Abstract
Microalgae of the genus Chlorella vulgaris are candidates for the production of lipids for biofuel production. Besides that, Chlorella vulgaris is marketed as protein and vitamin rich food additive. Its potential as a novel expression system for recombinant proteins inspired us to study its asparagine-linked oligosaccharides (N-glycans) by mass spectrometry, chromatography and gas chromatography. Oligomannosidic N-glycans with up to nine mannoses were the structures found in culture collection strains as well as several commercial products. These glycans co-eluted with plant N-glycans in the highly shape selective porous graphitic carbon chromatography. Thus, Chlorella vulgaris generates oligomannosidic N-glycans of the structural type known from land plants and animals. In fact, Man5 (Man5GlcNAc2) served as substrate for GlcNAc-transferase I and a trace of an endogenous structure with terminal GlcNAc was seen. The unusual more linear Man5 structure recently found on glycoproteins of Chlamydomonas reinhardtii occurred - if at all - in traces only. Notably, a majority of the oligomannosidic glycans was multiply O-methylated with 3-O-methyl and 3,6-di-O-methyl mannoses at the non-reducing termini. This modification has so far been neither found on plant nor vertebrate N-glycans. It’s possible immunogenicity raises concerns as to the use of C. vulgaris for production of pharmaceutical glycoproteins.
Similar content being viewed by others
Introduction
Chlorella is a well-known member of the taxonomically enormously diverse group of microalgae. It enjoys considerable attention as a production system for various lipids, either as biofuel source1,2,3,4,5 or as food and feed additives such as carotenoids or astaxanthin6,7. C. vulgaris and “C. pyrenoidosa” - a still applied but unfortunately outdated classification8,9 - are offered as dietary supplements with diverse assertions of health benefits10,11. Chlorella species are also being studied as production platforms of recombinant proteins12,13,14. This warrants interest in the potential of Chlorella and other microalgae used for this purpose to conduct post-translational modifications, in particular protein glycosylation.
High-mannose N-glycans have been found in various microalgae such as the diatom Phaeodactylum tricornutum15 but also unusual structures such as 6-O-methyl mannose on the red alga Porphyridium – a taxonomic group rather unrelated to green plants - have been discovered16. Euglena – sometimes referred to as microalga – but actually a member of a separate phylum (or group) to which i.a. the trypanosomes belong – were found to contain oligomannosidic structures partially modified with aminoethylphosphonate moieties17. The green alga Chlamydomonas reinhardtii was reported to produce glycoproteins with mammalian-type N-glycans even containing sialic acids18. Later, C. reinhardtii was shown to contain oligomannose glycans – or better low-mannose glycans due to their limited size of hardly more than 5 mannoses - but also glycans with up to two xylose residues and with 6-O-methyl mannose19. A glycopeptide based study on Botryococcus brauni – a green algae belonging to the class of Trebouxiophyceae just as Chlorella - discovered N-glycopeptides with up to three GlcNAc residues indicating action of GlcNAc-transferase I (GnTI)20. Furthermore, methyl-hexose and pentose were found by CID-MS/MS of glycopeptides. This small number of papers on glycoprotein structures of microalgae (including just a few green algae) can be rated as a sign of ignorance given their ecological significance and their growing role as biofactories. In particular, C. reinhardtii and Chlorella species are regarded as promising production hosts for proteins and glycoproteins14,21 and the diatom microalgae Phaeodactylum tricornutum has recently been demonstrated to produce a fully functional anti-hepatitis antibody with high-mannose glycans22. However, in the green alga C. reinhardtii the Man5GlcNAc2 N-glycan assumed to represent a substrate for recombinant GnTI turned out as having an unusual, more linear structure inaccessible for GnTI23.
In this work we investigated the N-glycosylation of Chlorella vulgaris strains from culture collections as well as of commercial products. MALDI-TOF MS, chromatography on graphitic carbon and amide silica, gas chromatography of constituent sugars and action of GlcNAc-transferase I (GnTI) were applied to characterize the N-glycans of C. vulgaris.
