Abstract
The main aim of the present investigation is modeling and optimization of a new culture medium for in vitro rooting of G×N15 rootstock using an artificial neural network-genetic algorithm (ANN-GA). Six experiments for assessing different media culture, various concentrations of Indole – 3- butyric acid, different concentrations of Thiamine and Fe-EDDHA were designed. The effects of five ionic macronutrients (NH4+, NO3−, Ca2+, K+ and Cl−) on five growth parameters [root number (RN), root length (RL), root percentage (R%), fresh (FW) and dry weight (DW)] were evaluated using the ANN-GA method. The R2 correlation values of 0.88, 0.88, 0.98, 0.94 and 0.87 between observed and predicted values were acquired for all five growth parameters, respectively. The ANN-GA results indicated that among the input variables, K+ (7.6) and NH4+ (4.4), K+ (7.7) and Ca2+ (2.8), K+ (36.7) and NH4+ (4.3), K+ (14.7) and NH4+ (4.4) and K+ (7.6) and NH4+ (4.3) had the highest values of variable sensitivity ratio (VSR) in the data set, for RN, RL, R%, FW and DW, respectively. ANN-GA optimized LS medium for G×N15 rooting contained optimized amounts of 1 mg L−1 IBA, 100, 150, or 200 mg L−1 Fe-EDDHA and 1.6 mg L−1 Thiamine. The efficiency of the optimized culture media was compared to other standard media for Prunus rooting and the results indicated that the optimized medium is more efficient than the others.
Similar content being viewed by others
Introduction
In recent decades, interspecies hybrids of the Prunus genus have been widely used as a rootstock in developing countries, which has solved major problems in stone fruit trees1. Garnem (G×N15) is one of its improved outcomes, which is a hybrid between the almond and peach [Prunus amygdalus (Garfi) × Prunus persica (Nemared)] that developed at the Center of Investigation and Technology Agrifood of Aragon in Spain2,3. Garnem is a strong, early bearing rootstock compatible with all types of soils with good drainage, tolerant to salinity, drought, waterlogging, iron chlorosis, nematodes, and soil-borne diseases which are suitable for both irrigated and non-irrigated areas2,4,5,6. In order to create mechanized orchards, its propagation via tissue culture is essential. However, rooting is a hard step in tissue culture propagation of many fruit trees such as Prunus rootstocks and varieties7.
Different physiological, biochemical, and genetic factors such as genotype/cultivars, medium composition, plant growth regulators, and also physical factors affect rooting8. Diverse culture media have different effects on rooting stage because of their different nutrient concentrations, so applying a specific medium depends on the plant species9. To improve the in vitro rooting in most plants, the macro elements have been reduced from the medium and also various vitamin combinations have been applied10,11. Exogenously added auxins such as indole-3-butyric acid (IBA), 1-naphthalene acetic acid (NAA), and indole-3-acetic acid (IAA), are able to induce adventitious roots. However, this requirement is only at early stages of the rooting process for promoting the adventitious roots12,13. In the next stages of root development, it can change or even inhibit the development of the rooting system14. Depending on the plant species, different kind of auxins might be effective on rooting11,15. The composition of root initiation medium such as hormonal content or the presence of active charcoal could influence the rooting process. When the medium contained active charcoal, the effect of NAA was reduced probably because of the adsorption of additional NAA by active charcoal11.
The hormonal composition of the last proliferation medium could influence the rooting ability of in vitro micro-shoots10. Although the high level of IBA can inhibit the rooting, but when a higher concentration of IBA (0.3 mg L−1) was applied in combination of different cytokinins in the last proliferation medium, it tended to enhance the root number independent of IBA concentration16. It has been expressed that phloroglucinol would increase the rooting due to its auxin activity and ½ MS medium supplemented with 0.2 mg L−1 IBA and 40 mg L−1 phloroglucinol have had the maximum rooting8. In a micropropagation study of Prunus domestica using ½ MS supplemented with 1 mg L−1 NAA, 0.1 mg L−1 GA3 and 20 g sucrose, 85% rooting was reported17. Also, different sources of iron have various effects on in vitro rooting of fruit trees. Substituting the iron source from Fe-EDDHA to FeCl3 improved the in vitro rooting of GF677 rootstocks, and increased the root fresh weight and dry weight18. In a study that investigated the effect of Fe-EDDHA and ascorbic acid on in vitro rooting of GF677 rootstock, the best rooting results were observed after four weeks of culture with 280 mg L−1 Fe-EDDHA. They also reported that ascorbic acid had no distinct stimulating effect on rooting19.
