Abstract
The area of saline soils accounts for 8% of the earth’s surface, making these soils an important terrestrial carbon sink. Soil organic carbon (SOC), microbial biomass carbon (MBC), dissolved organic carbon (DOC), soil enzyme activity, and soil bacterial abundance and biodiversity were measured in four successive coastal tidal flat ecosystems representing: bare saline soil (BS), Suaeda glauca land (SL), Imperata cylindrica grassland (IG), and Jerusalem artichoke field (JF). A decrease in soil salt content resulted in increased SOC content. With vegetation succession, MBC and DOC concentrations showed a positive trend, and activities of soil urease, catalase, invertase and alkaline phosphatase increased. A next-generation, Illumina-based sequencing approach showed that Proteobacteria, Acidobacteria, Chloroflexi, Bacteroidetes, Gemmatimonadetes, Actinobacteria, Nitrospirae and Planctomycetes were the dominant bacterial communities (a total of 597 taxa were detected, and 27 genera showed significant differences among the vegetation communities). Bacterial diversity at two soil depths was enhanced with the succession of vegetation ecosystems, with the increases in operational taxonomic units (OTUs) and the Shannon and Chao1 indices ranked in the order: JF > IG > SL > BS. The SOC and C/N were the most determinant factors influencing diversity of bacterial communities in the succession ecosystems.
Similar content being viewed by others
Introduction
The acceleration of greenhouse gas emissions is one of the primary concerns in the twenty-first century1. An increase in atmospheric temperatures and corresponding climate change and variability are attributed to the increasing levels of atmospheric carbon dioxide (CO2) and other greenhouse gases. The concentration of CO2 has increased by 31% from 280 μL/L in 1850 to 380 μL/L in 2004. The Earth surface temperature has increased between 0.4 and 0.8 °C (0.6 ± 0.2 °C) since the late 19th century2. An estimate of the expected rise in average surface air temperature globally is between 1 and 3.5 °C by year 21003. Carbon sequestration in soil reduces the rate of enrichment of atmospheric CO2 concentration, which can contribute to mitigating climate change4.
Soil salinization is an important process, affecting about 8.31 × 108 ha of soil resources worldwide5. The total area of saline soil in China is about 3.6 × 107 ha, accounting for 4.9% percent of total available land in the country6. The studies on sequestering atmospheric carbon in China have been focused mainly on forest, grassland and farmland soils7, with little knowledge on soil carbon sequestration in coastal alkali-saline soils.
Soil contains the largest pool of terrestrial organic carbon in the biosphere, storing more carbon than plants and atmosphere combined8, with 73% of soil C contained in soil organic matter9. It is estimated that the soil carbon reserve is about 2.5~3.0 times the vegetation carbon reserve in terrestrial ecosystems, and 2~3 times the carbon reserve in the atmospheric carbon pool. Therefore, relatively small variations in the C reserves in the soil organic C pool may influence substantially the concentration of CO2 in the atmosphere10.
Some studies indicated that vegetation succession can improve soil carbon sequestration capacity11. In addition, many studies on soil carbon dynamics agreed that the storage capacity and relative distribution of soil carbon are related to the vegetation type12. The reason is mainly due to different plant forms influencing soil physical and chemical properties, litter chemistry, detritus input and rooting depth13. However, such knowledge on the relationships between soil carbon and soil biotic and abiotic characteristics in different vegetation types in coastal alkali-saline soils is lacking.
Jiangsu Province (China) has abundant tidal flats with a total area of 6.0 × 105 hectares, representing a quarter of the total area of tidal flats in the country14. The coastal tidal flats in Dafeng area are an important part of tidal flats in Jiangsu Province, representing the largest tidal wetland in eastern Asia. Moreover, tidal flats in this area still expand at the rate of 50~200 m per year due to a large amount of sediment deposition by Yellow River and Yangtze River14,15. The experimental areas were undisturbed and maintain the original natural state.
The hydromorphic soils are easily affected by seawater intrusion and groundwater recharge and are vulnerable to becoming saline. Halophytic vegetation forms on saline soils, with salt-tolerant Suaeda glauca being a pioneer plant species16. Its residues cover soil surface, increase soil organic matter, improve soil fertility and alleviate the effects of salt accumulation on the soil surface. With a gradual decrease in soil salinity, communities of herbaceous perennials (e.g. Imperata cylindrica) appear in succession and begin to dominate17. In such circumstances, soil salinity gradually decreases, and cultivation of salt-tolerant Helianthus tuberosum (Jerusalem artichoke) becomes possible as the advanced stage in the process of vegetation succession. However, there is little knowledge about soil carbon sequestration along the sequence of vegetation succession in the tidal flats.
Soil microbial community plays a key role in mediating ecosystem C and N cycling18. However, plant community structure, soil pH, moisture, organic carbon, temperature, and climatic and environmental factors may affect soil microbial community composition and biomass19. Specific soil bacterial assemblages were responsible for decomposition of individual soil organic compounds. However, there is little knowledge about soil microbial communities in different native vegetation types in coastal saline alkali soils, particularly regarding the effects of specific microbial communities on soil carbon sequestration.
This study was aimed at determining soil physicochemical and biological properties along a vegetation succession in the coastal tidal flats in Dafeng area, China. In particular, we characterized changes in SOC, MBC, DOC, soil enzyme activities and soil bacterial diversity as influenced by the process of vegetation succession.