Results
MALDI-TOF MS profiles of Chlorella vulgaris N-glycans
N-glycans from the live strain SAG 211-11b were isolated from the complete bulk material by a succession of pepsin digestion, cation exchange, PNGase A digestion and another cation exchange step. MALDI-TOF MS of the resulting oligosaccharides revealed a rather complex pattern with five prominent groups of peaks. The smallest masses within each group had compositions from Man5GlcNA2 to Man9GlcNAc2 (Man5 to Man9) (Fig. 1A). These well-known compounds were followed by peaks spaced by 14.018 Da indicating series of methyl groups (Fig. 1B). Essentially the same profiles were obtained when samples were repeatedly (>four times) analyzed as in the case of SAG 211-11b and GreenGem.
N-glycan profiles of Chlorella vulgaris culture collection strains and several commercial Chlorella products. MALDI-TOF MS patterns of reduced (panel A and B) and native N-glycans (all other samples) are shown for the culture collection strains Chlorella vulgaris SAG 211-11b and 211-8 l (A–C), and UTEX 395 (D) and for commercial products (panels E to H).
The same compounds albeit in different proportions were found in the C. vulgaris strains UTEX 395 (Fig. 1C) and SAG 211-8 l (Fig. 1D). Very similar MALDI-TOF MS patterns were found for several commercial Chlorella products (Fig. 1E–H). Remarkably, some of these strains were designated as C. pyrenoidosa, although this taxonomic name has been dismissed some time ago and the respective strains have been assigned as other Chlorella species and lines or even as other genera8. Many commercial products nevertheless bear this species name and it still occurs in the scientific literature. It shall not be concealed that other “C. pyrenoidosa” products exhibited different N-glycan profiles that apparently contained pentoses, O-methyl groups and possibly deoxyhexoses but these shall be subject of a future study.
Location of methyl groups
To characterize the nature of glycan methylation, N-glycans from SAG211-11b and from GreenGem tablets were hydrolyzed. The monosaccharides were reduced, peracetylated and subjected to GC-MS together with suitable partially methylated standards24. This revealed the presence of 3-O-methyl mannose (24% compared to the mannose peak) and smaller amounts of 3,6-di-O-methyl mannose (4%) (Fig. 2) in C. vulgaris 211-11b. Similar values were found for the GreenGem sample, whereby the only semiquantitative nature of this figures shall be conceded as reference compounds for quantitative analysis of the methyl hexoses were not available.
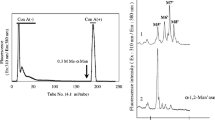
MALDI-TOF LIFT MS/MS spectra of underivatized glycans showed little useful detail about the location of the methyl groups. In order to obtain ESI-MS/MS spectra without the risk of hybrid spectra with precursors of differing degree of methylation (7 Th mass difference at charge state 2), we attempted HILIC fractionation, which led to a preparation of suitably isolated trimethylated Man9. The ESI-MS/MS spectrum of Me3Man9GlcNAc2 was in perfect agreement with the assumption that all methyl groups were attached to terminal mannose residues (Fig. 3). Y-ions or GlcNAc-truncated y-ions showed +14 Da increments only at a size range that possibly contained terminal Man residues. The pattern is particularly consistent with the assumption of a major fraction with all terminal residues being mono-methylated and a minor part with a non-, mono- and dimethylated mannose each. In fact, the presence of at least three isomers of Me3Man9GlcNAc2 is indicated by PGC chromatography (Fig. 4A). As a detail out of many, the large dimethylated Man9 peak (Fig. 4A) generates B-ions for a mono-methyl-mannose m/z = 339.1), whereas the later eluting, smaller one that for a di-methyl-mannose m/z = 353.1).
Analysis of C. vulgaris N-glycans by PGC-LC-ESI-MS. Panels (A,B) show the elution profiles of Chlorella glycans Man9 and Man8, respectively. Isomeric structures were deduced from coelution with bean N-glycans. The EIC traces of di- to penta-methylated glycans are shown in the background. Peaks labeled with “e” represent epimerization artefacts of the major peaks. Panel (C) is the EIC for Chlorella Man5 with an unusual early eluting peak in addition to the regular Man5 structure. Panel (D) gives the elution pattern of the Man5 isomer from an ALG3 deficient Arabidopsis line27. Panels (E,F) demonstrate the effect of GnTI on Chlorella Man5 in the absence (E) and presence (F) of UDP-GlcNAc.
Then again, the smaller glycans down to Man5 also were decorated with up to 4 methyl-groups. I.e. residues other than those modified in Man9 must have born the methyl groups. This insight has an interesting repercussion as to the biosynthesis of these glycans: The methyl groups are transferred to the mature N-glycans.
