Abstract
Recent conceptual and practical advances in phylogenetic species delimitation have enabled progressively robust biodiversity studies. Delimiting species in widespread taxa is an intriguing problem; the edible operculated land snail Cyclophorus volvulus (Müller, 1774) is a good example since it shows a high degree of shell and color variation along with a widespread distribution throughout Thailand. Taxonomic boundaries for C. volvulus were examined and clarified using a combined morphological and phylogenetic approach, the latter of which was based on both nuclear and mitochondrial gene sequences. Moreover, three species delimitation analyses were applied: Poisson tree processes (PTP), automatic barcode gap discovery (ABGD), and generalized mixed Yule-coalescent (GMYC). All phylogenetic trees revealed that C. volvulus was polyphyletic and comprised of three clades that coincided with their geographic distribution. The three species delimitation analyses concurred with the phylogenies and formed at least three groups. According to the results, C. volvulus s.l., as currently recognized, consists of three distinct species in Thailand: C. volvulus s.s., C. occultus sp. nov., and C. borealis sp. nov., which are described herein. Moreover, several of these highly distinct C. volvulus evolutionarily significant units (ESU) are likely to require urgent conservation attention.
Similar content being viewed by others
Introduction
Cyclophorus Monfort, 1810 is an operculated land snail genus whose members are widely distributed in Southeast Asia1,2,3. Traditionally, Cyclophorus snails have been classified based only on their shell morphological characters1,2,3,4. However, shell characteristics can be extremely variable, a phenomenon caused by phenotypic plasticity5,6,7,8, especially in widely dispersed species9. Cyclophorus are among the most confused in terms of taxonomy. They are one of the largest and most diverse groups of Cyclophoridae, with more than 100 nominal species1,2,3,4,6,7. However, some Cyclophorus species show high morphological variation, which can make their identification very difficult.
Cyclophorus volvulus (Müller, 1774) is one Cyclophorus species that shows high morphological variability. It is widely distributed in limestone habitats in Asia, including India, Thailand, Malaysia, China, and Vietnam1,3,6,10.C. volvulus is common in Thailand and resides in almost all parts of the country6,11,12. For over 4,000 years, this snail has been considered edible by local Thai, Laotian, and Vietnamese people13. However, in the past ten years, the number of Cyclophorus species, including C. volvulus, appear to be declining14, particularly in Eastern and Central Thailand. This phenomenon could be caused by overharvesting, climate change, and/or habitat destruction, among others. The high degree of intra-specific variation in shell morphology and geographically disjunct populations may confound taxonomy, either by lumping different species or by oversplitting species on the basis of geographic population differentiation6. Thus, the taxonomic status of this snail remains muddled, and additional methods are required to help resolve the taxonomic status of C. volvulus.
Molecular phylogenetic analyses and species delimitation approaches are powerful tools for resolving taxonomy, including cryptic species. These techniques have aided in understanding the evolutionary history of several land snail taxa6,7,9,15,16. The molecular techniques are useful to identify valuable morphological characteristics or evaluate features that are inappropriate for taxonomy17. Thus, this study aimed to determine the validity of species boundaries in C. volvulus within Thailand by combining molecular phylogeny, species delimitation, and morphological approaches. The recognition of cryptic species is necessary to evaluate the true diversity and inform conservation management of the regional status of this snail in limestone habitats.
Results
Molecular and phylogenetic analyses
The 16S ribosomal RNA (rRNA) (462 bp), cytochrome oxidase I (COI) (660 bp), 28S rRNA (585 bp), and concatenated dataset of all three DNA regions (1,707 bp) were aligned for the 29 individual C. volvulus samples, which represented 15 populations from Thailand, along with Cyclotus spp. and Leptopoma vitreum as outgroups. The concatenated dataset had a 44.55% GC content. Heterogeneity in base composition between sequences is known to affect phylogenetic inference, so the variations in base pair composition among sequences for the concatenated datasets were tested using χ2 analysis (χ2 = 29.54, degrees of freedom = 120, P = 1.000). The dataset included 609 variable sites and 443 parsimony-informative sites. The partition homogeneity test, performed using PAUP 4.0b10 with 100 replicates, showed no significant incongruence between the gene fragments (P = 0.097), while the K2P-distance between the taxa (without outgroups) ranged from 0.000 to 0.120 (inter- and intra-specific K2P-distances were 0.079 and 0.012, respectively).
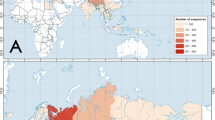
The phylogenetic relationships of C. volvulus samples are shown in Fig. 1. There was significant statistical support for a phylogenetic tree where certain clades coincided with certain morphological groups. However,C. volvulus was polyphyletic; it was divided into three well-supported main clades that represented three geographical regions, i.e., Northern (clade A), East-Central (clade B), and Western (clade C) Thailand. The sister taxon for clade A was C. subfloridus, while the sister taxa for clade B and C were C. labiosus and C. fulguratus, respectively. Clade A was strongly supported by 100% neighbor-joining (NJ) and maximum-likelihood (ML) bootstrap replicates and 1.00 posterior probability (PP) of Bayesian inference (BI). Clade B was supported by 94% NJ and 96% ML bootstrap replicates and 1.00 PP of BI, and clade C by 100% of NJ and ML bootstrap replicates and 1.00 PP of BI (Fig. 1). The K2P-distances among clades A, B, and C ranged from 0.104–0.113 for 16S rRNA, 0.146–0.152 for COI, and 0.008–0.019 for 28S rRNA (Tables 1 and 2).
