Abstract
In this paper, we present a novel Pt/CuO/Pt metal-oxide-metal (MOM) glucose sensor. The devices are fabricated using a simple, low-cost standard photolithography process. The unique planar structure of the device provides a large electrochemically active surface area, which acts as a nonenzymatic reservoir for glucose oxidation. The sensor has a linear sensing range between 2.2 mM and 10 mM of glucose concentration, which covers the blood glucose levels for an adult human. The distinguishing property of this sensor is its ability to measure glucose at neutral pH conditions (i.e. pH = 7). Furthermore, the dilution step commonly needed for CuO-based nonenzymatic electrochemical sensors to achieve an alkaline medium, which is essential to perform redox reactions in the absence of glucose oxidase, is eliminated, resulting in a lower-cost and more compact device.
Similar content being viewed by others
Introduction
Diabetes, which is one of the most commonly diagnosed diseases at this time, is predicted to be the 7th most deadly disease by 20301,2,3. It occurs either due to a deficiency in the production of insulin or due to the inability of a body to utilize the available insulin3,4,5. Continuous monitoring of glucose levels in patients is essential to manage treatment and avoid critical complications or conditions such as cataracts, foot damage, and loss of vision6,7,8. Thus, the acquisition of a low-cost, miniature glucose measuring device can help patients and clinicians manage the disease.
Available glucose sensors can be divided into two main types, namely, enzymatic and nonenzymatic9, as illustrated in Fig. 1. Enzyme-based sensors are developed using glucose dehydrogenase (GDH) or glucose oxidase (GOx), which interacts with glucose molecules and results in an electrical response that is correlated to the concentration of glucose. Although enzymatic glucose sensors have been widely used and developed in the literature3, their short-term stability is affected by operating temperature, pH level, and humidity, in addition to the high fabrication cost. Both of these issues have encouraged the development of nonenzymatic glucose (NEG) sensors10,11,12,13,14,15.
NEG sensors16,17,18,19,20,21,22,23 allow glucose to be oxidized directly on the surface of the sensor, where the atoms at the surface act as the electrocatalysts, resulting in high stability, repeatability and cost-effective fabrication24,25,26. Two main models of glucose oxidation in NEG sensors have been proposed and explained in the literature. The first model was proposed by Pletcher27 and is known as the activated chemisorption model. In this model, the adsorption of the glucose molecule on the surface initiates glucose oxidation and enables the glucose molecule to form a bond with the atoms on the surface. On the other hand, the incipient hydrous oxide/adatom mediator (IHOAM) model proposed by Burke28,29 is associated with the active metal atoms on the electrode surface. These atoms have a low lattice stabilization and an enhanced reactivity, which aid the premono- layer oxidation step and facilitate glucose oxidation29,30,31.
Different materials have been used to develop NEG sensors, as shown in Fig. 1. This includes metals and metal compounds, alloys and bimetallic composites, metal oxide composites, polymer modified composites, and carbon materials. Although each material type has its own advantages and limitations, metals (e.g., Pt, Au, Ni, Cu, and Ag)9 and metal oxides (e.g., NiO, Cu2O, CuO, TiO2, ZnO, SnO2, MnO2, and Co3O4)32,33,34,35,36,37,38,39 have attracted the most attention recently for use as NEG sensors. This is due to the well-developed understanding of the electrocatalytic mechanism of glucose oxidation in such structures.
NiO has been commonly utilized in NEG sensors because of its catalytic properties, for which Ni(II) and Ni(III) are responsible for the required redox reaction3. To improve the stability and sensing performance reported by the available Ni-based sensors34,40,41,42,43, NiO-based hybrids have been investigated. Nanoparticle-assembled NiO nanosheets prepared using graphene oxide film, which is used as a template, have been recently explored for glucose sensing44. Although this system shows enhanced stability and selectivity over the available NiO-based sensors, a smaller linear detection range has been reported (0.001 mM – 0.4 mM). It is noteworthy that an alkaline medium (pH > 7) is needed for NiO/NiO hybrid-based sensors to accomplish the redox reaction3,45. As a cost-effective material with negligible toxicity, ZnO has been widely used for fabricating enzymatic glucose sensors13,45. Dar et al.46 were the first to report ZnO nanorods working as NEG sensors. The fabricated device was able to detect glucose at a neutral pH. However, the obtained linear range was very small (0.001 mM – 0.01 mM). To enhance the sensing performance, a combination of ZnO with NiO or CuO has been shown to be an effective approach to improve the overall catalytic performance of the fabricated sensor45. Nevertheless, the sensing medium must be diluted to achieve alkaline conditions and consequently attain the synergistic effects of the combined materials.