Isomer structure of oligomannosidic N-glycans in C. vulgaris and detection of Man5Gn
The isomeric structures of oligomannosidic glycans of C. vulgaris SAG 211-11b by PGC-HPLC were studied by PGC-LC-ESI-MS. Comparison with the N-glycans of white kidney beans revealed co-elution of almost all non-methylated glycans as shown for Man8 and Man9 (Fig. 4). An EIC for the mass of Man5, however, gave two peaks, one at the position of the common (M6M3)M and another one eluting much earlier. At first, we verified the structure of the second peak by incubation of a HILIC enriched Man5 fraction with recombinant GnTI. The peak was converted to Man5Gn (Fig. 4B and Supplementary Fig. 1) and so elution time and transferase specificity argued for the classic (M6M3)M structure [for an explanation of nomenclature consult reference25]. Further support for this conclusion came from the accessilibity of the Man5Gn product to core α1,6-fucosyl transferase (Supplementary Fig. 2).
The earlier eluting peak Man5 that was not converted by GnTI may have the unusual structure with two α1,2-linked mannoses on the 3-arm but no branching mannoses on the 6-arm recently described as the only Man5 glycan in Chlamydomonas reinhardtii23. This MM2 isomer would elute rather early on PGC25. To verify its nature, we prepared glycans from an alg3 and also mannosidase (mns123) mutant plant that contained by necessity the MM2 isomer26. Surprisingly, this isomer did not coelute with the strange Chlorella Man5 (Fig. 4).
A last point worth mentioning is that the control sample already contained a small amount of a compound with just the same mass and elution position as Man5Gn (Fig. 4).
Influence of methylation on chromatographic behavior
On the PGC columns, methylation increased retention in accordance with the view of graphitic carbon operating – at least in part – by a reversed-phase mechanism. Isobaric methyl isomers were remarkably well separated (Fig. 4). The effect on the HILIC column was opposite and more uniform. Methylation resulted in a strong forward shift roughly equivalent to one mannose residue (Supplementary Fig. 3).
Discussion
Oligomannosidic glycans with zero to five methyl groups on terminal mannose residue constitute the N-glycomes of Chlorella vulgaris type strains 211-11b, SAG 211-8 l and UTEX 235. A number of commercially available algal preparations also exhibited this pattern irrespective of the species name declared by the suppliers. It must be emphasized that other products as well as other species exhibited different N-glycan patterns (Supplementary Fig. 4). These differences may harbor a valuable means for strain or species differentiation but plumbing this option would by far exceed the scope of the present study.
On a Man9 N-glycan of SAG 211-11b, MS/MS showed the methyl groups to reside on the terminal mannose residues (Fig. 4). As methylation affected glycans of all size, we propose that it takes place after the mannosidase trimming as a finishing touch of glycan maturation. The idea of incorporation of already methylated mannose residues during precursor synthesis would require that both cytosolic GDP-mannose and ER luminal dolichol-P-mannose would exist in part in a methylated version and that the respective transferases would accept these donors. Notably, C. vulgaris features 3-O-methyl rather than 6-O-methyl mannose as found for C. reinhardtii19. A possible purpose of methylation might be to confer resistance to unwelcome mannosidase trimming by competing organisms. In fact, it did confer resistance to jack bean α-mannosidase (Supplementary Fig. 5).