Materials and Methods
Study area
The studied area is located at Dafeng Nature Reserve of Jiangsu Province (33°00.688′N, 120°50.755′E) with an area of 78,000 hectares, of which core area is 2,668 hectares, buffer zone is 2,220 hectares, and experimental area is 73,112 hectares. The nature reserve faces the Yellow Sea and became land about 50 years ago. It is a typical coastal wetland. The main wetland types include tidal flats, seasonal rivers, and partially constructed wetlands. There are a large number of woodlands, reeds, and bare land, which provide habitat for a large number of animals and plants. The area has the typical monsoon climate transitioning from the warm-temperate zone to the north subtropical zone, with 2,280 h of average annual sunshine, 14.4–15.5 °C average annual temperature, and 214 frost-free days. The annual precipitation ranges from 785–1310 mm (most occurring from late June to August). In this research, the spatio-temporal substitution method was used to monitor changes in plants and soils occurring along a vegetative chronosequence20. The typical vegetations in this area for characterizing succession were Jerusalem artichoke field (JF), Imperata cylindrica grassland (IG), Suaeda glauca land (SL) and bare saline alkali soil (BS) chosen as control. The BS, SL and IG areas were undisturbed. Jerusalem artichoke was planted with inter-row (60 cm) and intra-row distance between plants (40 cm) on the natural saline-alkaline land 8 years ago after mowing the grass meadows; there was no fertilizer and no irrigation used ever. Jerusalem artichoke tubers were never collected; instead, tubers left in the ground germinated and grew unaffected by humans. Three representative sample plots 5 × 5 m were selected as replicates in each vegetation type.
Sample collection and pretreatment
In April of 2014, soil samples were taken randomly at five points in an “S” pattern in each replicate plot using a soil auger (6-cm diameter) at two depths (0–10 and 10–20 cm). Soil samples were packed in individual sterile plastic bags, and transferred on ice to the laboratory. Soil samples were air-dried, passed through a 2-mm sieve, and stored at room temperature for measuring physicochemical properties (EC, pH, total N, total P, SOC). Soil bulk density was measured using the separately collected soil cores (100 cm3) by the volumetric ring method.
In July of 2014, soil samples were collected as described above and placed into DNA-free polythene bags. The samples were kept on dry ice for transport to the laboratory, and were then stored at −20 °C for subsequent DNA extraction, microbial biomass carbon (MBC) and dissolved organic carbon (DOC) analyses. The remaining part of each soil sample was air-dried, passed through a 2-mm sieve, and stored at room temperature for soil organic carbon (SOC), soil enzyme activity, total N, and total P analyses.
Analytical methods
Soil physicochemical properties
Electrical conductivity (EC) and soil pH were measured in 1:5 (soil:water) suspension after end-over-end shaking for 15 minutes at 25 °C. Total nitrogen (TN) was analyzed by Kjeldahl method21. Total phosphorus (TP) was extracted with HF-HNO3-HClO4 and then determined by molybdenum antimony blue colorimetry22.
Soil organ carbon (SOC), microbial biomass carbon (MBC) and dissolved organic carbon (DOC)
SOC was measured by dichromate oxidation23. Carbon (C) from the microbial biomass was extracted using the chloroform fumigation and extraction method24. For this, 12.5 g of soil (fresh weight basis) were subjected to 48-h fumigation with chloroform in a glass desiccator. Triplicate subsamples of fumigated and non-fumigated soils from each of the three field soil samples were shaken for 30 min at 200 strokes per minute with 0.5 M K2SO4 at a ratio of 5:1 (extractant to fresh soil weight) and filtered through medium-speed quantitative filter paper, then placed at −15 °C until measurements. Organic carbon concentration in the filtrate was measured by a Shimadzu TOC-5000A analyzer (Shimadzu Corp., Kyoto, Japan). MBC was calculated as the difference between fumigated and non-fumigated samples. DOC concentration in soil samples was determined by a TOC analyzer (Elementar Analysensysteme GmbH, Germany)25.
Soil enzymes
Urease activity (URE) was determined by measuring released NH4+-N from the soil amended with urea26. Catalase activity (CAT) determination was based on the volume of KMnO4 necessary for titration of unused H2O227. Alkaline phosphatase (ALP) activity was measured within 24 h of sampling according to the published method28. Invertase activity was assessed using colorimetric determination of reducing sugars that react with 3,5-dinitrosalicylic acid upon incubation of soil in buffered (0.17 M modified universal buffer, pH 5.5) sucrose solution and toluene at 37 °C for 24 h29.
DNA extraction
Soil DNA extraction was performed on three samples randomly collected in each field site in July. Total soil DNA was extracted from 0.35 g of soil (after it had passed through a 1-mm sieve) using the PowerSoil DNA Isolation Kit (MO BIO Laboratories Inc., Carlsbad, CA, USA) according to the instructions provided by the manufacturer. Genomic DNA concentration and purity were determined by NanoDrop spectrophotometry (Thermo Scientific, Wilmington, DE, USA). DNA samples were stored at −80 °C30.
PCR amplification of bacterial 16S rRNA genes
PCR amplification of the V3-V4 region of 16S rDNA was conducted using the universal primers, 577 F (5′-AYTGGGYDTAAAGNG-3′) and 926 R (5′-CCGTCAATTCMTTTRAGT-3′). Amplification reactions were performed in 25-μL volume containing 12.5 μL Premix Ex TaqTM Hot Start Version (Takara Biotechnology Co. Ltd, Dalian, China), 0.1 μM of each primer, and 20 ng of template. PCR was conducted under the following conditions: 98 °C for 3 s, 35 cycles of denaturation at 98 °C for 10 s, annealing at 54 °C for 30 s, extension at 72 °C for 45 s, and a final extension at 72 °C for 10 min, followed by cooling to 4 °C. Amplicon pyrosequencing was performed on an Illumina MiSeq platform at LC-Bio Technology Co., Ltd, Hangzhou, Zhejiang Province, China.