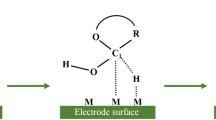
Among the metal oxide materials used, CuO is considered one of the best materials to be used in NEG sensing. This is due to its natural abundance, low production cost, high stability and appropriate redox potential. Equations (1) and (2) describe the dominant reactions taking place in CuO-based NEG sensors to allow electro-oxidation of glucose9. Furthermore, a substantial number of nonenzymatic CuO-based glucose sensors47,48,49,50,51,52,53,54,55,56,57 require a high pH (≥13) medium to perform glucose sensing.
In this paper, we present a CuO-based glucose sensor structure, named MOMSense. The structure is capable of differentiating dissolved glucose levels in a liquid sample from as low as 2.2 mM to at least 10 mM when the liquid sample is at neutral pH. Achieving glucose sensing at a neutral pH is essential to improve the sensitivity of the detection unit. Tang et al.58 showed that performing sensing at a pH outside the neutral level affects the accuracy of the results, especially at diabetic glucose levels. Moreover, eliminating the dilution step needed for the sensing devices that work in an acidic or alkaline medium results in a cost-effective and compact device. The ability of the sensor to operate at a neutral pH facilitates its integration with other blood substance sensors. The ability to operate at a neutral pH is advantageous for the development of future lab-on-chip structures for real-time health monitoring.
As shown in Fig. 2, MOMSense can be integrated into a microfluidic platform that serves as a miniature lab-on-chip59,60,61,62. The selective sample preparation and preconcentration steps enhance the sensitivity of the detection method. The improved selectivity starts by using a human fluid that is fed to the sensor through a microfluidic channel, where glucose molecules are extracted using a suitable separation technique. After this, separated fluid samples with glucose molecules are processed by the MOMSense device. The electrical response is measured and analyzed by the measurement and processing units to calculate the corresponding glucose level. Electrochemical detection integrated with a microfluidic paper-based analytical device (µPAD) is well-studied in literature and it is shown to play a significant role in glucose sensing due to its low cost, high sensitivity and selectivity, minimal sample preparation and short response time63. The microfluidic separation suggested in Fig. 2 is in line with the glucose sensing device proposed in64. In contrast, in this framework, the µPAD allows detection of low glucose molecules levels by pushing these molecules to the surface of the MOMSense through utilizing the capillary action of the µPAD structure. As a result, the current passing through the device will change as function of glucose concentration in the sample.
The MOMSense device presented in this work is fabricated in a planar structure and can be mass produced using a wafer-style fabrication process, as shown in Fig. 3(a). Each device consists of a CuO layer and one pair of first and second Pt electrodes arranged on the oxide and separated by a gap containing the CuO layer, as shown in Fig. 3(b). The CuO surface extends around and below the metal electrodes and rests on a substrate layer, which can be any suitable inert structural layer, such as, but not limited to, glass. Figure 3(c) presents a scanning electron microphotograph of the device cross-sectional view, which shows a CuO thickness of 26.7 nm with another 20.8 nm Pt layer on a glass substrate.
(a) A macrograph of the fabricated MOMSense devices on a glass wafer. (b) Device schematic to show the planar Pt/CuO/Pt structure. (c) Scanning electron microphotograph of a physical MOMSense device cross-sectional view under secondary electron mode (accelerating voltage, 5 kV; magnification, 140 453x; working distance, 5 mm).
Results
MOMSense Glucose Test
For each measurement, an unused device is selected randomly from the same wafer to investigate the sensing ability of MOMSense devices for the following glucose concentrations: 3.9 mM, 5.6 mM and 7.8 mM. The two measurement steps used in performing these tests are illustrated in Fig. 4.
Step 1: A dc voltage of 1 V is applied across the MOMSense sensors, and this voltage is the minimum working voltage for the sensor. (i) The resulting current level passing through the device is recorded. (ii) Next, the electrical stability of the device is checked in the absence of glucose.
Step 2: Under the same dc value, a 2 µl drop of glucose solution is added on the top of the sensor. This solution covers the oxide area and is simultaneously allowed to touch both electrodes.
This testing mechanism follows the well-reported amperometric glucose sensing approach detailed in15, which mainly involves the application of a constant bias potential, followed by an electric current measurement. This current is linearly related to the glucose concentration. As presented in Fig. 5, MOMSense devices show an instantaneous response at t = 10 s, which is the time when the glucose solution is applied to the device surface. It is clear from these plots that the measured current level after addition of the solution depends on the glucose concentration. Despite the fact that each measurement is conducted across seven separate devices with different concentrations, the error bars for the variation in the measured average currents are statistically significant. Such variation in responses is expected due to the variation associated with the patch device fabrication. The error bars can be significantly reduced by careful optimisation of the patch fabrication process.