Location of C. volvulus complex sampling sites in Thailand and a BI tree. A phylogenetic tree of the C. volvulus complex and related species was constructed using 1707 nucleotide sites of the concatenated 16S, COI and 28S genes. Bootstrap support values (when >70%) and PP (when >0.70) for each node are shown on the tree (based on the NJ/ML/BI methods).
Species delimitation
The three Thai C. volvulus groups (clades A, B, and C; Fig. 1) were supported by all three species delimitation methods (GMYC, ABGD, and PTP). Moreover, the three methods were congruent and proposed the three C. volvulus clades as candidate species, hereafter denoted as Group-A, Group-B, and Group-C (Fig. 2). However, the PTP method went further by splitting the three clades into more restricted candidate species.
Species delimitation analyses of the C. volvulus complex based on the concatenated 16S rRNA, COI and 28S rRNA genes. Results of species delimitation analyses using ABGD41, GMYC42 and PTP16, respectively. PSHs are labeled with letters and numbers; The PP support values for each node are shown on this BI phylogenetic tree when greater than 0.70.
Geometric morphometrics of shell morphology
Clades A–C (Fig. 1) were provided as an a priori group for each canonical variate analysis (CVA), which supplied a graphical display of the shape differences between them from the relationship between the two obtained canonical variate (CV) variables (Fig. 3). The shape variability explained 66.64% of the variance in the first canonical axis (CV1), with an eigenvalue of 0.7039, while shell shape variability explained 12.78% of the variance in the second axis (CV2), with an eigenvalue of 0.8358. Overall, the shell shapes of the three clades showed some overlap. However, there were also significant differences among clades based on permutation tests for Mahalanobis and Procrustes distances (P < 0.0001 to 0.0018 for C. volvulus). Accurate shell classification for each clade, along with cross-validation of discriminant function analysis, was 78% for clade A, 67% for clade C, and 63% for clade B (Fig. 1), with a 69% overall rate of reliability for identifying each C. volvulus individual. The overall significance tests were based on pooled variances (i.e., the average variance across all groups).
Geometric-morphometric study of shell shape variation in C. volvulus (type specimen; square symbol). (A) The measurements and landmarks (13 black spots). (B) Wireframe pictures of C. volvulus shell showing the shape deformations (solid line) from the consensus configuration (dotted line) to each extreme negative and positive CVs. (C) Plots of individual scores for the canonical variates (CVs) that were derived from the canonical variate analysis (CVA).
A wireframe modification (Fig. 3) defined the shape change from the consensus configuration along the two CVs. For CV1, individuals located in the positive portion of the axis had a wider shell compared to those in the negative portion (represented by shifts in landmarks 6 and 8 for C. volvulus). CV1 showed differences in aperture, where individuals in the positive portion of the axis had a narrower shell aperture compared to the negative portion. The CV2 of the C. volvulus complex showed differences in shell shape, prominently defined by landmarks 7 and 9, which represent the side of the last whorl twist, compared to the other landmarks as the score decreased.
In the present study, most of the examined C. volvulus samples showed a high degree of similarity in shell size and protoconch and jaw patterns (Fig. 4). However, we found slightly different characteristics among clades A–C, such as shell and umbilicus color patterns. However, the possible type specimen was placed in clade C; its shell was most similar to C. volvulus.
Discussion
In this study, we confirmed the existence of three highly divergent C. volvulus s.l. lineages in Thailand; these genetically differentiated and geographically isolated populations were confirmed using multi-loci species delimitation. These lineages (Clades A, B and C in Northern, East-Central, and Western Thailand, respectively; Fig. 1) were first recognized in a previous study6, and further verified here with the analysis of new samples as highly divergent populations (Figs 1 and 2). Therefore, the three gene loci that we analyzed were generally consistent with the previous study on the relationship of the genus Cyclophorus6. The individual gene trees showed some differences in topology and support for each lineage. However, individual gene trees are often different from the species tree due to retention and incomplete sorting of ancestral polymorphisms, especially in the case of recently diverged species or populations6,18,19,20. All three species delimitation analyses revealed the three C. volvulus lineages identified in the phylogenetic trees (Fig. 1) and clearly reflected three-to-thirteen distinct evolutionary significant units (ESUs; Fig. 2). PTP species delimitation analysis revealed additional candidate species within C. volvulus relative to the ABGD and GMYC methods (number of candidate species: PTP = 13, ABGD = 3, and GMYC = 3). Intra-specific K2P divergences for mitochondrial DNA in the three main candidate species ranged from 0.011–0.041, while the other Cyclophorus species ranged from 0.000–0.021. These species delimitation analyses were responsible for the high average intra-specific K2P genetic divergence observed for all candidate species. This relatively high intra-specific genetic divergence suggests that there are at least three separate species (clades A–C). Additionally, the results highlight differences in evolutionary patterns for the three nucleotide regions during inter-specific divergence and following demographic changes in candidate species. Notably, similar genetic distances were inferred to be indicative of distinct species in studies of other land snails7,21.
