at 25.5°, 37.9°, 48.2°, and 55.0° are attributed to (101), (004), (200) and (211) planes, respectively, which is indexed to anatase TiO2 (PDF card 21−1272, JCPDS). Besides, peaks at about 2θ = 27.5°, 41.3°, 56.8°, 62.9°, and 69.2° are also observed, which are attributed to the (110), (111), (220), (002) and (301) crystal faces of rutile TiO2, respectively. After the Au NPs solution was added to the TiO2 precursor, as shown in samples Au(5)/TiO2, Au(10)/TiO2 and Au(15)/TiO2, additional diffraction peaks with 2θ values of 38.2°, 44.3°, and 77.9° appeared, which are corresponding to (111), (200) and (331) crystal planes of cubic Au, respectively (PDF card 04–0784, JCPDS). The above XRD results show that the prepared samples are composites of Au and TiO2.
The morphologies of the as-prepared nanofibers were measured by SEM and shown in Fig. 2. Figure 2a shows that the pure TiO2 fibers are randomly aligned with the diameter ranging from 100 to 200 nm, and the randomly orientated nanofibers form a 3D network with macropores. SEM image (Fig. 2b) with higher magnification reveals that these TiO2 nanofibers have a glossy and homogeneous surface. After adding Au nanoparticles, the Au/TiO2 composite remained as a randomly aligned fiber network-like morphology, as shown in Fig. 2c. Because of the small size and low concentration of Au NPs, the surface of Au/TiO2 nanofibers remained unchanged (inset of Fig. 2c).
For purpose of further studying the microstructure of the Au/TiO2 nanofibers, the TEM and HRTEM images were measured. The low magnification TEM image of the Au/TiO2 samples also shows network nanofibers inlaid with particles (Fig. 2d). Meanwhile, a high-resolution image of the Au/TiO2 fiber (Fig. 2e) indicates that the nanofibers are composed of many granular particles in size of 10–20 nm. The lattice spacing of 0.235 nm is observed on the HRTEM image (Fig. 2f), corresponding to the (111) planes of Au, which indicates Au nanoparticles were successfully embedded with TiO2 particles.
UV-vis diffuse reflectance spectra
Figure 3 indicates the UV-Vis absorption spectra of the pure TiO2, Au(5)/TiO2, Au(10)/TiO2, Au(15)/TiO2 nanofibers. It can be observed that the pure TiO2 nanofiber has a sharp absorption edge at 420 nm, which is corresponding to TiO2 band gap excitation. The Au/TiO2 nanofibers were fabricated by incorporation of as-prepared Au nanoparticles during the electrospinning of TiO2 nanofibers and subsequent calcination in air. The main problem in this method maybe lead to the uneven distribution of Au nanoparticles and the Au nanoparticles were embedded in the TiO2 nanofibers, which showed no obvious absorption peak from Au nanoparticles in Au/TiO2 nanofibers. Furthermore, as shown in Table 1, the actual Au contents of Au(x)/TiO2 nanofibers is not high, which maybe lead to no obvious absorption peak as well.
However, the absorption edges of Au/TiO2 nanofibers show slightly red-shift, comparing with pure TiO2 nanofiber, which indicates an enhanced light absorption for Au/TiO2 nanofibers through incorporation of Au nanoparticles.
Photocatalytic activity
The photocatalytic performance of these Au/TiO2 nanofibers were measured by detecting the quantity of the RB degradation in water and the degradation effect of the Au/TiO2 nanofiber catalysts was labeled as C/C0, where C and C0 were marked the remainder and initial concentration of RB, respectively. The pure TiO2 nanofibers were acted as a photocatalytic performance reference. As shown in Fig. 4, The control experimental designs of different conditions were as follows: (1) the RB solution with photocatalysts without UV-Vis light irradiation; (2) the RB solution with UV-Vis light irradiation without photocatalysts; (3) the RB solution with photocatalysts with UV light irradiation; (4) the RB solution with photocatalysts with visible light irradiation. After 6 h without UV-Vis light irradiation or without nanofiber photocatalysts, the results (Fig. 4a) showed that there is barely degradations of RB.