A chart illustrating the measured current across the fabricated MOMSense devices over time when subjected to an applied voltage of 1 V and when supplied directly with a glucose-containing sample of known glucose concentrations of 3.9 mM, 5.6 mM, and 7.8 mM at t = 10 s. These concentrations span low, medium, and high blood glucose levels for an adult human. For each glucose concentration, the illustrated data represent the average result obtained from seven fresh identical devices, with error bars for the measured current variation over time.
After confirming the repeatability, reproducibility and stability of MOMSense devices, the study is expanded to determine their linear range. This is achieved by testing a set of fresh devices using the following glucose concentrations: 2.2 mM, 3.9 mM, 5.6 mM, 7.8 mM, 10.0 mM and 12.2 mM. As presented in Fig. 6(a), MOMSense devices show instantaneous responses at t = 10 s, which is the time when the glucose solution is applied to the device. The current level for each concentration is relatively stable after one second of glucose application. The current value is read at t = 18 s and plotted versus the corresponding glucose concentration, as presented in Fig. 6(b). This set time point is selected because it provides the best linear fitting at the shortest time. The sensor has a linear characteristic between 2.2 mM and 10.0 mM, where the measured current consistently increases with the increase in glucose concentration. Moreover, it can be observed that the device sensitivity saturates at glucose concentrations above 10 mM (180 mg/dl). This is due to the high dependency of glucose adsorption on the available sensor surface area. Anion competition limits the extent of glucose oxidation, and therefore, the linearity of the oxidation current to the glucose concentration substantially degrades when the sensor surface is saturated9,15,65,66. It is clear that the empirical equation provided in Fig. 6(b) shows a nonzero passing model, which means that MOMSense devices have a different regime for lower concentrations.
(a) A chart illustrating the measured current across MOMSense devices over time while 1 V is applied across the electrode and the glucose-containing sample. The results are shown for concentrations of 2.2 mM, 3.9 mM, 5.6 mM, 7.8 mM, 10.0 mM and 12.2 mM. These concentrations represent extremely low, low, medium, high and extremely high blood glucose levels for an adult human. As shown, the detected currents for each sample increase over time according to glucose concentration. Each concentration sample is tested on identical fresh devices. (b) A chart illustrating current data obtained with respect to Fig. 6(a) at a set time point of 18 s as a function of glucose concentration. It is clear that the response of the MOMSense devices saturates at a glucose concentration of 10.0 mM (180 mg/dl).
Table 1 summarizes the CuO-based NEG sensors available in the literature. It is clear that MOMSense devices exhibit a wide linear range and high sensitivity at a neutral pH. The concept of an integrated lab-on-chip separation and detection platform presented in Fig. 2 would facilitate employing excessively corrosive environments to increase the sensitivity and maintain the chemical stability of the device.
Figure 7 shows the equivalent circuit diagram of a MOMSense device, where
-
resistor RP represents the resistance of the Pt electrodes; this resistance is not affected by the added glucose, as the electrical current always passes through the conducting metal;
-
resistor RS is the CuO interface resistance, which is affected by the added glucose solution, and its value depends on the following electrochemical reactions;
-
glucose and Pt are in volumes Vol1 and Vol3; and
-
glucose, Pt and CuO are in volume Vol2.
Circuit diagram of the fabricated MOMSense when a voltage source, V, is applied across the two electrodes and a glucose sample applied. RP is the electrodes resistance, while RS is the modified resistance of the CuO due to the sample application. This figure shows the three volumes that can affect the electrochemical reaction. These are Vol1 and Vol3, which refer to the Glucose and Pt volume, and Vol2 refers to the Glucose, Pt and CuO volume.
To identify the roles of the Pt electrodes and the oxide material (CuO) used in MOMSense devices, two different systems, Pt/glass/Pt and Cu/CuO/Cu, are fabricated, and their glucose sensing abilities are tested.
Pt/Glass/Pt devices
To confirm the role of the CuO layer deposited underneath and between the platinum electrodes in MOMSense devices, 3 mm \(\times \) 3 mm Pt electrodes are deposited directly on the glass substrate, as illustrated in the inset of Fig. 8(a). Three fresh devices from the aforementioned system are tested with 3.9 mM, 5.6 mM and 7.8 mM glucose concentrations. The same testing procedure described and followed for the MOMSense devices is used for this investigation. Figure 8(a) shows a random small jump in the electric current level when a drop of solution is applied, indicating no sensing ability to the applied glucose.
Cu/CuO/Cu devices
In this structure, the Pt electrodes in the MOMSense device are replaced by Cu electrodes to investigate the sensitivity of the device in the absence of platinum. This is realized by depositing 3 mm \(\times \) 3 mm Cu electrodes on the CuO layer synthesized using the same process described for MOMSense devices and detailed in the Methods section. As presented in Fig. 8(b), there is no trend to relate the increase in the current passing through the device to the glucose concentration in the added drop. This confirms the role of Pt electrodes that act as catalytic electrodes that easily distinguish the number of electron transfers and consequently result in an electron flow that is proportional to the number of existing glucose molecules67.