Generally, cryptic diversity is common among land snails that are widely distributed and/or fragmented in distribution12. As time passes reduced gene flow and/or a complete lack of genetic exchange between populations would promote new species due to allopatric processes. Indeed, morphology comparison among the three clades with their sister taxa underscored this supposition. Specifically, clade A was similar to C. subfloridus in aperture shape but different in shell shape, size, and color pattern, clade B was similar to C. labiosus in color pattern but different in aperture shape, and clade C was similar to C. fulguratus in shell size but different in color pattern.C. volvulus clades A–C (Fig. 1) and their sister taxa may have recently undergone allopatric fragmentation within the large geographic distribution between the different localities7,22. Alternatively, they may be under similar selective pressure or niches that cause them to undergo convergent evolution23. Intriguingly, our PTP analysis provided a different number of candidate species compared to ABGD and GMYC methods. This discrepancy may demonstrate that neutral genetic variation in the snail is influenced by the environment. Compared to the three consistent candidate species identified with all delimitation methods, the remaining ten PTP-derived candidate species exhibited relatively little difference in shell morphological characteristics (e.g., size, shape, and color pattern). We conclude that the intra-specific genetic divergence for these additional PTP-identified species is too low to currently consider them as distinct species7,21, but the relatively high genetic divergence indicates they may split into different species in the future.
Although the external C. volvulus s.l. morphology among the three clades was highly similar, there were slight differences. Shell shape geometric morphometrics revealed significant divergence among these groups as well as considerable overlap in the shell shape, results that support their cryptic morphology. The possible type specimen, considered most related to C. volvulus in shell shape, was placed in clade C. In addition to the high degree of external morphology similarity, the genital systems of these groups also could not be used for classification and identification6,11.
In this study, we provided a robust provision for the previously reported relationships and conservation management of Cyclophorus6. We also identified priorities for ongoing and future investigations. Further, an extensive taxonomic revision of this genus would be valuable. The evidence presented in this study strongly suggests that the three geographic populations of the C. volvulus complex in Thailand should be recognized as three distinct species: C. volvulus s.s. (clade C) and the undescribed C. occultus sp. nov. (clade B), and C. borealis sp. nov. (clade A).
Systematics
Family Cyclophoridae Gray, 1847
Genus Cyclophorus Montfort, 1810
Cyclophorus volvulus (Müller, 1774)
Figure 1 (clade C), 4 (A, D, G) and 5 (A, B)
Helix volvulus Müller, 1774: 82. Type locality: unknown.
Cyclophorus volvulus — Kobelt, 1911: 143, 1441. Nantarat et al., 2014: 99–111, Figs 3 and 4B (clade 1v)6.
Material examined
Possible syntypes ZMUC ex. Spengler coll. (1 shell; Fig. 5A). Lan-Sang, Tak: CUMZ 1376 (3 shells) [N 16°46′57″ E 99°01′08″], CUMZ 1669 (18 shells) [N 16°46′57″ E 99°01′08″], Wang Chao, Tak Province: CUMZ 1749 (2 shells)[N 16°30′16″ E 99°09′40″], Doi Hau Mod, Tak Province: CUMZ 1747 (20 shells) [N 15°57′36.5″ E 98°51′22.1″], Erawan Waterfall, Kanchanaburi Province: CUMZ 1830 (2 shells) [N 14°22′30.9″ E 99°08′39.4″], Khao Rong, Phetchaburi: CUMZ 1713 (4 shells) [N 13°01′14″ E 99°54′53″], Aow Noi, Prachuapkhiri Khan: CUMZ 1806 (11 shells) [N 11°51′39.2″ E 99°49′18″].
Measurements
Shell height: ranges 23–27 ± 0.03 mm Shell width: ranges 29–37 ± 0.03 mm.
Description
Shell medium, thick, solid with low conical shape, smooth or with variously developed spiral ribs. Periostracum thin, with white umbilicus and narrow white spiral band on the last whorl. Aperture round or oblique; lip whitish with expanded and reflected. Umbilicus rather wide and deep. Operculum thin corneous, round, dark brown with multispiral.
Radula and jaw
Taenioglossan radula, each row contains 7 teeth with formula 2-1-1-1-2. Central tooth symmetrical semicircular shape composed of 5 denticles; central cusp well developed, largest and pointed tip; inner lateral cusps small, triangular shape with pointed cusp; outer lateral cusps very small with dull cusps. Lateral and inner marginal teeth tricuspid consisted 3 cusps; central cusp large, convex shape, and flanked with smaller and pointed head of one inner and one outer lateral cusps. At the edge of ribbon, outer marginal teeth bicuspid consisted 2 cusps; inner cusp small with pointed head; outer cusp very large triangular shape with pointed (Fig. 4D).
Jaw consists of two symmetrical parts, each part thin and rhomboid shape. Sculpture with very thin longitudinal parallel ridges, connected to each other with strong parallel slanting transverse ridges, which made sculpture of jaw like underside of leaf blade (Fig. 4G).
Distribution
The ranges of this species are demarcated to Southeast Asia including Southern China, Hong Kong and India.
Cyclophorus occultus Nantarat and Panha, sp. nov.
urn:lsid:zoobank.org:act:63358F60-1801-4A13-B75C-AA1857370309
Figures 1 (clade B), 4(B,E,H) and 5C
Cyclophorus volvulus — Nantarat et al., 2014: 99–111, Figs 3 and 4B (clade 2v)6.
Type materials
Holotype CUMZ 1171 (Fig. 5C; height 25 mm, width 32 mm, 5 whorls). Paratypes CUMZ 1762 (17 shells) and NHMUK (2 shells).
Type locality
Sao Wa Luk camp, Nakhonratchasima Province (N 14°35′45″ E 101°23′31″).