A recent work on Chlamydomonas reinhardtii revealed the surprising fact that the N-glycan Man5 (Man5GlcNAc2) did not have the common and expected structure (M6M3)M (see reference25 for explanation) but rather the isomer MM2-2 with an untruncated 3-arm23. This isomer is formed in the absence of ALG3 = Dol-P-Man:Man5GlcNAc2-PP-Dol α1,3-mannosyltransferase. As a consequence, heterologous expression of GnTI did not result in any change of the glycosylation profile23. While Chlamydomonas and Chlorella belong to different classes (Chlorophyceae and Trebouxiophyceae, respectively), they both find themselves in the phylum Chlorophyta/green algae and thus may share relevant features of N-glycan processing. However, while C. reinhardtii humbles itself with Man5 as the largest high-mannose glycan19,23, C. vulgaris presents mainly large high-mannose glycans that - apart from methylation - have the same structures as kidney bean glycans as judged from PGC-elution profiles. Man5, however, gave two peaks, one at the elution time of the common (M6M3)M and a peak eluting significantly earlier. The “linear” Man5 isomer MM2-2 described for C. reinhardti23 was isolated from an ALG3 deficient Arabidopsis plant and expected to coelute with this other Man5 isomer. However, it did not do so. A possible explanation that matches the effect of different mannose residues on retention could be that this Man5 peak is M3M2 the result of only partial alg12 action. The later eluting peak was readily converted to Man5Gn by GnTI and represented the classic isomer (M6M3)M. The GreenGem control sample lacking UDP-GlcNAc nevertheless already contained a trace of a substance with exactly the same m/z, elution time and also fragment spectrum as Man5Gn. The presence of Man5Gn in C. vulgaris is not unlikely given the presence of a gene of high homology to Arabidopsis thaliana MAGT (Uniprot # A0A2P6TDC6 _CHLSO). Compounds downstream of Man5Gn – if present - did not occur as major products, at least not under the applied growth conditions and not in the C. vulgaris lines studied. Genes/proteins with high homology to ALG3, ALG12 and ALG9 of Arabidopsis thaliana can be found in Chlorella species (Uniprot # A0A087SGA3 _AUXPR, E1Z9Y6_CHLVA or A0A2P6U3R5_CHLSO and E1ZH92_CHLVA or A0A2P6TST7_CHLSO, respectively). These proteins are responsible for the formation of the oligomannose precursor. Together with the identical elution positions of Chlorella and bean oligomannose glycans this strongly supports the idea of conserved pathway up to Man5 and very probably even Man5Gn. Highly homologous genes have recently been identified even in a red microalga27 and a diatom species15.
The current work certainly shows that microalgae, i.e. Chlorellas, can harbor the complete Glc3Man9 pathway as Man5 to Man9 structures indistinguishable from plant N-glycans were found. No ALG3 bottle neck exists in Chlorella as is the case in C. reinhardtii23, but C. vulgaris would have been but a slightly better choice for heterologous expression of GnTI, as only about 1% of the total N-glycome existed as the GnTI substrate (M6M3)M. The occurrence of traces of Man5Gn, i.e. the initial product of GnTI in C. vulgaris is backed up by glycoproteomic evidence for the occurrence of terminal HexNAc in an other Trebouxiophyceae species20.
Utilization of C. vulgaris as a production host for glycoproteins would at first require to identify the O-methyltransferase acting on terminal mannose residues. While the immunogenicity of methylated N-glycans has not yet been demonstrated, it is arguable that only methyltransferase knock-out microalgae could be considered for the expression of therapeutic glycoproteins. Such knock-out lines could also answer the scientific question as to the biological purpose of N-glycan methylation.
Methods
Sources of biological samples
Culture collection strains and commercial tablets as collected in 2016 to 2017 are listed in (Table 1). Live algae were grown for 15–21 days in 50 mL Bold’s Basal Medium in sterile Erlenmeyer flasks, in the presence of Tetracyclin (final concentration 10 μg/mL). Autotrophic cultivation was carried out at 22 °C, under continuous illumination by the built-in light source (Osram T8 L 36 W 830 G13 Lumilux, Munich, Germany) and shaking on 160 rpm. Microalgae concentration was determined by optical density measurement at 682 nm. Cells were harvested at the end of the exponential growth phase by centrifugation (5000 g; 15 min) and were subjected to further analysis immediately. Leaves of an alg3 mutant Arabidopsis thaliana line (mns123 alg3 quadruple knock out) were kindly provided by Richard Strasser (see Related manuscript).
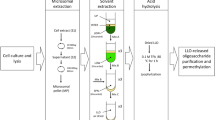
Extraction and fractionation of N-glycans
N-glycans were isolated by a combination of pepsin digestion, cation exchange based capturing of peptides and glycopeptides, digestion with peptide:N-glycosidase A (Europa Bioscience Ltd, Cambridge, UK), repeated cation exchange and polishing by reversed phase solid phase extraction as described albeit on smaller scale28.
HILIC on a TSK Amide80 column (4 × 250 mm, 5 µm; Tosoh Bioscience GmbH, Griesheim, Germany) was performed on underivatized glycans for preparative purposes28. Fractions of 0.5 mL were analyzed by MALDI-TOF MS. This led inter alia to a fraction of Me3Man9GlcNAc2 that could be used for ESI-MS/MS without any danger of interference by adjacent peaks with more or less methyl groups (Supplementary Fig. 3).