Glucose oxidation in CuO system
Copper oxide is well documented as a multiplex electrochemical catalyst in an aqueous medium due to the various oxidized/hydroxylated species that can be present within the neutral to alkaline pH range, depending on the applied potential68. A widely accepted nonenzymatic mechanism associates the electro-oxidation of glucose with the presence of the redox active couple Cu2+/Cu3+ in alkaline conditions (e.g., pH 11–13) in the form of CuO/CuO(OH) species3. Accordingly, the oxidation of glucose has been widely explained as per the following two-step process:
First, a half-oxidation reaction of Cu2+ to Cu3+ occurs under a sufficient voltage supply:
Second, a nonenzymatic oxidation-reduction reaction between the formed CuIII oxyhydroxide species and the adsorbed glucose takes place, allowing for further regeneration of CuO species:
In addition to being widely accepted for alkaline conditions, a recent work57 also claimed this mechanism for establishing glucose oxidation on graphene-modified CuO particles in neutral pH.
On the other hand, a thorough analytical study of the electrochemical CuO system by Barragan et al.69 pinpointed several controversies of the widely accepted mechanism above to justify a new hypothesis for the electrocatalytic behavior of CuO that claims little to no role of Cu3+ species in the electro-oxidation process of glucose. Barragan et al. attributed the electron transfer process to the synergistic role between the adsorbed hydroxide ions and the semiconductive behavior of the CuO system that involve ion-pairing and partial charge transfer models rather than direct involvement of Cu3+ ions.
As for MOMSense devices, some initial experiments (see Fig. 9) are carried out with our devices under alkaline conditions (pH = 13). These results show that a glucose sensing signature with enhanced sensitivity can be established for an increased pH level, where the ratio between the responses of the blank and the glucose sample is enhanced from 1.1 to 1.9 for a pH = 7 and pH = 13, respectively. This indicates that some of the hypotheses reported in the literature can still be applicable, and it also corroborates the electrocatalytic behavior of CuO. We believe that other redox active couples, such as Cu+/Cu2+, could be highly involved under neutral conditions. In fact, the involvement of the cupric ions Cu2+ (i.e., Cu(OH)2 and CuO species) in the electrochemical oxidation of carbohydrates is a well-known metabolic pathway, which is also the basis of several biochemical tests for glucose sensing, including Fehling’s test and Benedict’s test70,71. However, explaining the mechanism at pH = 7 with the novel MOM structure reported in this work requires further study of the fabricated CuO layer to identify the exact nature of the electrochemical reactions taking place.
Discussion
We successfully presented the design, fabrication and testing of an efficient nonenzymatic biomedical sensor that is capable of detecting different glucose concentrations ranging from 2.2 mM – 10.0 mM. It was demonstrated that the novel planar Pt/CuO/Pt structure enables the nonenzymatic sensing mechanism. The MOMSense device exhibits a synergistic role for the interfaces between the Pt electrodes and the CuO surface to act as electrocatalysts and consequently facilitate the glucose oxidation needed for glucose detection in the absence of GDH or GOx. The role of the CuO layer and Pt electrodes in the sensing process was demonstrated through fabricating and testing Pt/Glass/Pt and Cu/CuO/Cu structures. These results confirm the synergistic contribution of the Pt electrodes attached to CuO in MOMSense devices. CuO is reported as a promising material to be deployed in NEG sensors. It can perform glucose oxidation on modified CuO-based electrodes in an alkaline solution34,72,73,74. As our goal in this work is to perform glucose testing at a neutral pH, the fabricated Cu/CuO/Cu devices presented in the preceding section are incapable of differentiating glucose concentrations. On the other hand, the Pt electrodes used in MOMSense devices enable the glucose oxidation to take place in neutral solution. The cyclic voltammetry reported in62 for Pt electrodes in the presence of glucose at a pH of 7 showed three different oxidation peaks that reflect the electrochemically oxidized glucose at a platinum electrode. However, using Pt electrodes solely in glucose detection has been limited due to the many drawbacks of the material15,62,65. The sensing mechanism associated with MOMSense devices fabricated and presented in this paper generally provides new perspectives on the design and testing approaches for biomedical sensors and for glucose sensing specifically. Furthermore, the presented properties of MOMSense devices are in line with the requirements for a viable nonenzymatic glucose sensor65 in terms of sensitivity, stability, accuracy, ability to meet the ISO standard (International Organization for Standardization), no oxygen dependency, low cost and ease of fabrication. Evaluating the combined detection of MOMSense devices with µPAD using actual blood samples is beyond the scope of current work and is considered as a future work.