Etymology
The specific epithet ‘occultus’ is from the Latin word meaning “hidden or, concealed” with reference to the cryptic species. Description of this new species is here attributed to the first and the last author, Nantarat and Panha, respectively.
Other material examined
Wang Kan Luang Waterfall, Lopburi Province: CUMZ 1618 (12 shells) [N 15°6′48″ E 101°6′48″], Tum Sun Ti-Suk Temple, Lopburi Province: CUMZ 1784 (3 shells) [N 15°12′10″ E 100°39′50″], Dao Khao Kaeo Cave, Saraburi Province: CUMZ 1716 (3 shells) [N 14°53′05″ E 101°20′49″], Ban Ta Sao, Saraburi Province: CUMZ 1617 (3 shells) [N 14°42′16.8″ E 101°4′43.4″], Phu-Kae Botanic Garden, Saraburi Province: CUMZ 1629 (17 shells) [N 14°40′24″ E 100°53′22″], Khao Chakan, Sra Kaeo Province: CUMZ 1756 (15 shells) [N 13°39′28.9″ E 102°05′18.6″].
Measurements
Shell height: ranges 23–25 ± 0.02 mm. Shell width, ranges 30–34 ± 0.05 mm.
Description
Shell medium, thick, solid with low conical shape, apex acute; whorls 5 to 6, convex; suture deep and wide. Periostracum exhibits color variation from dark to pale brown. Protoconch with thin narrowly discernible transverse ridges (Fig. 4B). Last whorl rounded, enlarge and with whitish colour. Shell colour usually light to dark brownish colour, sometime dark zigzag streak present; on periphery with narrow dark spiral band; lower periphery with wide striated stripe surrounded umbilicus. Aperture circular, slightly oblique; lip little expanded and reflected with white colour. Umbilicus widely open and deep. Operculum corneous, multi-spiral and little concaved center (Fig. 5C).
Radula and jaw
Central tooth big and semicircular with 5 denticles, large at center and smaller arrangement of the other. Lateral teeth bicuspid, endocone small with sharp cusp, ectocone large. Marginal teeth bicuspid, have different arrangement into two layers of inner marginal teeth locates the same level of central tooth, the large sharp inflated ectocone, the outer marginal teeth locates at the edge of the ribbon with a little lower position from lateral teeth, with almost the same shape as inner marginal teeth.
General structure of jaw similar to that of C. volvulus s.s. (Fig. 4H) The difference characters are sculptured with thicken and strong longitudinal parallel ridges and connected to each other with very strong parallel slanting transverse ridges (Fig. 5C).
Distribution
This new species is known from several localities in the northeastern Thailand. The application of the taxon name in previously publications by Nantarat et al.6 as the C. volvulus s.l. are here reconsidered as the new species.
Remark
This new species can be distinguished from C. volvulus s.s. and C. borealis sp. nov. by having uniform brown to brownish shell colour, with light brown zigzag streaks. Aperture lip is a little bit more oblique than C. volvulus s.s. Shell pattern around umbilicus with wide spiral band and light brown zigzag streaks almost umbilicus area. This new species also supported by phylogenetic tree (Fig. 1) and geometric morphometrics (Fig. 3).
Cyclophorus borealis Nantarat and Panha, sp. nov.
urn:lsid:zoobank.org:act:62369102-DE9A-4323-B420-3E54AD961922
Figures 1 (clade A), 4(C,F,I) and 5D
Cyclophorus volvulus — Nantarat et al., 2014: 99–111, Figs 3 and 4B (clade 3v)6.
Type materials
Holotype CUMZ 1218 (Fig. 5D; height 25 mm, width 31 mm, 5 whorls). Paratypes CUMZ 1673 (22 shells) and NHMUK (2 shells).
Type locality
Tum Ra Kang, Sukhothai Province (N 17°09′54″ E 99°33′35″).
Etymology
The specific epithet is from the Latin word ‘boreas’ meaning “northern”. It refers to the new species that strictly appears in northern Thailand. Description of this new species is here attributed to the first and the last author, Nantarat and Panha, respectively.
Other material examined
Tum Ra Kang, Sukhothai Province: CUMZ 1673 (24 shells) [N 17°09′54″ E 99°33′35″], Pa Ma-Muang Temple, Phitsanulok Province: CUMZ 1770 (15 shells) [N 16°34′1.8″ E 100°40′30.4″].
Measurements
Shell height: 24–26 ± 0.04 mm. Shell width, ranges 29–37 ± 0.07 mm.
Description
Shell medium, funnel-shaped, umbilicate, solid, smooth surface, brownish, apex acute; whorls 5, convex; suture deep and wide. Periostracum thick corneous with brownish colour. Protoconch with thin closely discernible transverse ridges (Fig. 4C). Remain whorls with only thin irregular growth lines. Last whorl rounded, expand, with white colour background. Shell colour brownish, with dark zigzag streak present; on periphery with narrow dark brown spiral band; on lower periphery with narrow striated stripe surrounded umbilicus. Aperture circular, slightly oblique; lip little expanded with white colour. Umbilicus widely open and deep. Operculum corneous, multi-spiral and little concaved center (Fig. 5D).