Although there are several biological processes that can be easily observed in plant tissue culture, none of them are linear and also would be affected by numerous other factors; therefore, the appropriate modeling can effectively predict the in vitro growth kinetics, and the plant biomass20,21. Conventional analytical techniques based on mathematical models are questionable for these purposes because these methods do not conform to the non-idealities of in vitro culture process22. Our previous report indicate that artificial neural network-genetic algorithm (ANN-GA) is better than traditional regression methods such as forward, backward or stepwise to predict and optimize new culture media23. Artificial neural networks (ANN) are inspired by the human brain functions24. Although ANN has shown significant progress in biological processes, they have rarely been used in complicated plant tissue culture systems. ANN based modeling methods are more flexible and useful in dealing with non-linear relationships existing in tissue culture practices. ANN doesn’t need any previous knowledge concerning the construction or interrelationships between input and output signals which is one of its benefits22. Other advantages of ANN are predicting the plant biomass25 and clustering the in vitro regenerated plantlets. Also, affecting the growth and quality of regenerated plants by controlling CO2, light, ventilation, and air temperature inside the culture vessels could be expressed as ANN advantages22.
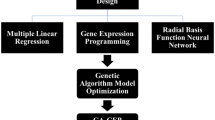
Understanding the relationship between the culture conditions and plant growth parameters is a foundation towards the development of high quality in vitro plantlets. In a modeling study for direct rooting and acclimatization of grape (Vitis vinifera L.) by neurofuzzy logic approach, the input data were three variables including types of auxin, auxin concentration and sucrose concentration, and the output data were root length and root number26. In several studies of micropropagation using bioreactors, input data such as pH, volume of growth medium, sucrose content, nitrate concentration, temperature, time of inoculation, size, fresh weight, and number of explant per flask in output of root weight and biomass using neural networks for modeling and optimization of rooting have been reported27,28,29. In our previous works, we found ANN-GA as a very precise and powerful modeling technique to optimizing nutrients for pear rootstocks30 and nutrients and hormonal composition for G×N 15 Prunus rootstock20,23. Recently, this technique has been also used successfully for predicting and optimizing the effect of various media components on Pistacia vera proliferation31. But there is lack of information concerning the effectiveness of this efficient method (ANN-GA) to find the complicated and non-linear relationships among important culture medium components and rooting. Establishing an efficient root structure on shoots grown in vitro is essential for massive commercial micropropagation purposes and plays a vital role in acclimatization of in vitro plantlets. As the most plantlet mortality occur during this phase and imposes large economical losses to the plant producers. So, we did this research distinctly on rooting to find the best composition of nutrients in general and in combination with thiamin vitamin and the chelated form of iron i.e. ethylene diamine di-o-hydroxyphenyl acetic acid (Fe-EDDHA) (6% Fe) as well as rooting hormone IBA which are well-known as effective factors in producing large number of high quality in vitro roots18. Furthermore, the present comprehensive study about effective factors on in vitro rooting using ANN-GA modeling and optimization method was followed with practical testing of the optimized medium which could confirm our theoretical results (Fig. 1).
Considering the above mentioned studies20,23,30,31 on proliferation stage of micropropagation process, the hypothesis of the present investigation is that different compositions of culture media supplemented with different concentrations of three important factors affecting on rooting including IBA, Thiamine and Fe-EDDHA determine the in vitro rooting efficiency. So, the ultimate goal of the current study is to develop a precise ANN-GA model for efficient and reproducible protocol by achieving an optimal nutrient culture medium supplemented with optimal concentrations of IBA, Thiamine and Fe-EDDHA concentrations.