(a) Photodegradation rate of RB in the presence of the pure TiO2 nanofibers, Au(5)/TiO2 nanofibers, Au(10)/TiO2 and Au(15)/TiO2 nanofibers in the absence of the nanofiber photocatalysts in the dark and with UV-vis light irradiation; (b) Photodegradation rate of RB for different nanofibers under visible light irradiation (300 mw/cm2); (c) Kinetic linear simulation curves of RB degradation under visible light for different nanofibers.
TiO2 is well known as a very efficient UV light active photocatalyst. In this work, we firstly examined the photocatalytic performance of our prepared TiO2 and Au/TiO2 nanofibers under UV light irradiation of 100 mw/cm2. As expected, all the TiO2 and Au/TiO2 nanofibers exhibited good photocatalytic activity for degrading RB solution. The TiO2 nanofibers could degrade almost 100% RB solution in 30 min under UV irradiation, and Au/TiO2 nanofibers even exhibited faster degrading rate than pure TiO2 nanofibers. It is worth mentioning that this degrading rate of our Au/TiO2 nanofibers can surpass most of the reported TiO2 and other UV-activated photocatalysts, as listed in Table 2.
Exploring TiO2 as a visible light activated photocatalyst is of great importance for potential applications. We further evaluated the photocatalytic activity of our prepared nanofibers under visible light irradiation of 300 mw/cm2. Not surprising, pure TiO2 nanofibers showed a bad degradation rate in visible light, with only degradation rate 5% in 120 min, as shown in Fig. 4b. However, Au/TiO2 nanofiber photocatalysts exhibited much enhanced photocatalytic activity in visible light, and the degradation effect of RB was about 35, 42 and 9% after 120 min for the sample of Au(5)/TiO2, Au(10)/TiO2 and Au(15)/TiO2 nanofibers, respectively. The kinetic analysis of degradation of RB which illustrates the photocatalytic efficiency was also evaluated. Because of the initial concentration of RB solution was low (C0 = 10 mg/L) in our experiment, and the kinetics linear emulation curve of the photocatalytic performance of these Au/TiO2 nanofibers followed the first order kinetics model of Langmuir-Hinshelwood. The explanation is depicted below36:
where C means the concentration of RB after the reaction (mg/L), t means ultraviolet or visible light illumination time, k means the reaction velocity constant (mg/(L min−1)), K means the adsorption index of the reactant (L/mg) and Kapp means the apparent first-order rate constant (min−1). The determined Kapp for four catalysts is summarized in Fig. 4c. It is revealed that the photocatalytic performances followed the order: Au(10)/TiO2 nanofibers >Au(5)/TiO2 nanofibers >Au(15)/TiO2 nanofibers >TiO2 nanofibers.
The photocatalytic activity of the as-prepared pure TiO2 and Au(10)/TiO2 nanofibers were further compared by RB degradation under natural light irradiation (15 mw/cm2). It can be seen that the Au(10)/TiO2 photocatalysts exhibited superior photocatalytic performance for degrading RB solution compared with the pure TiO2 nanofibers. As shown in Fig. 5a, the degradations of RB by using Au(10)/TiO2 nanofibers as photocatalysts reached almost 100% in 270 min irradiation. In the same condition, the degradation of RB by using pure TiO2 nanofibers was just 40%.
Stability is an important factor of the catalyst in the practical application. In order to test the stability of the Au(10)/TiO2 nanofibers, three recycling experiments were carried under identical conditions. As shown in Fig. 5b, after a three cycle experiment in UV irradiation, the photocatalytic degradation efficiency barely changes, with the degradation rate of about 93% during the third experiment.
Possible mechanism on the photocatalytic activity
As is well known that the main reaction substances include hole (h+), hydroxyl radical (•OH) and superoxide radical (•O2−) in the process of photocatalytic oxidation. For differentiating the role of reactive oxygen species in RB degradation and explaining the reaction mechanism, ammonium oxalate (AO), isopropanol (IPA) and benzoquinone (BQ) were chosen as quenching agents for h+, •OH and •O2−, respectively37,38,39. The experimental results (Fig. 6a) revealed that with the addition of AO and BQ, the photodegradation efficiency of RB decreases from 40% to 27.0% and 28.3%, implying that hole (h+) and •O2− act as the main reactive oxygen species in the process of photodegradation.