Illumina MiSeq sequencing of 16S rRNA genes
QIIME (Quantitative Insights Into Microbial Ecology) quality filters were used for filtering the reads. The CD-HIT pipeline was used to pick operational taxonomic units (OTUs) and make OTU table. The sequences were assigned to OTUs with similarity of 97%. The representative sequences were chosen for each OTU, and RDP (Ribosomal Database Project) classifier was used to assign taxonomic data to each representative sequence31. In order to estimate Alpha Diversity, the OTU table was rarified, and four metrics were calculated: Chao 1 index to estimate the richness, the Observed OTUs metric as the count of unique OTUs found in the sample, and Shannon and Simpson indices to estimate diversity32.
Statistical analysis
Soil samples in each vegetation ecosystem and all measurements were replicated thrice. The mean values of all parameters were taken from the three field replicates, and the standard error of the means was calculated. Statistical analyses were performed using Microsoft Excel 2010 and SPSS Statistics19.0 (IBM, Armonk, New York, USA). One-way Analysis of Variance (ANOVA) was performed to compare the mean values for the different field sites. Correlation analyses were done using the Pearson correlation method with significance defined at the 0.05 level unless otherwise stated. Individual means were compared using the least significant difference test at 5% significance level. Redundancy discrimination analysis (RDA) was used to detect the bacterial community distribution in relation to environmental explanatory variables using Vegan 2.3.0, a package of R functions for community ecology.
Results
Soil physicochemical properties
Soil properties at each site were presented in Table 1. There was a decline in soil pH from the bare soil to Suaeda glauca land and Jerusalem artichoke field at both soil depths. EC in both soil layers decreased with the succession of vegetation: BS > SL > IG > JF.
The content of TN as well as SOC in both soil layers increased along the vegetation sequence from BS to JF (Table 1). In contrast, soil bulk density at both soil depths decreased in the order: BS > SL > IG > JF.
Soil organic carbon (SOC), microbial biomass carbon (MBC) and dissolved organic carbon (DOC)
Dynamics of SOC content under different vegetation types in July was presented in Table 2. As expected, SOC increased along the vegetation sequence from BS to JF at both soil depths.
The mean values of MBC and DOC are presented in Table 3. At 0–10 cm soil depth, JF soil showed the highest MBC, followed by IG, SL and BS. A similar ranking was also obtained at 10–20 cm soil depth, except there was no difference between SL and BS. Regarding DOC, JF and IG showed higher values than SL and BS at both soil depths. The DOC content was higher in IG than JF at 0–10 cm soil depth, but reverse was true at 10–20 cm depth.
Soil enzyme activity
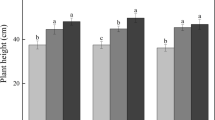
Soil enzyme activities tended to increase along vegetation succession (Fig. 1). At 10–20 cm depth, all four enzymes showed higher activities in JF and IG soils than SI and BS. A similar situation was recorded for the 0–10 cm depth as well, except that no difference between IG and SL was noted for urease and alkaline phosphatase activities.
The urease (A), catalase (B), invertase (C) and alkaline phosphatase (D) activities in soil at four different sites along vegetation succession in Dafeng coastal saline alkali land. The scale of the Y-axis differs in different graphs. Different letters in each graph at each soil depth denote significant differences among sites (P ≤ 0.05).
Alkaline phosphatase activity was correlated with urease (0.58, p ≤ 0.05), and catalase activity was correlated with invertase (0.79, p < 0.01) (Table 4). The SOC content had a significant positive correlation with urease (0.64, p < 0.01) and invertase activity (0.89, p < 0.01). In contrast, soil EC had a significant negative correlation with urease (−0.87, p < 0.01), alkaline phosphatase (−0.66, p < 0.01) and invertase activity (−0.82, p < 0.01).
Soil bacterial diversity and richness indices
We obtained a total of 9,831,155 valid reads through a sequence optimization process. A median sequence length of each read was 16.77 M based on the quality filtering. More than 10,000 reads were selected randomly from each sample to determine rarefaction curves, richness and diversity. Rarefaction curves determine the extent of species diversity through a slope. A total of 367,316 OTUs were identified at a 97% similarity cutoff point.
The rarefaction curves for BS and SL at 0–10 cm layer and BS at 10–20 cm did not tend toward a plateau, suggesting that the surveying effort did not cover the full extent of taxonomic diversity (Fig. 2a,b). The numbers of OTUs were ranked in the order: JF > IG > SL > BS at either soil depth (Fig. 2c,d).
(a,b) Calculated rarefaction curves of observed OTU richness at four different sites at soil depths 0–10 cm and 10–20 cm. (c,d) Venn diagram depicting operational taxonomic units (OTUs) detected in soil under different vegetation types at soil depths 0–10 cm and 10–20 cm. Numbers in parentheses indicate the total number of OTUs detected at each site.
Bacterial richness (Chao 1) and diversity (Shannon and Simpson) indices were shown in Table 5. The Chao 1 index was highest in JF and lowest in BS at 0–10 cm depth, whereas the ranking of the sites at 10–20 cm depth followed the order JF > IG = SL > BS. The Shannon index at 0–10 cm soil depth showed a decreasing (albeit non-significant) trend from JF to IG to SL, with these three being significantly higher than BS. At 10–20 cm, Shannon index was greater in JF than IG, whereas BS showed the significantly lower Shannon index than the other three sites. Simpson index of diversity showed no appreciable difference among the four sites at the two soil layers (Table 5).