Results
According to the analysis of variance for the first experiments, the interaction of culture medium and hormone significantly affects the rooting factors such as the root number (RN), root length (RL), root percentage (R%), and root fresh weight (FW) and dry weight (DW). The maximum RN with an average of 13.2 (the number of new roots per explant) in LS culture medium containing 1 mg L−1 IBA and the minimum RN in EM culture medium containing 0.5 mg L−1 of IBA was observed (Table 1). Maximum R% with an average of 80% in LS culture medium containing 1 mg L−1 IBA and the minimum R% in the MS culture medium containing 2 and 1.5 mg L−1 of IBA was observed. The highest FW with an average of 0.59 g was observed in LS culture medium containing 1 mg L−1 IBA (Table 1).
Results from the second experiment indicate that Thiamine and Fe-EDDHA and also their interactions significantly affect the RN, RL, R% and FW and DW. The maximum RN with an average of 14 (the number of new roots per explant) observed in 150 mg L−1 Fe-EDDHA and 1.6 mg L−1 Thiamine and the minimum RN with an average of 2.6 obtained in 200 mg L−1 Fe-EDDHA and 4 mg L−1 Thiamine. Also, the maximum and the minimum rooting with an average of 100% and 25% was obtained from 100 and 150 mg L−1 Fe-EDDHA in combination with the 1.6 mg L−1 Thiamine and 200 mg L−1 Fe-EDDHA in combination with 4 mg L−1 Thiamine, subsequently. Treatment containing 150 mg L−1 Fe-EDDHA combining with 1.6 mg L−1 Thiamine had the maximum fresh root weight with an average of 0.48 g (Table 2).
Modeling and evaluation
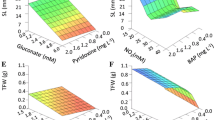
The plots of ANN model-predicted vs. the observed values of the RN, RL, R%, FW, and DW are shown in Figs 2–6. The fitted simple regression lines indicate good agreement between the observed and predicted values of the RN, RL, R%, FW, and DW for both the training and testing sets. Using high squared determination coefficients (R2) fitting method and based on the ANN models obtained, ten graphs were generated to show the variation with RN, RL, R%, FW and DW of NH4+, NO3−, Ca2+, K+, and Cl− (Figs 2–6). The graphs may be useful for understanding the complete nutrient-response relationship and to evaluate the combined effects of LS medium modified mineral nutrients. The goodness-of-fit statistical values derived from the ANN model to predict the RN, RL, R%, FW and DW are shown in Table 3. The ANN models could accurately (R2 > 0.88, 0.88, 0.98, 0.94 and 0.87) predict the RN, RL, R%, FW and DW of the testing data sets, which were not used during the training processes (Figs 2–6). Moreover, the trained ANN models of RN, RL, R%, FW and DW had balanced statistical values for the two subsets of training and testing (Table 3). Overall, statistical values (Table 3) revealed that the ANN-based models could efficiently fit data on the responses of G×N15 micro-shoots during in vitro rooting to LS medium with modified mineral nutrients.
Sensitivity analysis of the models
The comparative rank of input variables was determined using the entire 216 lines of data (training and testing) to calculate the overall VSR. The VSR obtained for the model output (RN, RL, R%, FW, and DW), with respect to modified mineral nutrients of LS medium are shown in Table 4. Analysis of RN indicated that the number of micro-shoots of G×N15 were more sensitive to K+ concentration, followed by NH4+, Ca2+, Cl−, and NO3− (Table 4). In RL model, the feed efficiency of G×N15 showed more sensitivity to K+ concentration, followed by Ca2+, NH4+, Cl−, and NO3− (Table 4). Analysis of R% indicated that the R% of G×N15 micro-shoots were more sensitive to K+ concentration, followed by NH4+, Cl−, Ca2+, and NO3− (Table 4). In FW, the feed efficiency of G×N15 micro-shoots showed more sensitivity to K+ concentration, followed by NH4+, Cl−, Ca2+, and NO3− (Table 4). Analysis of DW indicated that the DW of G×N15 micro-shoots were more sensitive to K+ concentration, followed by NH4+, Ca2+, Cl−, and NO3− (Table 4). This suggests that ion concentration (inputs) levels can significantly influence the performance of G×N15 micro-shoots rooting. However, the effect of K+, NH4+ and Ca2+ levels was more pronounced than that of Cl− and NO3−. Several researchers have suggested that the responses of Prunus rootstocks to K+, NH4+, Ca2+, Cl− and NO3− are different.