Based on the aforementioned experimental results, a feasible scheme (Fig. 6b) is proposed. In our case, the Au/TiO2 nanofibers were irradiated by UV and visible light, respectively. As shown in Scheme 1 for the situation under visible light irradiation, photo-generated electron-hole pairs are appeared at Au NPs because of surface plasmon resonance (SPR)40. The conduction band energy of TiO2 is lower that the Femi level of Au, but the sprayed electrons coming from gold can transfer to the conduction band of TiO2. In our work, The TiO2 nanofibers have anatase/rutile phases. O2 reduction by the photo-induced electrons on the rutile surface is inefficient but the anatase is more active for O2 reduction. As a result, the electrons prefer transfer from rutile to anatase can effectively suppresses the recombination of photogenerated electron-hole pairs and accelerates the photodegradation procedure41. The photogenerated electrons transfer to the rutile of TiO2 and further transfer to anatase to initiate reacting with the dissolved oxygen and the holes (·Au+) react with H2O or OH−, avoiding the recombination of electron-hole pairs, which can enhance the photocatalytic effect of TiO2. The migration of photogenerated electrons is very fast on TiO2, also indicating that the photogenerated electron-hole pairs can be effectively separated42. In other words, the combination of gold and TiO2 is supposed to generate a charge separation condition with relatively mild oxidation (positive gold) and same reduction (TiO2 conduction band) potentials as TiO243. The process of RB degradation can be further illustrated as following: the photo-induced electrons are injected into the TiO2 conduction band (Eqs (2) and (3)). The electrons can combine with the dissolved oxygen molecules and produce •O2− (Eq. (4)), then the HOO• are produced by protonation (Eq. (5)), the HOO• and captured electrons react to generate H2O2 (Eq. (6)), and finally •OH are produced (Eq. (7)). At the same time, the h+ can combine with OH− or H2O in the solution to form •OH (Eqs (8) and (9)). The RB solution was degraded by •O2− and •OH to CO2, H2O and other environmental pollutants44 ((Eqs (10) and (11)).
Conclusion
In summary, using a facile electrospinning method followed by calcinations, Au/TiO2 nanofibers were successfully fabricated. The nanofibers presented a network-like three-dimensional (3D) structures with macropores and Au particles with a size of 10–20 nm were well dispersed on the TiO2 fibers. The prepared Au/TiO2 nanofibers exhibited much enhanced photocatalytic activity by degradation of RB under UV, Vis and natural light irradiation. It is believed that the enhanced photocatalytic performance is due to the high utilization of Au particles on the fibers with a three-dimensional network structures which worked as a framework for providing high available Au active sites for degrading RB, and to the efficient charge separation through Au/TiO2 heterojunction structure. This study highlights the potential use of electrospinning technique to fabricate TiO2 nanofibers as noble metal supports for photcatalysis.
Data Availability
The authors declare that data in our manuscript are available.
References
Ochiai, T. & Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applicationfor environmental purification. J. Photoch. Photobio. C 13, 247–262 (2012).
Pi, M., Wu, T., Zhang, D., Chen, S. & Wang, S. Facile preparation of semimetallic WP2 as a novel photocatalyst with high photoactivity. RSC Adv. 6, 15724–15730 (2016).
Asahi, R., Morikawa, T., Ohwaki, T. & Aoki, K. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Sci. 293, 269–271 (2001).
Ma, Y. et al. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 114, 9987–10043 (2014).
Schneider, J. et al. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 114, 9919–9986 (2014).
Al-Shahry, M. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Sci. 297, 2243–2245 (2002).
Pelaez, M. et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Cata. B. 125, 331–349 (2012).
Christoforidis, K. C. & Fernández-García, M. Photoactivity and charge trapping sites in copper and vanadium doped anatase TiO2 nano-materials. Catal. Sci. Technol. 6, 1094–1105 (2016).
Asahi, R., Morikawa, T., Irie, H. & Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 114, 9824–9852 (2014).
Nowotny, J. et al. Defect chemistry and defect engineering of TiO2-based semiconductors for solar energy conversion. Chem. Soc. Rev. 44, 8424–8442 (2015).
Li, B., Hao, Y., Zhang, B., Shao, X. & Hu, L. A multifunctional noble-metal-free catalyst of CuO/TiO2 hybrid nanofibers. Enery Environ. Sci. 7, 3431–343 (2014).