Methods
Device fabrication
A low-cost standard photolithography fabrication process is followed in fabricating the MOMSense devices. As illustrated in Fig. 10, 99.9% pure Cu is sputtered on a 4″ Borofloat glass wafer using a Q300T T coating tool by Quorum Technologies. To form the CuO layer, the wafer is heated at 500 °C on a hot plate for three hours. After cooling to room temperature, the lithography step is performed by spin coating 1.4 µm thick MICROPOSIT™ S1813™ positive photoresist. Prior to photoresist deposition, an HMDS primer is used to improve adhesion. A UV exposure system (KLOE 650) is used to pattern the photoresist layer on the wafer, followed by a one-minute development step using an appropriate developer. Next, 99.99% pure Pt is sputtered onto the wafer. Finally, the photoresist layer is lifted off using acetone to produce the final wafer presented in Fig. 3(a).
Device Characterization
The cross section of a sample MOMSense device is inspected using high-resolution scanning electron microscopy (FEI Nova NanoSEM 650). A Keithley 4200-SCS Parametric Analyzer (Tektronix) is used to perform an amperometric test using voltage pulse mode. The prepared devices are mounted on a probe station and are electrically tested by applying one volt across the Pt electrodes. The compliance current (cc) is set to the instrumental maximum level (i.e., 0.1 A).
References
Danaei, G. et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2· 7 million participants. The Lancet 378(9785), 31–40 (2011).
World Health Organization. Global status report on noncommunicable diseases 2010. Geneva: World Health Organization (2011).
Tian, K., Prestgard, M. & Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Materials Science and Engineering: C 41, 100–118 (2014).
Nichols, S. P., Koh, A., Storm, W. L., Shin, J. H. & Schoenfisch, M. H. Biocompatible materials for continuous glucose monitoring devices. Chemical reviews 113(4), 2528–2549 (2013).
Shaw, K. M., & Cummings, M. H. (Eds). Diabetes: Chronic Complications. John Wiley & Sons (2012).
Pearson-Stuttard, J., Blundell, S., Harris, T., Cook, D. G. & Critchley, J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. The Lancet Diabetes &. Endocrinology 4(2), 148–158 (2016).
Shah, M., & Vella, A. Understanding Diabetes Mellitus: Pathophysiology. In Metabolic Syndrome and Diabetes. Springer, New York, NY, 33–45 (2016).
Lv, Y., Jin, S., Wang, Y., Lun, Z. & Xia, C. Recent advances in the application of nanomaterials in enzymatic glucose sensors. Journal of the Iranian Chemical Society 13(10), 1767–1776 (2016).
Wang, G. et al. Non-enzymatic electrochemical sensing of glucose. Microchimica Acta 180(3-4), 161–186 (2013).
Tang, J. et al. Sensitive enzymatic glucose detection by TiO2 nanowire photoelectrochemical biosensors. Journal of Materials Chemistry A 2(17), 6153–6157 (2014).
Gao, Z. D., Qu, Y., Li, T., Shrestha, N. K. & Song, Y.-Y. Development of Amperometric Glucose Biosensor Based on Prussian Blue Functionalized TiO2 Nanotube Arrays. Sci. Rep. 4, 6891 (2014).
Wang, H. C. & Lee, A. R. Recent developments in blood glucose sensors. Journal of food and drug analysis 23(2), 191–200 (2015).
Ahmad, R. et al. Highly efficient non-enzymatic glucose sensor based on CuO modified vertically-grown ZnO nanorods on electrode. Scientific Reports 7(1), 5715 (2017).
Liu, S., Hui, K. S. & Hui, K. N. Flower-like copper cobaltite nanosheets on graphite paper as high-performance supercapacitor electrodes and enzymeless glucose sensors. ACS applied materials & interfaces 8(5), 3258–3267 (2016).
Zhu, Z. et al. A critical review of glucose biosensors based on carbon nanomaterials: carbon nanotubes and graphene. Sensors 12(5), 5996–6022 (2012).
Ahmad, R., Vaseem, M., Tripathy, N. & Hahn, Y. B. Wide linear-range detecting nonenzymatic glucose biosensor based on CuO nanoparticles inkjet-printed on electrodes. Analytical chemistry 85(21), 10448–10454 (2013).
Pandya, A., Sutariya, P. G. & Menon, S. K. A non enzymatic glucose biosensor based on an ultrasensitive calix [4] arene functionalized boronic acid gold nanoprobe for sensing in human blood serum. Analyst 138(8), 2483–2490 (2013).
Pang, H., Lu, Q., Wang, J., Li, Y. & Gao, F. Glucose-assisted synthesis of copper micropuzzles and their application as nonenzymatic glucose sensors. Chemical Communications, 46 ( 12 ) (2010).