Mass spectrometric methods
MALDI-TOF MS of glycan pools was performed with dihydroxybenzoic acid as the matrix on a Bruker Autoflex MALDI-TOF instrument in the positive ion reflectron mode. Usually, unreduced samples were analyzed, but in some cases reduction with 1% sodium borohydride was done to readily discriminate glycan from non-glycan peaks.
Reduced glycans were analyzed by LC-ESI-MS with a porous graphitic carbon (PGC) column (0.32 µm x 150 mm) operated by an Ultimate RSLC (Thermo Scientific, Vienna) connected to a Maxis 4 G Q-TOF MS (Bruker, Bremen, Germany)25. N-glycans from white kidney beans were used as reference25. MS/MS was performed in positive mode.
The monosaccharide constituents were analyzed after hydrolysis of glycan pools of fractions with 4 M trifluoroacetic acid at 100° for 4 h. Sugars were reduced with NaBD4, peracetylated and analyzed on an Agilent J&W HP-5ms GC Column (30 m x 0.25 mm, 0.25 µm) installed in a GC-MS system (GC 7820 A & MSD 5975, Agilent, Waldbronn, Germany). Partially methylated alditol acetates were available from a previous study29 and their relative retention times were additionally confirmed by literature data30.
GlcNAc-transferase reaction
Man5 substrates were prepared from the N-glycan pools of either Green Gem tablets or kidney beans by size separation on an amide column as described31. Rabbit GlcNAc-transferase I (GnTI) lacking the N-terminal 105 amino acids32 was expressed with an N-terminal His6-tag using a pVT-Bac vector and the baculovirus insect cell system33. The enzyme was purified by metal chelate chromatography34. The purified enzyme was added to 0.45 nmol of Man5 from GreenGem tablets or kidney beans in 50 mM MES buffer (pH 7.0) containing 500 nmol MnCl2 and 10 nmol UDP-GlcNAc (Kyowa Hakko, Tokyo) and incubated overnight at 37 °C. The glycans in the mixtures were purified using carbon solid phase cartridges (Multi-Sep Hypercarb 10 mg, Thermo Scientific, Vienna) as described35. The eluate was dried, taken up in pure water and analyzed by PGC-LC-ESI-MS as described above.
Data Availability
The datasets (MS files) generated during the current study are available from the corresponding author on request.
References
Rosenberg, J. N. et al. Comparative analyses of three Chlorella species in response to light and sugar reveal distinctive lipid accumulation patterns in the Microalga C. sorokiniana. PLoS One 9, e92460, https://doi.org/10.1371/journal.pone.0092460 (2014).
Huang, J., Xia, J., Jiang, W., Li, Y. & Li, J. Biodiesel production from microalgae oil catalyzed by a recombinant lipase. Bioresour Technol 180, 47–53, https://doi.org/10.1016/j.biortech.2014.12.072 (2015).
Chen, C. L. et al. Biodiesel production from wet microalgae feedstock using sequential wet extraction/transesterification and direct transesterification processes. Bioresour Technol 194, 179–186, https://doi.org/10.1016/j.biortech.2015.07.021 (2015).
He, Q., Yang, H. & Hu, C. Culture modes and financial evaluation of two oleaginous microalgae for biodiesel production in desert area with open raceway pond. Bioresour Technol 218, 571–579, https://doi.org/10.1016/j.biortech.2016.06.137 (2016).
Baroukh, C., Munoz-Tamayo, R., Steyer, J. P. & Bernard, O. A state of the art of metabolic networks of unicellular microalgae and cyanobacteria for biofuel production. Metab Eng 30, 49–60, https://doi.org/10.1016/j.ymben.2015.03.019 (2015).
Huang, W., Lin, Y., He, M., Gong, Y. & Huang, J. Induced High-Yield Production of Zeaxanthin, Lutein, and beta-Carotene by a Mutant of Chlorella zofingiensis. J Agric Food Chem 66, 891–897, https://doi.org/10.1021/acs.jafc.7b05400 (2018).
Mao, X., Wu, T., Sun, D., Zhang, Z. & Chen, F. Differential responses of the green microalga Chlorella zofingiensis to the starvation of various nutrients for oil and astaxanthin production. Bioresour Technol 249, 791–798, https://doi.org/10.1016/j.biortech.2017.10.090 (2018).
Champenois, J., Marfaing, H. & Pierre, R. Review of the taxonomic revision of Chlorella and consequences for its food uses in Europe. J Appl Phycol 27, 1845–1851 (2015).