Model optimization
The final goal was to analyze the ANN models to address the question of what levels of elements NH4+, NO3−, K+, Ca2+, and Cl− may be used to achieve maximum R%. The purpose of this study, as in many other studies, was not to develop profit (economic) optimization, but to optimize nutrient requirements for the maximum R% of G×N15 micro-shoots. The results of optimization are summarized in Table 5. The process of optimization was conducted in the range of values from the data set (Table 7).
In conclusion, a platform of ANN-based models with sensitivity analysis and optimization algorithms was used successfully in this study to integrate published data on the responses of in vitro rooting of G×N15 rootstock to macro element nutrient concentration. Analyses of the ANN models for RN, RL, R%, FW and DW from a compiled data set suggested that the K+, NH4+ and Ca2+ concentrations were more important than the NO3−and Cl− concentrations. According to the preliminary results obtained by the ANN-GA, optimal productivity (R%) may be achieved with modified LS medium containing 42.9 mM of NO3−, 18.1 mM of NH4+, 10.3 mM of K+, 2.9 mM of Ca2+ and 1.2 mM of Cl−. Finally, modified LS medium was compared with other media, such as MS, EM, ½ MS and LS that are commonly applied for Prunus in vitro rooting (Table 10).
Validation experiment (assessment of optimum productivity of new media formulated)
When MS, EM, ½ MS, LS and modified LS were used, multifactorial analysis of variance showed that different types of media had significant effects (P < 0.001) on RN, RL, R%, FW and DW of G×N15 rootstock after four weeks in culture. The best root formation was obtained on modified LS medium. Modified LS medium including 1 mg L−1 IBA resulted in production of an average 15.4 new root per treated explant which is significantly higher than that produced by using the same concentration of IBA in other media. LS and modified LS media were the most productive media and, MS and EM media were the poorest performer for in vitro rooting of G×N15 rootstock. The highest average RL (8.10 cm) obtained by modified LS medium was also significantly higher than those with MS, EM, ½ MS and LS media. Modified LS medium supplemented with 1 mg L−1 IBA resulted in the highest R% with an average 95%, which was significantly higher than that produced by other media. The highest FW with an average 0.70 g per explant was obtained in modified LS medium supplemented with 1 mg L−1 IBA. Based on the above results, it can be inferred that modified LS medium was more efficient than other media.
The effects of Fe-EDDHA and Thiamine in modified LS medium optimized by ANN-GA on in-vitro rooting of G×N15 rootstock
Fe-EDDHA, Thiamine and also their interaction could significantly affect the RN, RL, R%, FW and DW in the modified LS medium. 150 mg L−1 Fe-EDDHA and 1.6 mg L−1 Thiamine produced the maximum RN with an average of 16 (the RN per explant) whereas, the minimum RN with an average of 3.4 observed in treatments with 200 mg L−1 Fe-EDDHA and 4 mg L−1 Thiamine (Table 7). Also, the maximum R% with an average of 100% observed in 100, 150, and 200 mg L−1 Fe-EDDHA along with 1.6 mg L−1 Thiamine and the minimum R% with an average of 25% belonged to 200 mg L−1 Fe-EDDHA combined with 4 mg L−1 thiamine (Table 7). Also, in the treatments containing 150 mg L−1 Fe-EDDHA and 2.8 mg L−1 Thiamine, the R% with an average of 100% was observed. Treatments containing 150 mg L−1 Fe-EDDHA and 1.6 mg L−1 thiamine had the maximum FW with an average of 0.57 g per each rooted micro-shoot.