Radula and jaw
Central tooth semicircular 5 denticles with large center and smaller arrangement of the other. Lateral teeth bicuspid, endocone small with sharp cusp, ectocone large. Marginal teeth bicuspid, have different arrangement into two layers of inner marginal teeth locates the same level of central tooth, the large sharp inflated ectocone, the outer marginal teeth locates at the edge of the ribbon with a little lower position from lateral teeth, with almost the same shape as inner marginal teeth (Fig. 5F).
General structure of jaw similar to that of C. volvulus s.s. The difference characters are sculptured with strong longitudinal parallel ridges, connected to each other with solid parallel curving transverse ridges (Fig. 5I).
Distribution
This new species is known from the northern Thailand. The application of the taxon name in previously publications by Nantarat et al.6.
Remark
This new species can be distinguished from C. volvulus s.s. and C. occultus sp. nov. by having uniform brown to dark brownish shell colour, with dark brown zigzag streaks. The last whorl is relatively larger than C. volvulus s.s. and C. occultus sp. nov. Shell height is relatively more than the other two species. Shell pattern around umbilicus with solid band outside and light brown spiral line with light brown zigzag streaks about ¾ of umbilicus. This new species also supported by phylogenetic tree (Fig. 1) and geometric morphometrics (Fig. 3).
Conclusion
Morphologically cryptic species among land snails are an important problem for taxonomists, ecologists, and biogeographers. Study of these species provides valuable information for communicating the unique properties of separate evolutionary lineages and conservation management. The presented phylogenetic interpretation of C. volvulus s.l., based on mtDNA (16S rRNA and COI) and nuDNA (28S rRNA) sequence data, was conducted using multiple approaches, including molecular phylogeny, species delimitation, geometric morphometrics, and morphological data. All methods supported the presence of at least three geographically distinct candidate species in C. volvulus s.l. over broad regions (clades A, B, and C from Northern, East-Central, and Western Thailand, respectively). We clarified the species boundaries of C. volvulus and the two newly described species. We unveiled cryptic diversity in a biodiversity hotspot: the identification of these (at least) three cryptic lineages suggests additional species diversity. The new species may comprise the portion of the potential niche that is actually occupied, given that historical and biotic factors pose further restriction on new species’ distribution24. Differences among new species’ niches could reflect their evolution, an actual change in the potential niche, and/or a mechanism that results in isolation and speciation. However, these differences could also reflect morphological plasticity, where some PTP candidate species possess the same potential niche despite ostensibly expressing a different niche. This study also provides useful information to evaluate ESUs for conservation, especially in some parts of Northeast and East-Central Thailand where the snail populations have declined dramatically. Understanding the high level of lineage diversity in an operculated land snail in Thailand, is within an area designated as one of the biodiversity hotspots and a world conservation priority25.
Methods
Taxon sampling and morphology
Cyclophorus volvulus s.l. was collected from 15 populations (Tables 3 and 4). Species determination was based on the publications of Kobelt1, Reeve3, Müller10, and Benthem Jutting26,27. The shells were subsequently compared with the relevant type specimens and reference collections, including those at the Natural History Museum of Denmark (Zoological Museum), University of Copenhagen, Denmark (ZMUC), The Natural History Museum, London (NHMUK), and Chulalongkorn University, Museum of Zoology, Bangkok, Thailand (CUMZ). Living specimens were preserved. The foot tissues were fixed and preserved in 95% (v/v) ethanol, while the remaining parts of each specimen were preserved in 70% (v/v) ethanol for morphological study. In total, 51 specimens of C. volvulus and related species were examined. Adults shell were measured with Vernier calipers to the nearest 0.1 mm and photographed with a digital camera.
Details of the shell surface, protoconch, jaw and radula morphology (form and formula of radula teeth) were observed using scanning electron microscopy (SEM; JSM-5410 LV) at the Scientific and Technological Research Equipment Centre (STREC), Chulalongkorn University.
DNA extraction, amplification and sequencing
Genomic DNA was extracted from the foot tissue using a DNAeasy Tissue Kit (QIAGEN Inc.). The mitochondrial 16S ribosomal RNA (16S rRNA) region and cytochrome c oxidase subunit I (COI) gene region were PCR amplified using the 16sar and 16sbr28 and the LCO1490 and HCO219829 primer pairs, respectively. The nuclear 28S ribosomal RNA (28S rRNA) region was PCR amplified using primers 28SF4 and 28SR530. The thermal cycling conditions for amplification of the 16S rRNA and 28S rRNA were 2 min at 94 °C, followed by 36 cycles of 30 s at 94 °C, 30 s at 50 °C and 90 s at 72 °C, and then a final 5 min at 72 °C. That for the amplification of COI was performed in the same condition except that the annealing stage was changed to 2 min at 42 °C and the extension time to 2 min. Products were resolved and visually checked by 1% (w/v) agarose gel electrophoresis with SYBR Safe staining and blue light transillumination. A QIAquick purification Kit (QIAGEN Inc.) was used to purify the PCR products before being sent for commercial automatic cycle-sequencing at Macrogen, Inc. (Seoul, South Korea).
Phylogeny inference
The mitochondrial DNA (approximately 462 bp of 16S rRNA and 660 bp of COI) and nuclear DNA (approximately 585 bp of 28S rRNA) sequences were edited and aligned using MUSCLE version 3.631, a sub-program within the MEGA 6.01 software32, and then improved manually. The unambiguous of 16S rRNA, COI (all codon positions) and 28S rRNA fragments were aligned across all samples. All base frequencies and molecular character statistics were calculated using MEGA 6.0132,33.