De Clerck, O., Guiry, M. D., Leliaert, F., Samyn, Y. & Verbruggen, H. Algal taxonomy: a road to nowhere? J Phycol 49, 215–225, https://doi.org/10.1111/jpy.12020 (2013).
Panahi, Y., Darvishi, B., Jowzi, N., Beiraghdar, F. & Sahebkar, A. Chlorella vulgaris: A Multifunctional Dietary Supplement with Diverse Medicinal Properties. Curr Pharm Des 22, 164–173, https://doi.org/10.2174/1381612822666151112145226 (2016).
Furbeyre, H., van Milgen, J., Mener, T., Gloaguen, M. & Labussiere, E. Effects of oral supplementation with Spirulina and Chlorella on growth and digestive health in piglets around weaning. Animal, 1–10, https://doi.org/10.1017/S1751731118000125 (2018).
Rasala, B. A. & Mayfield, S. P. Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth Res 123, 227–239, https://doi.org/10.1007/s11120-014-9994-7 (2015).
Yan, N., Fan, C., Chen, Y. & Hu, Z. The Potential for Microalgae as Bioreactors to Produce Pharmaceuticals. Int J Mol Sci 17, https://doi.org/10.3390/ijms17060962 (2016).
Yang, B., Liu, J., Jiang, Y. & Chen, F. Chlorella species as hosts for genetic engineering and expression of heterologous proteins: Progress, challenge and perspective. Biotechnol J 11, 1244–1261, https://doi.org/10.1002/biot.201500617 (2016).
Baiet, B. et al. N-glycans of Phaeodactylum tricornutum diatom and functional characterization of its N-acetylglucosaminyltransferase I enzyme. J Biol Chem 286, 6152–6164, https://doi.org/10.1074/jbc.M110.175711 (2011).
Levy-Ontman, O. et al. Unique N-glycan moieties of the 66-kDa cell wall glycoprotein from the red microalga Porphyridium sp. J Biol Chem 286, 21340–21352, https://doi.org/10.1074/jbc.M110.175042 (2011).
O’Neill, E. C. et al. Exploring the Glycans of Euglena gracilis. Biology (Basel) 6, https://doi.org/10.3390/biology6040045 (2017).
Mamedov, T. & Yusibov, V. Green algae Chlamydomonas reinhardtii possess endogenous sialylated N-glycans. FEBS Open Bio 1, 15–22, https://doi.org/10.1016/j.fob.2011.10.003 (2011).
Mathieu-Rivet, E. et al. Exploring the N-glycosylation pathway in Chlamydomonas reinhardtii unravels novel complex structures. Mol Cell Proteomics 12, 3160–3183, https://doi.org/10.1074/mcp.M113.028191 (2013).
Schulze, S. et al. Identification of methylated GnTI-dependent N-glycans in Botryococcus brauni. New Phytol 215, 1361–1369, https://doi.org/10.1111/nph.14713 (2017).
Mathieu-Rivet, E. et al. Protein N-glycosylation in eukaryotic microalgae and its impact on the production of nuclear expressed biopharmaceuticals. Front Plant Sci 5, 359, https://doi.org/10.3389/fpls.2014.00359 (2014).
Vanier, G. et al. Alga-Made Anti-Hepatitis B Antibody Binds to Human Fcgamma Receptors. Biotechnol J, https://doi.org/10.1002/biot.201700496 (2017).
Vanier, G. et al. Heterologous expression of the N-acetylglucosaminyltransferase I dictates a reinvestigation of the N-glycosylation pathway in Chlamydomonas reinhardtii. Sci Rep 7, 10156, https://doi.org/10.1038/s41598-017-10698-z (2017).
Altmann, F. et al. Genome Analysis and Characterisation of the Exopolysaccharide Produced by Bifidobacterium longum subsp. longum 35624. PLoS One 11, e0162983, https://doi.org/10.1371/journal.pone.0162983 (2016).
Pabst, M. et al. Isomeric analysis of oligomannosidic N-glycans and their dolichol-linked precursors. Glycobiology 22, 389–399, https://doi.org/10.1093/glycob/cwr138 (2012).
Veit, C., Altmann, F. & Strasser, R. Processing of the terminal alpha-1,2-linked mannose residues from oligomannosidic N-glycans is critical for proper root growth. Front Plant Sci in press https://doi.org/10.3389/fpls.2018.01807 (2018).