Redundancy discrimination analysis (RDA) was used to analyze the diversity distribution of bacterial community at the four sites in response to soil environmental variables (e.g., TN, TP, SOC, pH, C/N, soil bulk density and EC) (Fig. 3). The first two axes accounted for 32.7% and 10.0% of variation. RDA analysis indicated that SOC and C/N were the most influential factors effecting diversity of bacterial community in soils of the four sites, explaining respectively 97% (P < 0.01) and 61% (P < 0.01) of the total variation.
Redundancy discrimination analysis (RDA) depicting the relationship between the main soil physicochemical parameters and diversity distribution of bacterial communities of the four soil sites. Symbols of different colour represent the soil sites/depth and arrows denote physicochemical parameters. The length of each arrow represents the strength of correlation between the environmental factors and community distribution. (A) 0–10 cm; (B) 10–20 cm depth.
OTUs and Chao 1, Shannon and Simpson diversity indices each had a significant negative correlation with EC (p < 0.01), but they were positively correlated with SOC, soil moisture, DOC and alkaline phosphatase activity (p < 0.01) (Table S1). MBC had positive correlation with Chao 1 index (0.72, p < 0.01). Urease activity had a positive correlation with OTUs, Shannon index and Simpson diversity. Invertase activity showed a positive correlation with OTUs and Chao 1 index (p < 0.01) (Table S1).
Composition and relative abundance of bacterial communities
The relative abundances of the phyla detected are presented in Fig. 4. Soil bacterial community composition included 16 phyla in both soil layers and all four sites. In decreasing order of abundance these 16 phyla were: Proteobacteria > Acidobacteria > Chloroflexi > Bacteroidetes > Gemmatimonadetes > Actinobacteria > Nitrospirae > Planctomycetes > Cyanobacteria > Fimicutes > WS3 > NC10 > Verrucomicrobia > SBR1093 > Deinococcus-thermus > Caldithrix > other unidentified phyla. The first eight phyla were the dominant bacterial communities, accounting for 52.8, 18.1, 6.7, 4.6, 4.4, 2.8, 2.3 and 1.4% of total bacteria, respectively. Other phyla each accounted for 0.3–0.7% of the total bacteria community (Fig. 4).
The distribution of each phylum varied among the four sites and with soil depth. Proteobacteria was the most abundant phylum in soils following the order SL = IG > JF > BS in the surface 0–10 cm. Compared with BS samples, those from SL, IG and JF had a significantly higher percentage of Acidobacteria (respectively, 2.8, 2.9 and 3.3 times in the topsoil 0–10 cm, and also 2.1, 2.6 and 2.7 times in the subsoil 10–20 cm). The relative abundance of Verrucomicrobia in the two soil layers followed the order: JF = IG > SL > BS. In the early stage of vegetation succession, the relative abundance of Actinobacteria and Planctomycetes was higher in SL than BS, but declined in IG. The relative abundance of Actinobateria was higher in JF than IG, but the opposite was true for Planctomycetes. In contrast, the relative abundances of Chloroflexi, Bacteroidetes, Fimicutes and Cyanobacteria in the two soil layers were significantly higher in BS than the other three sites (BS > SL > IG > JF).
The relative abundances of 16 phyla differed in the two soil layers. At all four sites, Verrucomicrobia and Deinococcus-thermus were more abundant in the topsoil than subsoil. In contrast, Chloroflexi, Gemmatimonadetes, Nitrospirae, Firmicutes, WS3 and NC10 were relatively more abundant in the subsoil than topsoil.
On a genus level, all 597 detected genera were found in all samples, except for Gp26, Ohtaekwangia, Saccharibacteria-genera-incertae-sedis, Streptophyta, Gemmatimonas, Ignavibacterium, Latescibacteria-genera-incertae-sedi, Phenylobacterium, Amaricoccus, Methylibium, Azoarcus, Candidatus-Entotheonella, Plesiocystis, Marinobacterium, Pseudomonas, Arenimonas, Lysobacter, Subdivision3-genera-incertae-sedis, and Steroidobacter that were not detected in BS, whereas Aliifodinibius, Gracilimonas, Salinibacter, Dehalogenimonas, GpIX-Truepera, Paenisporosarcina, NP25, Halanaerobium, Rhodoligotrophos, Marivita, Roseovarius, Novispirillum, Rhodovibrio, Desulfatiglans-Desulfococcus, Desulfosalsimonas, Haliea, Methylohalomonas, and Halomonas were detected only in BS. Gp1, Gp7, Acanthopleuribacter, Aciditerrimonas, Gaiella, Pontibacter, Sediminibacter, Flavitalea, Niastella, Terrimonas, Echinicola, Hyphomonas, Pedomicrobium, Mesorhizobium, Dongia, Piscinibacter, Limnohabitans, Rubrivivax, Geobacter, Kofleria, Pseudoxanthomonas, Opitutus, and WPS-2-genera-incertae-sedis were detected only in JF. Candidatus, Hydrogenedens, and Parcubacteria-genera-incertae-sedis were detected only in SL. Vadicella, Azospirillum and Thalassobaculum were detected only in IG.