All sequences were checked for ambiguous nucleotide sites and saturation before being subjected to phylogenetic analysis. The saturation graphs were plotted using uncorrected pairwise distances for transition and transversion substitutions to envision saturation and identify the taxa responsible. In the cases of accuracy of the combined data suffered relative to the individual partitions, the concatenate datasets of mitochondrial (16S rRNA and COI) and nuclear DNA (28S rRNA) were checked using the partition homogeneity test in PAUP 4.0b10, using 100 replicates34. The alignment was partitioned by gene fragments. However, the analyses were run for each gene fragment and also for the concatenated dataset. jModeltest 2.1.735 was used to calculate and select a GTR + G model that was then used in all analyses.
Phylogenetic trees (Fig. 1) were constructed using neighbor joining (NJ), maximum likelihood (ML) and Bayesian inference (BI). The parameters, including the rate matrix and gamma shape parameter (α) of the gamma distribution (based on 16 rate categories) were estimated. The alignment was partitioned by gene fragment. The NJ analysis was performed using PAUP* v4.0b1034, while the ML analysis was performed via the RAxML Web servers36. Bootstrap resampling37 with 1000 replicates was used to assess branch support. The BI analysis was performed using MrBayes version 3.2.538, where the tree space was explored using four chains of a Markov chain Monte Carlo algorithm (MCMC) and the optimum substitution model found above. The Bayesian analysis was run for 10 million generations (heating parameter = 0.03), sampling every 100 generations and then discarded 25% of the trees with burn-in. Convergence was monitored by showing the average standard deviation of the split frequencies (between 2 runs) was below 0.1%38. Support for nodes was defined as posterior probabilities (PP).
Species delimitation methods
Three species delimitation methods (Fig. 2) were performed39,40, since the support across multiple species delimitation approaches is generally superior to a single method40. Thus, the species boundaries of C. volvulus were investigated based on the (i) Poisson Tree Processes (PTP)16, (ii) Automatic Barcode Gap Discovery (ABGD)41 and (iii) Generalized Mixed Yule-Coalescent (GMYC)42,43 methods. While ABGD detects ‘barcode gaps’ in the distribution of pairwise genetic distances, GMYC and PTP use an input tree as a model to delineate the PSHs in speciation and coalescent processes40. The ABGD approach was run using the web server (http://www.abi.snv.jussieu.fr/public/abgd/abgdweb.html) and the default parameters. The PTP and GMYC43 methods were also performed on the concatenated 16S rRNA, COI and 28S rRNA genes.
The delimitated species tree was produced using the relaxed log-normal clock algorithm implemented in the BEAST v1.8.2 package44. The GTR + G model was applied to re-construct the tree for 1 × 107 generations with sampling every 100 steps. The MCMC output was examined by consideration of traces in Tracer 1.645 and analyzed with TreeAnnotator 1.7.4 using all trees. A PP limit of 0.5 with maximum clade credibility tree was set. The GMYC method was performed using both a single- and a multiple-threshold. The output tree was optimized using SPLITS v.1.0–19 package for R. The PTP method was implemented based on the output tree and run in Python using the Environment for Tree Exploration package46.
Geometric morphometric analysis
Photographs of 108 shells, including the shell-only collections and the 42 individuals for which we assembled the DNA sequence data from, were randomly ordered in tpsUtil v.1.4947. The shell photos were then digitized in tpsDig v. 1.4048 to capture the bi-dimensional coordination of 13 landmarks (Fig. 3A). Landmark coordinates for all specimens were evaluated in the software package MORPHOJ v1.06d49. All landmark data were superimposed by Procrustes Fit, with the data transformed into a common coordinate system to eliminate other variations, such as isometric size variation and orientation, except for shape from the data47. The allometric components/and the other effects of allometric shape variation were removed by using MORPHOJ v1.06d49. All statistical analyses were performed using MorphoJ49. Shell shapes were plotted against the centroid size which used as proxy of shell size to perform. Multivariate regression was used to discover any correlation among the centroid size and shape variable (p < 0.001; 10,000 replicates of permutation test) and its statistical significance. This allowed us to identify the modes of change that account for the highest degree of variation and provided linear numerical values for further analysis. Canonical variance analysis (CVA) was used to test the shell shape variation with candidate species recognized by molecular analyses as an a priori group. Mahalanobis and Procrustes distances between pairwise comparisons were calculated for significant differences by the permutation test (10,000 iterations). The correct classification percentage of each paired species was evaluated using the leave-one-out cross-validation, which was the implement of discriminant function analysis.
Nomenclatural acts
The electronic edition of this article fit with the requirements of the amended International Code of Zoological Nomenclature (ICZN), and hence the new names contained herein are available under that the Code rules. The published work and the nomenclatural acts it encloses have been registered in ZooBank (http://zoobank.org), the online registration system for the ICZN. The LSID for this publication is: urn:lsid:zoobank.org:pub:458492DC-E3F5-4293-AE27-FFCAB7287FC5. The electronic edition of this paper was published in a journal with an ISSN, and has been recorded and is available from PubMed Central.
References
Kobelt, W. Cyclophoridae, bearbeitet von dr. Wilhelm Kobelt. Mit 110 abbildungen und 1 landkarte. 16, (Berlin, R. Friedländer und Sohn, 1902).