Levy-Ontman, O. et al. Genes Involved in the Endoplasmic Reticulum N-Glycosylation Pathway of the Red Microalga Porphyridium sp.: A Bioinformatic Study. International Journal of Molecular Sciences 15, 2305–2326, https://doi.org/10.3390/ijms15022305 (2014).
Grass, J. et al. Discovery and structural characterization of fucosylated oligomannosidic N-glycans in mushrooms. J Biol Chem 286, 5977–5984, https://doi.org/10.1074/jbc.M110.191304 (2011).
Windwarder, M. et al. “Hypermethylation” of anthranilic acid-labeled sugars confers the selectivity required for liquid chromatography-mass spectrometry. Anal Biochem 514, 24–31, https://doi.org/10.1016/j.ab.2016.09.008 (2016).
Carpita, M. C. & Shea, E. M. In Analysis of Carbohydrates by GLC and MS (eds McGinnis, G. D. & Biermann, C. J.) Ch. 9, 157–181 (CRC Press, 1988).
Pabst, M. et al. Comparison of fluorescent labels for oligosaccharides and introduction of a new postlabeling purification method. Anal Biochem 384, 263–273, https://doi.org/10.1016/j.ab.2008.09.041 (2009).
Sarkar, M. et al. Removal of 106 amino acids from the N-terminus of UDP-GlcNAc: alpha-3-D-mannoside beta-1,2-N-acetylglucosaminyltransferase I does not inactivate the enzyme. Glycoconj J 15, 193–197 (1998).
Liebminger, E. et al. Class I alpha-mannosidases are required for N-glycan processing and root development in Arabidopsis thaliana. Plant Cell 21, 3850–3867, https://doi.org/10.1105/tpc.109.072363 (2009).
Strasser, R. et al. Molecular basis of N-acetylglucosaminyltransferase I deficiency in Arabidopsis thaliana plants lacking complex N-glycans. Biochem J 387, 385–391, https://doi.org/10.1042/BJ20041686 (2005).
Grunwald-Gruber, C., Thader, A., Maresch, D., Dalik, T. & Altmann, F. Determination of true ratios of different N-glycan structures in electrospray ionization mass spectrometry. Anal Bioanal Chem 409, 2519–2530, https://doi.org/10.1007/s00216-017-0235-8 (2017).
Acknowledgements
The authors acknowledge the Equipment GmbH of University of Natural Resources and Life Sciences for privileged access to the Bruker Maxis 4 G Q-TOF MS. They thank Ing. Karin Polacsek for Dr. Clemens Grünwald-Gruber for valuable help with mass spectrometric equipment and Dr. Stephan Hann for granting access to the GLC-MS instrument.
Author information
Authors and Affiliations
Contributions
R.M. conducted most of the experimental work, R.F. performed HILIC. and HILIC-M.S. analysis, C.T. established microalgae cultivation, R.S. contributed the Arabidopsis thaliana mns123 alg3 mutant sample, ES performed MALDI and monosaccharide analyses, M.W. performed relevant L.C.-M.S. and M.S.M.S. experiments, AT cloned and expressed recombinant glycosyltransferases and F.A. supervised the work and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mócsai, R., Figl, R., Troschl, C. et al. N-glycans of the microalga Chlorella vulgaris are of the oligomannosidic type but highly methylated. Sci Rep 9, 331 (2019). https://doi.org/10.1038/s41598-018-36884-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-36884-1
- Springer Nature Limited
This article is cited by
-
Particle-attached bacteria act as gatekeepers in the decomposition of complex phytoplankton polysaccharides
Microbiome (2024)
-
Golgi fucosyltransferase 1 reveals its important role in α-1,4-fucose modification of N-glycan in CRISPR/Cas9 diatom Phaeodactylum tricornutum
Microbial Cell Factories (2023)
-
Algal glycobiotechnology: omics approaches for strain improvement
Microbial Cell Factories (2021)
-
N-linked glycosylation enzymes in the diatom Thalassiosira oceanica exhibit a diel cycle in transcript abundance and favor for NXT-type sites
Scientific Reports (2021)
-
Thorsmoerkia curvula gen. et spec. nov. (Trebouxiophyceae, Chlorophyta), a semi-terrestrial microalga from Iceland exhibits high levels of unsaturated fatty acids
Journal of Applied Phycology (2021)