Parcubacteria-genera-incertae-sedis, GpXIII, Parvularcula, Loktanella-Erythrobacter, Geoalkalibacter, Marinobacter, and Salinisphaera were detected in BS and SL. Fulvivirga, Hoeflea, Bauldia, Labrenzia. Desulfopila, Haliangium, Photobacterium, and Spartobacteria-genera-incertae-sedis were detected in SL and IG. Gp11, Flavobacterium, Devosia, Nitratireductor, Rhodobacter, Porphyrobacter, Kaistobacter-Novosphingobium, Sphingobium, Azohydromonas, Hydrogenophaga, Dechloromonas, and Thauera were detected in IG and JF.
The 27 genera with significant differences among sites and soil depths were listed in Table 6. GP10, GP15, GP5, GP9 and Geminicoccus were more abundant in the subsoil than topsoil at the four sites. In contrast, GP16 was more abundant in the topsoil than subsoil at the four sites. The abundance of GP6, GP16, Rhodoplanes, Steroidobacter and Sphingomonas in the topsoil (0–10 cm) followed the order: JF > IG > SL > BS.
Discussion
The soil properties are influenced by parent materials, topography, climate, and organisms. Soil moisture, depth, structure, fertility, pH, and salinity are the main factors that influence plant community distribution and species composition33. In the study reported here, the effects of vegetation succession in coastal saline-alkali land on soil physical and chemical properties were obvious. EC and soil bulk density decreased with the vegetation succession. In contrast, SOC and TN increased (Table 1). This indicated that vegetation succession could improve the physical and chemical properties of coastal saline-alkali soil.
SOC is the largest terrestrial organic carbon pool and plays a vital role in the global C cycle34. SOC is mainly derived from litter, root and plant residues and root exudates and is affected by species composition in the vegetation, land use and management35. Our study showed that SOC content was significantly lower in BS than in SL, IG and JF. The largest increase in SOC occurred between BS and SL, and smaller increases were recorded with the vegetation succession (Table 2), which was consistent with related studies36.
MBC represents an important living part of soil organic matter, and is involved in the processes influencing soil development37. Any changes in soil MBC have a significant impact on soil carbon, nitrogen, phosphorus, plant species and the dynamics of the terrestrial ecosystems38. It can be used as the early prediction index of soil quality and the change of soil total organic matter39. Our results showed that there was an increasing trend of soil MBC with the succession of vegetation community (Table 3). This may be explained by an increase in soil fertility and increased rhizodeposition through vegetation succession40.
DOC is composed of organic compounds mainly derived from root exudates, microbial biomass and the decomposition of plant litter and soil organic matter41. DOC is the most active form of soil organic carbon and plays an important role in the global carbon cycling42. In this study, DOC concentrations showed an increasing trend with the succession of vegetation communities (Table 3). DOC had positive correlation with OTUs and the Shannon, Simpson diversity and Chao 1 indices (Table S1), suggesting that the process of vegetation succession results in an increase in root exudates and other easily soluble carbon components, which enhances microbial growth and diversity.
Soil EC was significantly negatively correlated with SOC content, total P, urease and invertase activities, but positively correlated with soil bulk density (Table 4). These findings suggested that a decrease in soil salt content was associated with the succession of vegetation communities, resulting in an increase in SOC content and the improvement in soil fertility and soil structure.
Soil enzyme activity reflects the rate of soil nutrient cycling and utilization and may be an index of soil biodiversity, productivity and potential microbial activity43. Soil enzymes are linked with vegetation community variations44. In the present study, activities of soil urease, catalase, invertase and alkaline phosphatase at both soil depths were highest in JF, followed by IG and SL, with the lowest values measured in BS, suggesting vegetation succession enhanced soil nutrient transformation (Fig. 1). Soil EC was negatively correlated with activities of most soil enzyme in our study. In contrast, SOC was positively correlated with urease and invertase activity, whereas TP was positively correlated with catalase and invertase activity, suggesting that SOC and soil nutrient content had a substantial effect on soil enzyme activities. Alkaline phosphatase activity was correlated with urease activity, and that of catalase to invertase activity (Table 4), reflecting general inter-dependence of at least some soil enzymes in promoting the soil nutrient cycling.
Soil microbial communities play a central role by driving soil organic matter decomposition and nutrient cycling45. Bacteria are the most abundant and diverse group of microorganisms, playing an important role in maintaining soil health and ecological services46. The increases in the Chao 1 and Shannon indices were ranked generally in the order: JF > IG > SL > BS (Table 5), indicating that the richness and diversity of soil bacterial communities increased with the succession of vegetation in the coastal saline-alkali soil.
Diversity of microbial communities was controlled by biotic and abiotic factors47. Soil pH, SOC, C/N ratio, moisture, temperature, and soil types were the major determinants of the diversity of soil microbial community48. The RDA analysis showed that the diversity of soil bacteria in various stages of vegetation succession was related mainly to C/N and SOC (Fig. 3). The species richness (Chao 1) and diversity (Shannon and Simpson) indices had a significant positive correlation with SOC and DOC (p < 0.01) (Table S1), indicating that with the succession of plant communities (accompanied by an increase in SOC and DOC and a decrease in EC) soil became a more suitable environment for supporting abundant and diverse microbial communities to enhance the ecosystem stability in coastal saline-alkali land. However, other factors affecting distribution and abundance of bacteria cannot be excluded.
The 16S rRNA gene sequencing results indicated an increasing diversity of bacteria with vegetation community succession in coastal saline-alkali soil. Proteobacteria, Acidobacteria, Chloroflexi, Bacteroidetes, Gemmatimonadetes, Actinobacteria, Nitrospirae and Planctomycetes were the dominant bacterial phyla across the whole succession sequence. Carbon storage can be increased through N deposition, N2 fixation and N fertilizer supply to the ecosystem49. Proteobacteria are one of the largest phyla of soil bacteria and include many nitrogen-fixing bacteria50. JF had the highest percentage of Proteobacteria, followed by IG and SL, with the lowest in BS.