Kobelt, W. Die gedeckelten Lungenschnecken (Cyclostomacea). In Abbildungen nach der Natur mit Beschreibungen. Dritte Abteilung. Cyclophoridae I. Systematisches Conchylien-Cabinet von Martini und Chemnitz. 1(19) [3], (Nürnberg:Bauer und Raspe, 1908).
Reeve, L. Conchologia iconica, or, Illustrations of the shells of molluscous animals/by Lovell Augustus Reeve. v.13 1862, (London:Reeve, Brothers, 1862).
Gude, G. K. Mollusca III (land operculates). In: Shipley, A. E., Marshall, A. K. (Eds), The Fauna of British India including Ceylon and Burma. (London,Taylor and Francis). at, http://www.biodiversitylibrary.org/item/46616 (1921).
Albrecht, C., Wilke, T., Kuhn, K. & Streit, B. Convergent evolution of shell shape in freshwater limpets: The African genus Burnupia. Zool. J. Linn. Soc. 140, 577–586 (2004).
Nantarat, N. et al. Phylogenetic relationships of the operculate land snail genus Cyclophorus Montfort, 1810 in Thailand. Mol. Phylogenet. Evol. 70, 99–111 (2014).
Nantarat, N., Wade, C. M., Jeratthitikul, E., Sutcharit, C. & Panha, S. Molecular Evidence for Cryptic Speciation in the Cyclophorus fulguratus (Pfeiffer, 1854) Species Complex (Caenogastropoda: Cyclophoridae) with Description of New Species. PLoS One 9, 109785 (2014).
Bourdeau, P. E. et al. What can aquatic gastropods tell us about phenotypic plasticity? A review and meta-analysis. Heredity (Edinb). 115, 312–321 (2015).
Kameda, Y., Kawakita, A. & Kato, M. Cryptic genetic divergence and associated morphological differentiation in the arboreal land snail Satsuma (Luchuhadra) largillierti (Camaenidae) endemic to the Ryukyu Archipelago, Japan. Mol. Phylogenet. Evol. 45, 519–533 (2007).
Frederik, M. O. Vermivm terrestrium et fluviatilium, seu animalium infusoriorum, helminthicorum et testaceorum, non marinorum, succincta historia, auctore Othone Friderico Müller… v.2, (Havniæ,apud Heineck et Faber, 1774).
Kongim, B., Naggs, F. & Panha, S. Karyotypes of operculate land snails of the genus Cyclophorus (Prosobranchia: Cyclophoridae) in Thailand. Invertebr. Reprod. Dev. 49, 1–8 (2006).
Prasankok, P., Sutcharit, C., Tongkerd, P. & Panha, S. Biochemical assessment of the taxonomic diversity of the operculate land snail, Cyclophorus fulguratus (Gastropoda: Cyclophoridae), from Thailand. Biochem. Syst. Ecol. 36, 900–906 (2008).
Bellwood, P. & Dizon, E. 4000 Years of Migration and Cultural Exchange: The Archaeology of the Batanes Islands, Northern Philippines. (ANU E Press). at, https://books.google.co.th/books?id=W0l3AgAAQBAJ (2013).
Corporation, M. C. Endangered Wildlife and Plants of the World. (Marshall Cavendish Corporation). at, https://books.google.co.th/books?id=PYOV5PcWVAsC (2001).
Chiba, S. Species diversity and conservation of Mandarina, an endemic land snail of the Ogasawara Islands. Restoring Ocean. Isl. Ecosyst. Impact Manag. Invasive Alien Species Bonin Islands 117–125, https://doi.org/10.1007/978-4-431-53859-2_18 (2010).
Zhang, J., Kapli, P., Pavlidis, P. & Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinforma. 29, 2869–2876 (2013).
Paquin, P. & Hedin, M. The power and perils of ‘molecular taxonomy’: a case study of eyeless and endangered Cicurina (Araneae: Dictynidae) from Texas caves. Mol. Ecol. 13, 3239–55 (2004).
Heled, J. & Drummond, A. J. Bayesian Inference of Species Trees from Multilocus Data. Mol. Biol. Evol. 27, 570–580 (2010).
Avise, J. C., Shapira, J. F., Daniel, S. W., Aquadro, C. F. & Lansman, R. A. Mitochondrial DNA differentiation during the speciation process in Peromyscus. Mol. Biol. Evol. 1, 38–56 (1983).
Hoekstra, P. & Schilthuizen, M. Phylogenetic relationships between isolated populations of the limestone‐dwelling microsnail Gyliotrachela hungerfordiana (Gastropoda: Vertiginidae). J. Zool. Syst. Evol. Res. 49, 266–272 (2011).
Desouky, M. M. A. & Busais, S. Phylogenetic relationships of the land snail; Eobania vermiculata (Müller, 1774) from Egypt and Saudi Arabia. A combined morphological and molecular analysis. J. Basic Appl. Zool. 65, 144–151 (2012).
Detwiler, J. T., Bos, D. H. & Minchella, D. J. Revealing the secret lives of cryptic species: Examining the phylogenetic relationships of echinostome parasites in North America. Mol. Phylogenet. Evol. 55, 611–620 (2010).
Alizon, S., Kucera, M. & Jansen, V. A. A. Competition between cryptic species explains variations in rates of lineage evolution. Proc. Natl. Acad. Sci. USA 105, 12382–12386 (2008).
Ahmadzadeh, F. et al. Cryptic speciation patterns in Iranian rock lizards uncovered by integrative taxonomy. PLoS One 8, e80563 (2013).