Discussion
Micropropagation is a very complicated and time-consuming process which needs expensive chemicals to set a protocol for a plant. Specifically, fruit trees are harder to work with than herbal plants, so that producing tree plantlets such as Prunus rootstocks with high quality roots which could prevent plantlet losses during hardening phase is a very difficult step affected by different factors such as genotype, medium composition and plant growth regulators7,8. Various culture media have different effects at different stages of plant micropropagation because of their different nutrient concentrations and compositions. On the other hand, each plant species shows a different reaction to a special culture medium, so one has to optimize a unique medium for each unique plant species and each micropropagation phase. The importance of using ANN-GA mathematical modeling method as a very accurate procedure for optimizing culture media in plant tissue culture technique has been recently considered. Different media ingredients like nutrients, hormones and vitamins have been used as inputs of ANN for modeling and predicting their effects on proliferation stage of a few number of tree species like Pistacia vera31, pear rootstoks30 and G×N15 prunus rootstock20,23. All these researches found ANN-GA as a powerful tool in designing optimized proliferation culture media and predicting the proliferation indices by using different amounts of ingredients. Hence, in the present work, we tried to introduce a particular and optimized culture medium for rooting, as a fatal phase of plant in vitro regeneration, using ANN-GA which to our knowledge has not been previously done on any of the woody or herbal plants, although the neurofuzzy logic26 and ANN32 have been used for modeling Vitis vinifera in vitro rooting. Here, we practically tested the optimized medium in contrast to other media (Table 10), so, this work can be an important guide for future studies on in vitro rooting of other plant species such as hard-to-root ones.
The plants ability to produce lateral roots relies on the interaction of many external and internal factors such as hormone types and elements of culture media. Exogenously added auxins such as IBA, NAA, and IAA, are able to induce adventitious roots and are just needed in early stages of the rooting process for promoting the adventitious roots12,13. In the present investigation, the interaction between medium type and various hormones had different effects on rooting. The maximum R% was observed in LS medium containing 1 mg L−1 IBA condition. The results obtained here is in keeping with9 which mentioned that ½ MS culture medium is suitable for in vitro rooting of Prunus rootstock.
Analysis of rooting elements through ANN-GA indicates that potassium nitrate, ammonium nitrate, and calcium nitrate are the key components in the rooting medium. Also, a reduction in potassium nitrate to half in combination with the full amount of ammonium nitrate in MS and calcium nitrate in QL33 would significantly increase the rooting.
Sensitivity analysis indicated that potassium and ammonium have the maximum impact on rooting, respectively, and also indicated that ammonium nitrate has little effect at the rooting stage. The nutrient concentrations in the media would affect the rooting. Also, a number of researchers have suggested that reducing the media nutrients by half would improve the rooting34. The desirable effects of reducing the organic elements in rooting medium through nitrogen reduction are justified in literature35 which is in keeping with our findings. Nutrient reduction to half provides a satisfactory condition for root growth by reducing the vegetative growth, on the other hand, due to the reduced concentrations of nutrients, the osmotic pressure would decrease, and subsequently rooting would facilitate36. Our results are in contrast with those who expressed that the reduction of all macro elements by half in QL, MS and WPM37 would improve the rooting of the Prunus genus8. The results of the present study showed that reducing potassium nitrate and ammonium nitrate have positive effects on rooting (Tables 4 and 6). The important role of potassium and ammonium (Table 4) on rooting in this study might be due to the adjustment of pH and osmotic potential in the medium38,39, which is in agreement with the studies that expressed the pH as a regulating agent on absorbing the nutrients40. It can be inferred that potassium by adjusting the osmotic potential and ammonium by reducing the pH in the media would improve the rooting. Successful rooting is a crucial step for in vitro micropropagation of plants and could be a problem in some plant species micropropagation and also could limit the acclimatization of plantlets.
The results from optimizing Fe-EDDHA and Thiamine concentrations indicate that the maximum rooting obtained from modified and optimized LS medium and by changing the medium elements in all three concentrations of 100, 150 and 200 mg L−1 Fe-EDDHA along with 1.6 mg L−1 Thiamine, 100% rooting was obtained. On the other side, in a concentration of 150 mg L−1 Fe-EDDHA along with 2.8 mg L−1 thiamine also 100% rooting acquired. These results indicate that changing the medium elements would influence the efficiency of other medium components such as vitamins. The efficiency of ANN-GA to optimize the in vitro rooting and acclimatization of grape has been reported26,32 which confirms the competence of ANN-GA as a tool for optimizing in vitro rooting. Similar to our results, IBA has been suggested as a suitable hormone for in vitro rooting of Prunus rootstocks9. This is because that IBA is more stable and less sensitive to auxin degrading enzymes, and would slowly be metabolized by the peroxidase enzyme41,42.