Huang, J. et al. High performance non-enzymatic glucose biosensor based on copper nanowires–carbon nanotubes hybrid for intracellular glucose study. Sensors and Actuators B: Chemical 182, 618–624 (2013).
Cheng, T. M. et al. (110)-exposed gold nanocoral electrode as low onset potential selective glucose sensor. ACS applied materials & interfaces 2(10), 2773–2780 (2010).
Guo, C., Wang, Y., Zhao, Y. & Xu, C. Non-enzymatic glucose sensor based on three dimensional nickel oxide for enhanced sensitivity. Analytical Methods, 5(7), 1644–1647 (2013).
Dong, X. C. et al. 3D graphene–cobalt oxide electrode for high-performance supercapacitor and enzymeless glucose detection. ACS nano 6(4), 3206–3213 (2012).
Fang, B. et al. Silver oxide nanowalls grown on Cu substrate as an enzymeless glucose sensor. ACS applied materials & interfaces 1(12), 2829–2834 (2009).
Zhou, C. et al. Ultrasensitive non-enzymatic glucose sensor based on three-dimensional network of ZnO-CuO hierarchical nanocomposites by electrospinning. Scientific reports 4, 7382 (2014).
Mani, S. et al. Hydrothermal synthesis of NiWO4 crystals for high performance non-enzymatic glucose biosensors. Scientific reports 6, 24128 (2016).
Sivakumar, M. et al. Low-temperature chemical synthesis of CoWO4 nanospheres for sensitive nonenzymatic glucose sensor. The Journal of Physical Chemistry C 120(30), 17024–17028 (2016).
Pletcher, D. Electrocatalysis: present and future. Journal of applied electrochemistry 14(4), 403–415 (1984).
Rahman, M. M., Ahammad, A. J., Jin, J. H., Ahn, S. J. & Lee, J. J. A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sensors 10(5), 4855–4886 (2010).
Burke, L. D. Premonolayer oxidation and its role in electrocatalysis. Electrochimica Acta 39(11-12), 1841–1848 (1994).
Wang, J. Amperometric biosensors for clinical and therapeutic drug monitoring: a review. Journal of pharmaceutical and biomedical analysis 19(1-2), 47–53 (1999).
Ernst, S., Heitbaum, J. & Hamann, C. H. The electrooxidation of glucose in phosphate buffer solutions: Part I. Reactivity and kinetics below 350 mV/RHE. Journal of Electroanalytical Chemistry and Interfacial Electrochemistry 100(1-2), 173–183 (1979).
Xu, S. et al. One-pot synthesis of Ag@ Cu yolk–shell nanostructures and their application as non-enzymatic glucose biosensors. CrystEngComm 16(38), 9075–9082 (2014).
Khan, R. et al. Glucose-assisted synthesis of Cu2O shuriken-like nanostructures and their application as nonenzymatic glucose biosensors. Sensors and Actuators B: Chemical 203, 471–476 (2014).
Liu, S., Yu, B. & Zhang, T. A novel non-enzymatic glucose sensor based on NiO hollow spheres. Electrochimica Acta 102, 104–107 (2013).
Luo, J., Jiang, S., Zhang, H., Jiang, J. & Liu, X. A novel non-enzymatic glucose sensor based on Cu nanoparticle modified graphene sheets electrode. Analytica chimica acta 709, 47–53 (2012).
Li, X. et al. Nickel/Copper nanoparticles modified TiO2 nanotubes for non-enzymatic glucose biosensors. Sensors and actuators B: Chemical 181, 501–508 (2013).
Sun, A., Zheng, J. & Sheng, Q. A highly sensitive non-enzymatic glucose sensor based on nickel and multi-walled carbon nanotubes nanohybrid films fabricated by one-step co-electrodeposition in ionic liquids. Electrochimica Acta 65, 64–69 (2012).
Zhao, J. et al. A non-enzymatic glucose sensor based on the composite of cubic Cu nanoparticles and arc-synthesized multi-walled carbon nanotubes. Biosensors and bioelectronics 47, 86–91 (2013).
Saei, A. A., Dolatabadi, J. E. N., Najafi-Marandi, P., Abhari, A. & de la Guardia, M. Electrochemical biosensors for glucose based on metal nanoparticles. TrAC Trends in Analytical Chemistry 42, 216–227 (2013).
Cao, F. et al. Nickel oxide microfibers immobilized onto electrode by electrospinning and calcination for nonenzymatic glucose sensor and effect of calcination temperature on the performance. Biosensors and Bioelectronics. 26(5), 2756–2760 (2011).
Wang, G. et al. Free-standing nickel oxide nanoflake arrays: synthesis and application for highly sensitive non-enzymatic glucose sensors. Nanoscale. 4(10), 3123–3127 (2012).