Tian, J., Hao, P., Wei, N., Cui, H. & Liu, H. 3D Bi2MoO6 Nanosheet/TiO2 Nanobelt Heterostructure: Enhanced Photocatalytic Activities and Photoelectochemistry Performance. ACS Catal. 5, 4530–4536 (2015).
Sood, S., Mehta, S. K., Sinha, A. S. K. & Kansal, S. K. Bi2O3/TiO2 heterostructures: Synthesis, characterization and their application in solar light mediated photocatalyzed degradation of an antibiotic, ofloxacin. Chem. Eng. J. 290, 45–52 (2016).
Gupta, S. M. & Tripathi, M. A review of TiO2 nanoparticles. Phys. Chem. 56, 1639–1657 (2011).
Etacheri, V., Di Valentin, C., Schneider, J., Bahnemann, D. & Pillai, S. C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photoch. Photobio. C. 25, 1–29 (2015).
Herrmann, J. M., Tahiri, H. & Ait-Ichoub, Y. Characterization and photocatalytic activity in aqueous medium of TiO2 and Ag-TiO2 coatings on quartz. Appl. Catal. B. 13, 219–228 (1997).
Evanoff, D. D. & Chumanov, G. Synthesis and Optical Properties of Silver Nanoparticles and Arrays. Phys. Chem. 6, 1221–1231 (2005).
Dai, H., Gong, J., Hakyong, K. & Doukrae, L. A novel method for preparing ultra-fine alumina-borate oxide fibres via an electrospinning technique. Nanotechnology. 13, 635–637 (2002).
Hao, Y. & Shao, X. Mesoporous TiO2 nanofibers with controllable Au loadings for catalytic reduction of 4-nitrophenol. Mat. Sci. Semicon. Proc. 40, 621–630 (2015).
Li, B., Hao, Y. & Shao, X. Synthesis of hierarchically porous metal oxides and Au/TiO2 nanohybrids for photodegradation of organic dye and catalytic reduction of 4-nitrophenol. J. Catal. 329, 368–378 (2015).
Choi, J., Park, H. & Hoffmann, M. R. Effects of Single Metal-Ion Doping on the Visible-Light Photoreactivity of TiO2. J. Phys. Chem. 114, 783–792 (2010).
Rosario, A. V. & Pereira, E. C. The role of Pt addition on the photocatalytic activity of TiO2 nanoparticles: The limit between doping and metallization. Appl. Catal. B. 114, 840–845 (2014).
Sun, H. et al. Visible light responsive titania photocatalysts codoped by nitrogen and metal (Fe, Ni, Ag, or Pt) for remediation of aqueous pollutants. Chem. Eng. J. 231, 18–25 (2013).
Amrollahi, R., Hamdy, M. S. & Mul, G. Understanding promotion of photocatalytic activity of TiO2 by Au nanoparticles. J. Catal. 319, 194–199 (2014).
Zielińska-Jurek, A. & Hupka, J. Preparation and characterization of Pt/Pd-modified titanium dioxide nanoparticles for visible light irradiation. Catal. Today. 230, 181–187 (2014).
Li, D., Wang, Y. & Xia, Y. Electrospinning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 3, 1167–1171 (2003).
Sigmund, W. et al. Processing and Structure Relationships in Electrospinning of Ceramic Fiber Systems. J. Am. Ceram. Soc. 89, 395–40 (2006).
Dai, Y., Liu, W., Formo, E., Sun, Y. & Xia, Y. Ceramic nanofibers fabricated by electrospinning and their applications in catalysis, environmental science, and energy technology. Adv. Technol. 22, 326–338 (2010).
Gołąbiewska, A. et al. Visible light photoactivity of TiO2 loaded with monometallic (Au or Pt) and bimetallic (Au/Pt) nanoparticles. Appl. Surf. Sci. 317, 1131–1142 (2014).
Ligon, C. & Latimera, K. Electrospun metal and metal alloy decorated TiO2 nanofiber photocatalysts for hydrogen generation. RSC Adv. 8, 32865–32876 (2018).
Tian, Y. & Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 127, 7632–7637 (2005).
Chen, Y. et al. Synergetic Integration of Cu1.94S–ZnxCd1–xS Heteronanorods for Enhanced Visible-Light-Driven Photocatalytic Hydrogen Production. J. Am. Chem. Soc. 138, 4286–4289 (2016).