In a rooting study on GF677 the concentration of 0.5 mg L−1 IBA caused the maximum rooting43. Inasmuch as the positive effect of dark has been proved44, the proliferated micro-shoots were kept in the dark for a week and then were transferred to the lighting conditions. Photoperiod is another factor that can affect the rooting process, the positive effects of low darkness periods on in vitro rooting have been reported45. Photoreceptors are the most important agents that cause the positive effect of dark on rooting. Phytochrome is a well-characterized plant photoreceptor, which controls the development of plants such as apical dominance and in vitro rooting9. In some species in vitro rooting only occurred in the presence of light. Similar to our findings in some other species the light plays as a rooting inhibitor and short-term maintenance in the dark would increase the rooting19,46. Dark environments inhibit the activity of phytochrome and in this condition the presence of auxins such as IBA could induce the cell division and subsequently increase the R%47. Removing light would protect auxin from degradation and reduce48 and reduces the activity of peroxidase which is an auxin degrading enzyme49.The positive effects of dark conditions in the present study could be derived from aforesaid reasons. According to our results, higher concentrations of IBA in rooting stage enhanced the callus formation which subsequently weakened or killed a high percentage of plants in the acclimatization stage. Callus formation at the end of the micro-shoots, impairs the vascular connection between roots and micro-shoots and prevents the absorption of water and nutrients.
In the present study the Fe-EDDHA and Thiamine compounds were used to increase the quality and R% and also to decrease the callus formation rate as well. Plantlets growth and survival during the acclimatization and reducing the losses at this stage is of great economic value. Literature indicates that, the Fe-EDTA chelate complex is not steady and causes the 45% loss of its initial Fe concentration at pH 5.819. Also, decrease in Fe availability, precipitation, and production of toxic compounds have been reported in the application of Fe-EDTA as an iron source18. Due to the positive effects of Fe-EDDHA and Thiamine on rooting reported from several studies, these two compounds were added to LS media for increasing the quality and quantity of rooting, which produced the maximum R%.
The positive effects of iron could be attributed to its vital roles in many metabolic pathways such as cytochromes, DNA biosynthesis, hormones, lipids, detoxification of reactive oxygen species (ROS), and nitrogen assimilation; which needs sufficient amount of iron50. Iron deficiency leads to morphological and physiological changes in root meristem especially the root tip meristems. Some of these changes include the increased cell division, root elongation inhibition, increased root diameter, and formation of hairy roots51. By replacing the Fe-EDDHA with Fe-EDTA the above problems can be overcome since this compound is more stable and is easily accessible for plant uptake. Thiamine has many positive effects on numerous physiological processes such as glycolysis, the pentose phosphate pathway, and the synthesis of nucleic acids. Also, in addition to its nutritional role thiamine has been found to act as secondary signaling messengers and have been reported to induce systemic acquired resistance in plants against infections caused by various pathogens52.
Conclusion
In conclusion, the results of the present study demonstrate that the type of culture media, Fe-EDDHA and Thiamine are effective factors for in vitro rooting of G×N15 rootstocks which have been optimized here step-by-step. The most commercial culture media for in vitro rooting of G×N15 obtained from the LS medium optimized by ANN-GA contained 1 mg L−1 IBA, 100, 150, or 200 mg L−1 Fe-EDDHA and 1.6 mg L−1 Thiamine which has been optimized through six experiments. Also, the results obtained here similar to those reported by23,30,31 indicate that the ANN-GA is an efficient method to formulate a new culture medium. Difference in nutritional requirements, Fe-EDDHA and Thiamine in G×N15 rootstock in comparison with the other varieties of the Prunus genus might be due to the different nutritional needs of various cultivars and rootstocks, internal hormone levels and physiological conditions of micro-shoots. Here, we optimized a special rooting culture medium by using ANN-GA method for the first time and increased the success of rooting G×N15 rootstock in vitro plantlets. The efficiency of this method for G×N15 was practically confirmed by achieving 100% rooting using the ANN-GA optimized medium. Further works are recommended to evaluate the ANN-GA method proficiency on micropropagation process of other plant species and on other components of culture medium like micro nutrients to increase the plant tissue culture technique commercial revenue.