Correlation analysis among soil properties and microbial diversity parameters indicated that OTUs and the Shannon, Chao 1 and Simpson diversity indices had a negative correlation with EC, but a positive correlation with soil moisture and SOC (Table S1). These results indicated that vegetation succession of coastal saline-alkali soil associated with decreased soil salinity might have resulted in increased abundance and diversity of bacterial communities, including those bacterial phyla with relatively low resistance to high soil salinity.
The percentages of Deinococcus-thermus, Fimicutes and Cyanobacteria showed a decreased trend, whereas the percentages of Acidobacteria and Proteobacteria revealed an increasing trend in the process of vegetation succession (Fig. 4). Cyanobacteria are widely distributed in freshwater, marine and terrestrial ecosystems, even in the most extreme niches51,52. In the present study, compared with SL, IG and JF at 0–10 cm soil depth, BS had a significantly higher percentage of Cyanobacteria (3.3, 11.4 and 26.7 times, respectively), indicating relatively extreme and unfavorable conditions in highly saline soil at the BS (bare soil) site. Acidobacteria are ubiquitous and play an important role in soil ecological processes53. Related research showed that abundance of Acidobacteria displayed a more significant positive correlation with soil carbon content than with soil pH54. In our study, the percentages of Acidobacteria increased with the succession of vegetation communities (Fig. 4), mimicking the trends in SOC (Tables 1 and 2) and DOC (Table 3).
The relative abundances of phyla exhibited an inconsistent response to soil layers. Verrucomicrobia are ubiquitous in soil but less frequent bacterial phyla55. Relative abundance of Verrucomicrobia was highest in the subsurface soils due to their oligotrophic strategies, which was consistent with related research56. More research is required to determine whether this phylum has some specific relationships with soil carbon content.
Salinibacter is a genus of extremely halophilic red bacteria that are among the most salt-tolerant and salt-requiring strains57,58. Similarly, Paenisporosarcina is a well-known genus in saline and hypersaline environments and Desulfosalsimonas is isolated from the extreme hypersaline sediments59. These genera were found in the present study only in BS due to hypersaline conditions. Some uncultured organisms such as Candidatus were found in SL with the succession of vegetation. Azospirillum was only detected in IG, representing the best-characterized genus of plant growth-promoting rhizobacteria that was found in the rhizosphere of several grasses60. Aciditerrimonas, Pontibacter and Flavisolibacter that are obligate aerobic genera61,62,63 were detected only in JF. These findings indicated that the process of vegetation succession resulted in differences in soil microbial functional diversity that would have influenced ecosystem nutrient fluxes and soil organic matter quality to a large extent64.
Conclusions
Vegetation succession was associated with the changes in the soil physicochemical properties in coastal saline-alkali soil (eg. decreased soil EC and soil bulk density, and increased SOC and TN) (Fig. S1). Active components of SOC such as DOC and MBC showed an increasing trend with vegetation succession. The SOC and DOC had positive correlations with OTUs and the Shannon, Simpson diversity and Chao 1 indices, suggesting an environment that would enhance bacterial growth and diversity. Activities of soil urease, catalase, invertase and alkaline phosphatase generally increased with vegetation succession. The SOC, TP and soil EC had significant effects on soil enzyme activity. Soil enzyme activity correlated with the species richness and diversity indices, reflecting the level of soil microbial activity. The changes in bacteria richness and diversity were associated with vegetation types, soil moisture, EC, SOC and DOC. Vegetation community succession in coastal saline-alkali soil was associated with increased bacterial diversity. Bacteria such as Proteobacteria (that include many nitrogen-fixing bacteria) and Acidobacteria (with a significant positive correlation with soil carbon content) may have contributed to promoting the storage of soil organic carbon.
References
Kane, R. L. & Klein, D. E. Carbon sequestration: an option for mitigating global climate change. Chem Eng Prog. 6, 75–88 (2002).
Lal, R. Agricultural activities and the global carbon cycle. Nutr Cycl Agroecosys. 70, 103–116 (2004).
Nelson, D. W., Sommers, L. E. Total carbon, organic carbon, and organic matter. (eds Page, A. L. et al.) 539–579 (American Society of Agronomy, Madison, 1982).
Triberti, L., Nastri, A. & Baldoni, G. Long-term effects of crop rotation, manure and mineral fertilisation on carbon sequestration and soil fertility. Eur J Agron. 74, 47–55 (2016).
Ghassemi, F., Jakeman, A. J. & Nix, H. A. Salinisation of land and water resources: Human causes, extent, management and case studies. 554 (UNSW Press, Sydney, 1995).
Wang, J. L., Huang, X. J., Zhong, T. Y. & Chen, Z. Review on sustainable utilization of salt-affected land. Acta Geographica Sinica. 66, 673–684 (2011).
Lal, R. Sequestering carbon in soils of agro-ecosystems. Food Policy. 36, S33–S39 (2011).
Xu, M., Zhang, J., Liu, G. B. & Yamanaka, Z. Soil properties in natural grassland, Caragana korshinskii planted shrubland, and Robinia pseudoacacia planted forest in gullies on the hilly Loess Plateau, China. Catena. 119, 116–124 (2014).
Eswaran, H., Berg, E. V. D. & Reich, P. Organic carbon in soils of the world. Soil Sci Soc Am J. 57, 192–194 (1993).