Zachos, F. E. & Habel, J. C. Biodiversity Hotspots: Distribution and Protection of Conservation Priority Areas. (Springer Berlin Heidelberg). at, https://books.google.co.th/books?id=MRp9NoUfLVwC (2011).
Van Benthem Jutting, W. S. S. Systematic studies on the non-marine Mollusca of the Indo-Australian Archipelago. Treubia 19, 539–604 (1948).
Van Benthem Jutting, W. S. S. On a collection of non-marine Mollusca from Malaya in the Raffles Museum, Singapore, with an appendix on cave shells. Bull. Raffles Museum, Singapore 19, 50–77 (1949).
Kessing, B. et al. The simple fool’s guide to PCR. (Department of Zoology, University of Hawaii, Honolulu, 1989).
Folmer, O., Black, M., Hoeh, W., Lutz, R. & Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 3, 294–299 (1994).
Morgan, J. A. T. et al. A phylogeny of planorbid snails, with implications for the evolution of Schistosoma parasites. Mol. Phylogenet. Evol. 25, 477–488 (2002).
Edgar, R. C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5, 113 (2004).
Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 (2013).
Kumar, S., Nei, M., Dudley, J. & Tamura, K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Brief. Bioinform. 9 (2008).
Swofford, D. L. PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods). (Sinauer Associates, Sunderland, Massachusetts, 2003).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: more models, new heuristics and parallel computing. Nat. Methods 9, 772 (2012).
Stamatakis, A., Hoover, P. & Rougemont, J. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 57, 758–771 (2008).
Felsenstein, J. Bootstraps and testing trees. Presentation 71 at, papers2://publication/uuid/FF574B71-EBF4-44B3-9C94-E3D6AC19ED83 (2008).
Ronquist, F. et al. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Syst. Biol. https://doi.org/10.1093/syosbio/sys029 (2012).
Hebert, P. D. N., Ratnasingham, S. & deWaard, J. R. Barcoding animal life: cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B Biol. Sci. 270, S96–S99 (2003).
Larson, E. R., Castelin, M., Williams, B. W., Olden, J. D. & Abbott, C. L. Phylogenetic species delimitation for crayfishes of the genus Pacifastacus. PeerJ 4, e1915 (2016).
Puillandre, N., Lambert, A., Brouillet, S. & Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 21, 1864–1877 (2012).
Pons, J. et al. Sequence-Based Species Delimitation for the DNA Taxonomy of Undescribed Insects. Syst. Biol. 55, 595–609 (2006).
Monaghan, M. T. et al. Accelerated species inventory on Madagascar using coalescent-based models of species delineation. Syst. Biol. 58, 298–311 (2009).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian Phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Rambaut, A., Suchard, M., Xie, D. & Drummond, A. J. Tracer v1.6. at, http://beast.bio.ed.ac.uk/Tracer (2014).
Huerta-Cepas, J., Dopazo, J. & Gabaldón, T. ETE: a python Environment for Tree Exploration. BMC Bioinformatics 11, 24 (2010).
Rohlf, F. J. Tpsutil Version 1.49. (New York: Department of Ecology and Evolution, State University of New York at Stony Brook, 2012).
Rohlf, F. J. Tpsdig Version 2.16. (New York: Department of Ecology and Evolution, State University of New York at Stony Brook, 2010).
Klingenberg, C. P. MorphoJ: An integrated software package for geometric morphometrics. Mol. Ecol. Resour. 11, 353–357 (2011).
Acknowledgements
This research was supported by Ratchadapisek Somphot Fund for Postdoctoral Fellowship, Chulalongkorn University (Nattawadee Nantarat). The main financial research support are from The Thailand Research Fund, TRF-DPG628001, and from Center of Excellence on Biodiversity, BDC-PG1-159005. This research work was partially supported by Chiang Mai University. We would like to express our profound gratitude to Asst. Prof. Dr. Pongpan Prasankok for helping us to create the map. We thank members of the Animal Systematics Research Unit members, Chulalongkorn University for assistance in collecting specimens. The authors are also grateful to NHMUK, London and ZMUC, Denmark for invaluable help with type specimens investigation.
Author information
Authors and Affiliations
Contributions
N.N. performed research. S.P., F.N. and C.W. designed the research study. C.S. and P.T. collected and provided samples. N.N. and C.W. analysed the data. N.N. and S.P. wrote the manuscript. All authors approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nantarat, N., Sutcharit, C., Tongkerd, P. et al. Phylogenetics and species delimitations of the operculated land snail Cyclophorus volvulus (Gastropoda: Cyclophoridae) reveal cryptic diversity and new species in Thailand. Sci Rep 9, 7041 (2019). https://doi.org/10.1038/s41598-019-43382-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43382-5
- Springer Nature Limited
This article is cited by
-
Slow and steady saves the race: molecular and morphological analysis of three new cryptic species of Iberus land snails from the Iberian Peninsula
Organisms Diversity & Evolution (2024)
-
Stramonita Genus Exhibits a New Uncovered Species: A Cryptic Species Collected from Accra, Ghana (Eastern Atlantic Ocean)
Thalassas: An International Journal of Marine Sciences (2023)
-
Phylogeography and molecular species delimitation reveal cryptic diversity in Potamolithus (Caenogastropoda: Tateidae) of the southwest basin of the Andes
Scientific Reports (2021)
-
New insights into the genetic diversity of the stone crayfish: taxonomic and conservation implications
BMC Evolutionary Biology (2020)