Methods
An overview of the various steps used in the present study to achieve an optimized in vitro protocol for rooting of G×N15 prunus rootstock has been shown in Fig. 1.
In vitro culture establishment
After disinfection, the explants were cultured on a medium for shoot induction, consisting MS medium53, 30 g L−1 sucrose, 0.25 mg L−1 BAP, 0.05 mg L−1 IBA, and 7 g L−1 agar. The pH of applied media was adjusted to 5.8 with 0.1 M NaOH before autoclaving at 121 °C under a pressure of 1.2 kg cm−2 for 15 min. The explants were maintained in a growth room with light intensity of 35–40 umol m−1 s−1, 25 ± 1 °C and 16/8 h photoperiod for four weeks. Shoots that originated from the explants were subcultured after 30 days. The new micro-shoot was transferred to Yadollahi, Arab and Shojaeiyan (YAS)20 medium supplemented with 1 mg L−1 BAP and 0.1 mg L−1 IBA. Before direct transferring the micro-shoots to the experimental media, proliferated explants pre-subcultured four times on free hormone YAS medium for 15–20 days in order to sterility screening and fifth subculture explants were used for rooting experiments. In all rooting experiments, in order to achieve the best medium and hormonal composition of in vitro rooting of G×N15 rootstocks, after culturing on different media, the explants (elongated micro-shoots) were kept in the dark for the first week of culture and then transferred to 8 hours of light and 16 hours of darkness condition.
Media preparation for in vitro rooting
Different culture media were applied: MS53, EM9 (Specific media), LS54 (Table 8),½MS modified LS predicted-optimized according to ANN-GA, and modified LS (second experiment) (Tables 6 and 9). The media were supplemented with 20 g L−1 sucrose and adjusted to pH 5.7–5.8 before the addition of the gelling agent (7.0 g L−1 \({\rm{agar}}\)). After autoclaving (121 °C, 1.2 kg cm−2, 30 min), the media were cooled to 65 °C in a water bath, and then the medium was distributed into glass baby food jars (250 ml) each containing 50 ml of medium.
Interaction of culture media and IBA concentrations on rooting
For the first experiment, 20 media (Table 1), differing in nutrient formulation (MS, EM, ½ MS and LS) and various concentrations of IBA as an auxin resource were evaluated. The IBA concentrations were variable, ranging from 0–2 mg L−1.
Interaction of Fe-EDDHA and thiamin vitamin on rooting
For the second experiment, 12 media (Table 2), micro-shoots were cultured in LS medium supplemented with different concentrations of thiamine (0.4, 1.6, 2.8 and 4 mg L−1) and Fe-EDDHA at various concentrations (100, 150 and 200 mg L−1). All media were supplemented with 1 mg L−1 IBA as auxin source.
Modeling and optimization of the obtained culture medium nutrients composition using ANN-GA
Preparation of LS culture media with modified macro elements concentrations
To investigate the effects of macro elements, Potassium Nitrate (KNO3), Ammonium Nitrate (NH4NO3), Calcium Nitrate (Ca(NO3)2) and Calcium Chloride (CaCl2), on optimizing the rooting of G×N15 rootstocks, 36 culture media were used (Tables 6 and 9). In order to prepare the different macro elements culture media, the LS culture medium was changed as follows: In this experiment the values of 1, 0.75 and 0.5 fold of KNO3 (950, 1425, 1900 mg L−1), values of 1, 0.75 and 0.5 fold of NH4NO3 (825, 1238, 1650 mg L−1) and values of 1 and 0.5 fold of Ca(NO3)2 (278 and 556 mg L−1) or values of 1 and 0.5 fold of CaCl2 (220 and 440 mg L−1) were combined with each other as factorial experiments based on a completely randomized design with 6 replicates each including four explants. Micro elements and vitamins used in all culture media were the same of LS medium and the amount of 1 mg L−1 IBA hormone was used in all culture media as well.