Dou, X., He, P., Cheng, X. & Zhou, W. Long-term fertilization alters chemically-separated soil organic carbon pools: Based on stable C isotope analyses. Sci Rep. https://doi.org/10.1038/srep19061.
Deng, L., Wang, K. B., Chen, M. L., Shangguan, Z. P. & Sweeney, S. Soil organic carbon storage capacity positively related to forest succession on the Loess Plateau, China. Catena. 110, 1–7 (2013).
Dinakaran, J., Hanief, M., Meena, A. & Rao, K. S. The Chronological advancement of soil organic carbon sequestration research: a review. P Natl Acad. Sci USA 84, 487–504 (2014).
Jobbágy, E. G. & Jackson, R. B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10, 423–436 (2000).
Huang, Q., Chen, F. & Zhu, D. Sustainable development and coastal management of tidal flat in Jiangsu Province, China. Chinese Geogr Sci. 8, 33–43 (1998).
Sun, L., Zhu, Z. S., Liu, Y. & Zhang, J. L. Evaluation of service values of the intertidal land ecosystem of Dafeng City (in Chinese). Rural Eco-environment. 20, 10–14 (2004).
Sun, H. X. & Zhou, D. W. Effect of dietary supplement of seed of a halophyte (Suaeda glauca) on feed and water intake, diet digestibility, animal performance and serum biochemistry in lambs. Livestock Sci. 128(1–3), 133–139 (2010).
Liu, F. X., Zong, S. X. & Huang, Z. Y. A study on the succession of beach vegetation in Jiangsu Province (in Chinese). Journal of Plant Resources and Environment. 1, 13–17 (1992).
Kramer, T. D., Warren, R. J. II, Tang, Y. & Bradford, M. A. Grass invasions across a regional gradient are associated with declines in belowground carbon pools. Ecosystems. 15, 1271–1282 (2012).
Drenovsky, R. E., Vo, D., Graham, K. J. & Scow, K. M. Soil water content and organic carbon availability are major determinants of soil microbial community composition. Microb Ecol. 48, 424–430 (2004).
Zhang, J., Zhao, H., Zhang, T., Zhao, X. & Drake, S. Community succession along a chronosequence of vegetation restoration on sand dunes in Horqin Sandy Land. Jarin Environ. 62, 555–566 (2005).
Qiu, Q. et al. Effects of plant-derived dissolved organic matter (DOM) on soil CO2 and N2O emissions and soil carbon and nitrogen sequestrations. Appl Soil Ecol. 96, 122–130 (2015).
Zhong, W. et al. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil. 326, 511–522 (2010).
Walkley, A. & Black, I. A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38 (1934).
Vance, E. D., Brookes, P. C. & Jenkinson, D. S. An extraction method for measuring soil microbial biomass C. Soil Biol Biochem. 19, 703–707 (1987).
Mabuhay, J. A., Nakagoshi, N. & Isagi, Y. Soil microbial biomass, abundance, and diversity in a Japanese red pine forest: first year after fire. Forest Res-Jpn. 11, 165–173 (2006).
Vlek, P. L. G., Stumpe, J. M. & Byrnes, B. H. Urease activity and inhibition in flooded soil systems. Nutr Cycl Agroecosys. 1, 191–202 (1980).
Roberge, M. R. Methodology of soil enzymes measurement and extraction (Academic Press, London, UK, 1978).
Ma, D. W. et al. Alkaline phosphatase activity in ornithogenic soils in polar tundra. Adv Polar Sci. 22, 92–100 (2011).
Frankeberger, J. W. T. & Johanson, J. B. Method of measuring invertase activity in soils. Plant Soil. 74, 301–311 (1983).
Rodrigues, J. L. M., Pellizari, V. H. & Mueller, R. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. P Natl Acad USA 110, 988–993 (2013).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. P Natl Acad Sci USA 108, 4516–4522 (2011).
Kuffner, M. et al. Effects of season and experimental warming on the bacterial community in a temperate mountain forest soil assessed by 16S rRNA gene pyrosequencing. Fems Microbiol Ecol. 82, 551–562 (2012).
Leonard, S. G., Miles, R. L. & Tueller, P. T. Vegetation-Soil relationships on arid and semi-arid rangelands (ed. Tueller, P. T.) 226–251 (Kluwer Academic Publishers, Dordrecht, The Netherlands. 1988).
Zhang W. et al. Relative contribution of maize and external manure amendment to soil carbon sequestration in a long-term intensive maize cropping system. Sci Rep. https://doi.org/10.1038/srep10791.
You, Y. M. et al. Differential controls on soil carbon density and mineralization among contrasting forest types in a temperate forest ecosystem. Sci Rep. https://doi.org/10.1038/srep22411.
Shen, Z. J., Wang, Y. P., Sun, Q. Y. & Wang, W. Effect of vegetation succession on organic carbon, carbon of humus acids and dissolved organic carbon in soils of copper mine tailings sites. Pedosphere. 24, 271–279 (2014).
Velmourougane, K. et al. Mandal C. Microbial Biomass Carbon Status in Agro-Ecological Sub Regions of Black Soils in India. P Natl A Sci India B. 84, 519–529 (2014).
Roy, S. & Singh, J. S. Consequences of habitat heterogeneity for availability of nutrients in a dry tropical forest. J Ecol. 82, 503–509 (1994).
Dalal, R. C., Henderson, P. A. & Glasby, J. M. Organic matter and microbial biomass in a vertisol after 20 yr of zero-tillage. Soil Biol Biochem. 23, 435–441 (1991).