Ge, M. et al. A review of one-dimensional TiO2 nanostructured materials for environmental and energy applications. J. Mater. Chem. A. 4, 6772–6801 (2016).
Li, B. & Zhang, B. Optimization of plasmon-induced photocatalysis in electrospun Au/CeO2 hybrid nanofibers for selective oxidation of benzyl alcohol. J. Catal. 348, 256–264 (2017).
Zhang, Z., Shao, C. & Li, X. Electrospun Nanofibers of ZnO−SnO2 Heterojunction with High Photocatalytic Activity. J. Phys. Chem. C. 114, 7920–7925 (2010).
Lee, M. S., Park, S. S., Lee, G.-D., Ju, C.-S. & Hong, S.-S. Synthesis of TiO2 particles by reverse microemulsion method using nonionic surfactants with different hydrophilic and hydrophobic group and their photocatalytic activity. Catal. Today. 101, 283–290 (2005).
Zhou, X., Hu, C. & Hu, X. Plasmon-Assisted Degradation of Toxic Pollutants with Ag-AgBr/Al2O3 under Visible-Light Irradiation. J. Phys. Chem. C. 114, 2746–2750 (2010).
Yang, Y., Zhang, G. & Yu, S. Efficient removal of organic contaminants by a visible light driven photocatalyst Sr6Bi2O9. Chem. Eng. J. 162, 171–177 (2010).
Li, Y., Wan, J., Yao, H. & Dang, L. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. J. Mol. Catal. A-Chem. 334, 116–122 (2011).
Wu, T., Liu, G. & Zhao, J. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of Rhodamine B under visible light irradiation in aqueous TiO2 dispersions. J. Phys. Chem. B 102, 5845–5851 (1998).
Yu, Y., Wen, W. & Qian, X. UV and visible light photocatalytic activity of Au/TiO2 nanoforests with Anatase/Rutile phase junctions and controlled Au locations. Sci. Rep. 7, 41253 (2017).
Furube, A. & Du, L. Ultrafast Plasmon-Induced Electron Transfer from Gold Nanodots into TiO2 Nanoparticles. Phys J. Am. Chem. Soc. 129, 14852–14853 (2007).
Primo, A., Corma, A. & García, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 13, 886–910 (2011).
Gerischer, H. & Heller, A. The role of oxygen in photooxidation of organicmolecules in semi-conductor particles. J. Phys. Chem. 95, 5261–5267 (1991).
Acknowledgements
This work is supported by the National Natural Science Foundation of China (NSFC) (grants 51672031) and the Fundamental Research Funds for the Central Universities (Project No. 2018CDJDWL0011, 106112017CDJQJ308820). We also acknowledge the support from the sharing fund of large-scale equipment of Chongqing University.
Author information
Authors and Affiliations
Contributions
Zhuojun Duan contributed to the preparation of Au/TiO2 nanofibers, photocatalytic test and drafting manuscript; Yingzhou Huang contributed to the preparation of Au nanoparticles Dingke Zhang and Shijian Chen contributed the design of research and data analysis. All authors were involved with reviewing the article. All authors have approved the final version of this article.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Duan, Z., Huang, Y., Zhang, D. et al. Electrospinning Fabricating Au/TiO2 Network-like Nanofibers as Visible Light Activated Photocatalyst. Sci Rep 9, 8008 (2019). https://doi.org/10.1038/s41598-019-44422-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44422-w
- Springer Nature Limited
This article is cited by
-
Unveiling the mechanism of enhanced methylene blue degradation using chromium-TiO2/carbon nanocomposite photocatalyst
Journal of Solid State Electrochemistry (2023)
-
Gold Nanoparticles/Titania/Graphene Oxide Composite as a New Efficient Aerobic Oxidation Photocatalyst
Iranian Journal of Science and Technology, Transactions A: Science (2021)
-
Ternary Pt@TiO2/rGO Nanocomposite to Boost Photocatalytic Activity for Environmental and Energy Use
Journal of Inorganic and Organometallic Polymers and Materials (2021)
-
Recent progress on Ag/TiO2 photocatalysts: photocatalytic and bactericidal behaviors
Environmental Science and Pollution Research (2021)
-
Analysis of resistance to bending of metal electroconductive layers deposited on textile composite substrates in PVD process
Scientific Reports (2020)