Soomro, R. A., Ibupoto, Z. H., Abro, M. I. & Willander, M. Electrochemical sensing of glucose based on novel hedgehog-like NiO nanostructures. Sensors and Actuators B: Chemical. 209, 966–974 (2015).
Sharifi, E., Salimi, A., Shams, E., Noorbakhsh, A. & Amini, M. K. Shape-dependent electron transfer kinetics and catalytic activity of NiO nanoparticles immobilized onto DNA modified electrode: fabrication of highly sensitive enzymeless glucose sensor. Biosensors and Bioelectronics. 56, 313–319 (2014).
Zhang, H. & Liu, S. Nanoparticles-assembled NiO nanosheets templated by graphene oxide film for highly sensitive non-enzymatic glucose sensing. Sensors and Actuators B: Chemical. 238, 788–794 (2017).
Zhu, H., Li, L., Zhou, W., Shao, Z. & Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. Journal of Materials Chemistry B. 4(46), 7333–7349 (2016).
Dar, G. N. et al. Fabrication of highly sensitive non-enzymatic glucose biosensor based on ZnO nanorods. Science of Advanced Materials 3.6, 901–906 (2011).
Ibupoto, Z. H., Khun, K., Beni, V., Liu, X. & Willander, M. Synthesis of novel CuO nanosheets and their non-enzymatic glucose sensing applications. Sensors 13(6), 7926–7938 (2013).
Reitz, E., Jia, W., Gentile, M., Wang, Y. & Lei, Y. CuO nanospheres based nonenzymatic glucose sensor. Electroanalysis: An International. Journal Devoted to Fundamental and Practical Aspects of Electroanalysis 20(22), 2482–2486 (2008).
Sun, S. et al. Hierarchical CuO nanoflowers: water-required synthesis and their application in a nonenzymatic glucose biosensor. Physical Chemistry Chemical Physics 15(26), 10904–10913 (2013).
Zhang, W., Li, R., Xing, L., Wang, X. & Gou, X. Carnation‐like CuO Hierarchical Nanostructures Assembled by Porous Nanosheets for Nonenzymatic Glucose Sensing. Electroanalysis 28(9), 2214–2221 (2016).
Medeiros, N. G., Ribas, V. C., Lavayen, V. & Da Silva, J. A. Synthesis of flower-like CuO hierarchical nanostructures as an electrochemical platform for glucose sensing. Journal of Solid State Electrochemistry 20(9), 2419–2426 (2016).
Gou, X. et al. A very facile strategy for the synthesis of ultrathin CuO nanorods towards non-enzymatic glucose sensing. New Journal of Chemistry 42(8), 6364–6369 (2018).
Meher, S. K. & Rao, G. R. Archetypal sandwich-structured CuO for high performance non-enzymatic sensing of glucose. Nanoscale 5(5), 2089–2099 (2013).
Lin, L. Y., Karakocak, B. B., Kavadiya, S., Soundappan, T. & Biswas, P. A highly sensitive non-enzymatic glucose sensor based on Cu/Cu2O/CuO ternary composite hollow spheres prepared in a furnace aerosol reactor. Sensors and Actuators B: Chemical 259, 745–752 (2018).
Dayakar, T., Rao, K. V., Bikshalu, K., Malapati, V. & Sadasivuni, K. K. Non-enzymatic sensing of glucose using screen-printed electrode modified with novel synthesized CeO2@ CuO core shell nanostructure. Biosensors and Bioelectronics 111, 166–173 (2018).
Dung, N. Q., Patil, D., Jung, H. & Kim, D. A high-performance nonenzymatic glucose sensor made of CuO–SWCNT nanocomposites. Biosensors and Bioelectronics 42, 280–286 (2013).
Foroughi, F., Rahsepar, M., Hadianfard, M. J., & Kim, H. Microwave-assisted synthesis of graphene modified CuO nanoparticles for voltammetric enzyme-free sensing of glucose at biological pH values. Microchimica Acta, 185 ( 1 ) (2018).
Tang, Z., Du, X., Louie, R. F. & Kost, G. J. Effects of pH on glucose measurements with handheld glucose meters and a portable glucose analyzer for point-of-care testing. Archives of pathology & laboratory medicine. 124(4), 577–582 (2000).
Flores, G., Perdigones, F., Aracil, C., Cabello, M. & Quero, J. M. Microfluidic platform with absorbance sensor for glucose detection. In Electron Devices (CDE), 2015 10th Spanish Conference on (pp. 1–4). IEEE (2015).
Agrawal, M., & Seshia, A. A. A microfluidic platform for glucose sensing using broadband ultrasound spectroscopy. In Frequency Control Symposium (IFCS), 2016 IEEE International (pp. 1–5). IEEE (2016).