Bajgai, Y., Kristiansen, P., Hulugalle, N., McHenry, M. & McCorkell, B. Soil organic carbon and microbial biomass carbon under organic and conventional vegetable cropping systems in an Alfisol and a Vertisol. Nutr Cycl Agroeco. 101, 1–15 (2015).
Eckhardt, B. W. & Moore, T. R. Controls on dissolved organic carbon concentrations in streams, southern Quebec. Can Fish Aquat Sci. 47, 1537–1544 (1990).
Zhan, M. et al. Dynamics of methane emission, active soil organic carbon and their relationships in wetland integrated rice-duck systems in Southern China. Nutr Cycl in Agroecosys. 89, 1–13 (2011).
Williams, C. J. & Jochem, F. J. Ectoenzyme kinetics in Florida Bay: implications for bacterial carbon source and nutrient status. Hydrobiologia. 569, 113–127 (2006).
Caldwell, B. A., Griffiths, R. P. & Sollins, P. Soil enzyme response to vegetation disturbance in two lowland Costa Rican soils. Soil Biol Biochem. 31, 1603–1608 (1999).
Zhao, C. et al. Soil microbial community composition and respiration along an experimental precipitation gradient in a semiarid steppe. Sci Rep. https://doi.org/10.1038/srep24317.
Zhao, J. et al. Pyrosequencing reveals contrasting soil bacterial diversity and community structure of two main winter wheat cropping systems in China. Microb Ecol. 67, 443–453 (2014).
Hamidovic, S. et al. Seasonal Dynamic and Vertical Distribution of Microorganisms and Nutrients in Soils of Mostar Pit (Bosnia and Herzegovina). Agric. Conspec. Sci. 78, 107–111 (2013).
Mandic-Mulec, I. et al. Microbial Community Structure and Function in Peat Soil. Food Sci Biotechnol. 52, 180–187 (2014).
Soussana, J. F. & Hartwig, U. A. The effects of elevated CO2 on symbiotic N2 fixation: a link between the carbon and nitrogen cycles in grassland ecosystems. Plant Soil. 187, 321–332 (1995).
Spain, A. M., Krumholz, L. R. & Elshahed, M. S. Abundance, composition, diversity and novelty of soil Proteobacteria. Isme J. 3, 992–1000 (2009).
Stewart, I. & Falconer, I. R. Cyanobacteria and cyanobacterial toxins. (eds, Walsh, P. J. et al.) 271–296 (Academic Press, New York, 2008).
Griffiths, E. & Gupta, R. S. Identification of signature proteins that are distinctive of the Deinococcus-Thermus phylum. Int Microbiol. 10, 201–208 (2010).
Zhang, C., Liu, G., Xue, S. & Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biolo Biochem. 97, 40–49 (2016).
Liu, J. et al. Diversity and distribution patterns of acidobacterial communities in the black soil zone of northeast China. Biol Biochem. 95, 212–222 (2016).
Zhang, L. & Xu, Z. Assessing bacterial diversity in soil. J Soil Sediment. 8, 379–388 (2008).
Buckley, D. H. & Schmidt, T. M. Environmental factors influencing the distribution of rRNA from Verrucomicrobia in soil. FEMS Microbiology Ecology. 35, 105–112 (2001).
Bardavid, R. E. et al. Selective enrichment, isolation and molecular detection of Salinibacter and related extremely halophilic Bacteria from hypersaline environments. Hydrobiologia. 576, 3–13 (2007).
Oren, A. The genera Rhodothermus, Thermonema, Hymenobacter and Salinibacter (eds Dworkin, M. et al.) 712–738 (The Prokaryotes, Springer, New York, 2006).
Almeida, C. R., Alvarez, V. M., Marques, J. M., Jurelevicius, D. D. & Seidin, L. Exploiting the aerobic endospore-forming bacterial diversity in saline and hypersaline environments for biosurfactant production. BMC Microbiol. 15, 1 (2015).
Steenhoudt, O. & Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev. 24, 487–506 (2000).
Sun, W. et al. Diversity of the sediment microbial community in the Aha watershed (Southwest China) in response to acid mine drainage pollution gradients. Appl Environ Microb. 81, 4874–4884 (2015).
Joung, Y., Kim, H., Ahn, T. S. & Jon, K. Pontibacter salisaro sp. nov., isolated from a clay tablet solar saltern in Korea. J Microbiol. 49, 290–293 (2011).
Joo, E. S. et al. Flavisolibacter swuensis sp. nov. isolated from soil. J Microbiol. 53, 442–447 (2015).
Saetre, P. & Bååth, E. Spatial variation and patterns of soil microbial community structure in a mixed spruce-birch stand. Soil Biol Biochem. 32, 909–917 (2000).
Acknowledgements
The authors are grateful for the financial support of Jiangsu Agricultural Science and Technology Independent Innovation Fund Project [No. CX(15)1005], the National Key Research and Development Program of China (2016YFC0501207), the National Science and Technology Basic Work of China (2015FY110500), the National Key Project of Scientific and Technical Supporting Programs funded by Ministry of Science & Technology of Jiangsu Province (Nos. BE2017310-2, BY2015071-03, BY2016077-02 and BN2016145), and the Fundamental Research Funds for the Central University (KYZ201623, YZ2016-1 and KYYJ201703).
Author information
Authors and Affiliations
Contributions
N.L., X.L. and Z.L. designed the experiments. N.L. and T.S. performed the experiments. N.L., X.L. and T.S. analyzed the data. N.L., X.G., H.S. and Z.R. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, N., Shao, T., Zhu, T. et al. Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci Rep 8, 9728 (2018). https://doi.org/10.1038/s41598-018-28054-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28054-0
- Springer Nature Limited