Gonzalez, A., Estala, L., Gaines, M. & Gomez, F. A. Mixed thread/paper‐based microfluidic chips as a platform for glucose assays. Electrophoresis 37(12), 1685–1690 (2016).
Avoundjian, A., Jalali-Heravi, M. & Gomez, F. A. Use of chemometrics to optimize a glucose assay on a paper microfluidic platform. Analytical and bioanalytical chemistry 409(10), 2697–2703 (2017).
Liu, S., Su, W. & Ding, X. A review on microfluidic paper-based analytical devices for glucose detection. Sensors. 16(12), 2086 (2016).
Suresh, V., Qunya, O., Kanta, B. L., Yuh, L. Y. & Chong, K. S. Non-invasive paper-based microfluidic device for ultra-low detection of urea through enzyme catalysis. Royal Society Open Science. 5(3), 171980 (2018).
Toghill, K. E. & Compton, R. G. Electrochemical non-enzymatic glucose sensors: a perspective and an evaluation. Int. J. Electrochem. Sci 5(9), 1246–1301 (2010).
Park, S., Boo, H. & Chung, T. D. Electrochemical non-enzymatic glucose sensors. Analytica chimica acta 556(1), 46–57 (2006).
Yoo, E. H. & Lee, S. Y. Glucose biosensors: an overview of use in clinical practice. Sensors 10(5), 4558–4576 (2010).
Beverskog, B. & Puigdomenech, I. Revised Pourbaix diagrams for Copper at 5–150 C. Swedish Nuclear Power Inspectorate (1995).
Barragan, J. T., Kogikoski, S. Jr., da Silva, E. T. & Kubota, L. T. Insight into the Electro-Oxidation Mechanism of Glucose and Other Carbohydrates by CuO-Based Electrodes. Analytical chemistry 90(5), 3357–3365 (2018).
Singh, S. V., Saxena, O. C. & Singh, M. P. Mechanism of copper (II) oxidation of reducing sugars. I. Kinetics and mechanism of oxidation of D-xylose, L-arabinose, D-glucose, D-fructose, D-mannose, D-galactose, L-sorbose, lactose, maltose, cellobiose, and melibiose by copper (II) in alkaline medium. Journal of the American Chemical Society 92(3), 537–541 (1970).
Katoch, R. Analytical techniques in biochemistry and molecular biology. Springer Science & Business Media (2011).
Cao, F. & Gong, J. Nonenzymatic glucose sensor based on CuO microfibers composed of CuO nanoparticles. Analytica chimica acta 723, 39–44 (2012).
Liu, G. et al. Improvement of sensitive CuO NFs–ITO nonenzymatic glucose sensor based on in situ electrospun fiber. Talanta 101, 24–31 (2012).
Huang, F. et al. Nonenzymatic glucose sensor based on three different CuO nanomaterials. Analytical. Methods 5(12), 3050–3055 (2013).
Acknowledgements
This research was supported by Khalifa University KUSTAR-KUIRF L2 internal research grant and System on Chip Center RC2-2018-20.
Author information
Authors and Affiliations
Contributions
H.A. led device design, material selection, electrical characterization, glucose testing, in addition to manuscript preparation. B.M. oversaw the full project and contributed to data analysis. A.A. led device fabrication and contributed to data analysis. M.A.J. contributed to the material selection, device design and data analysis. M.A.Q. contributed to data analysis. S.A.H. contributed to material characterization. S.A.S. contributed to data analysis and critically revised the manuscript. H.A., B.M., A.A. M.A.J. M.A.Q. and S.A.S. commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abunahla, H., Mohammad, B., Alazzam, A. et al. MOMSense: Metal-Oxide-Metal Elementary Glucose Sensor. Sci Rep 9, 5524 (2019). https://doi.org/10.1038/s41598-019-41892-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41892-w
- Springer Nature Limited
This article is cited by
-
Silver Nanoclusters as Label Free Non-enzymatic Fast Glucose Assay with the Fluorescent Enhancement Signal
Journal of Fluorescence (2024)
-
Electrodeposition of Ni-Cu alloy electrodes in sulfate–citrate medium and their use for non-enzymatic glucose oxidation
Ionics (2024)
-
Reduced graphene oxide supported MXene based metal oxide ternary composite electrodes for non-enzymatic glucose sensor applications
Scientific Reports (2022)
-
High-Precision Nonenzymatic Electrochemical Glucose Sensing Based on CNTs/CuO Nanocomposite
Journal of Electronic Materials (2022)
-
Highly sensitive non-enzymatic electrochemical glucose sensor based on dumbbell-shaped double-shelled hollow nanoporous CuO/ZnO microstructures
Scientific Reports (2021)