ANN-GA modeling
Developing ANN models
Five data matrices including 36 mineral composition treatments with different concentrations of ions NO3−, NH4+, K+, Ca2+ and Cl− (Table 6) were developed for each of the five rooting measured parameters including RN, RL, R%, FW and DW to perform ANN modeling.
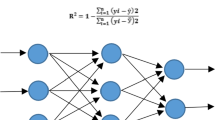
Developing an ANN is according to the operations summation in each neuron that constitute the system. A vector X i = (X1, X2, … Xn) is used for entering the information into the system. A mathematical function is used for processing the information of the input vector which transfers them to the first intermediate layer. The commitment of propagation function is adding all the input data and generating one response (Equation 1). where, N is the number of neurons in the first ANN layer, denominated input layer, w ni is the weight (indicating the importance of the connection) between neurons of the input layer (n) and neurons of the middle layer (i), and b is the biases related to the neurons in the middle layer (Equation 1).
The obtained values by the propagation function are applied by another mathematical function, named activation function (Equation 2), to make an output value (y) as a function of the internal state55 and is more than a threshold value33. Different activation functions can be used but in this work, Tansig (hyperbolic tangent sigmoid) (Equation 2) and purelin (linear) functions were applied as the transfer functions for hidden and output layers, respectively.
All entered information in the ANN are propagated to the output layer, where an output value is made (y0) which is compared with the observed value (d0), and the mean squared error (MSE) produced by the ANN (Eq. 3) can be estimated.
To train the network, a Levenberg-Marquardt algorithm for back-propagation with a gradient descent and momentum weight and bias learning function was applied56. The MSE leveling 0.01 was applied as performance function and training was ended after 800 epochs or iterations of the network. Different levels of five input variables including NO3−, NH4+, Ca2+, K+ and Cl- were applied as units in the input layer of the ANN model. Five models were developed separately for RN, RL, R%, FW and DW with the topology of 5–8–1. For training and testing of the network, 130 and 86 data lines were used. The data lines in train and test groups were randomly chosen before establishing the training model. Prior to training, the input and output data were normalized in the range of −1 to 1 to simplify the problem for the network, to reach fast convergence minimum mean square error, and to guarantee that the fall of targets (output data) into the certain range of the new feed forward network could be reconstructed56,57,58. The fitness of the ANN-model was assessed by R2, root mean square error (RMSE) and mean bias error (MBE) as follows:
where n is the number of data, O is the value of actual datasets, and M is value of predicted datasets. The ranges are 0 ≤ R2 ≤ 1, 0 ≤ RMSE ≤ + ∞ and −1 ≤ MBE ≤ +1. R2 values closer to 1 and RMSE values closer to 0 indicate better fit. The MBE value represents a positive or negative calculation error which indicate that the predicted values are more or less than observational values.
Optimization of input variables for the developed ANN models using GA
To determine the optimal values of input variables (KNO3, NH4NO3, Ca(NO3)2, and CaCl2) and maximize the RN, RL, R%, FW and DW, the prepared ANN models were exposed to a further process using GA, after the training process. Accordingly, the ANN models were applied as the fitness function for GA (Figs 7 and 8). For selecting the elite populations for crossover a roulette wheel selection method was applied. To attain the best fitness, the initial population of 50, generation number of 500, mutation rate of 0.1, and crossover rate of 0.85 were set59. This generational process was repeated until the number of generations was reached. To identify the important input variable in the model, the constructed ANN models were subjected to the sensitivity analysis. This analysis indicates which KNO3, NH4NO3, Ca(NO3)2, and CaCl2 concentration is more important to acquire the optimal number of root, root length, root percentage, and fresh and DW in G×N 15 rootstock micro-shoots.
Schematic diagram of an artificial neural network (ANN) model with 5 input neurons, 8 neurons in the intermediate layer and one output neuron (including one of RN: root number, RL: root length, R%: root percentage, root fresh weight: FW or root dry weight: DW) with the topology of 5–8–1. The connections between nodes are related to weights and biases.
Schematic diagram showing the relationship between ANN and GA to achieve an optimized model (adopted from20).
Sensitivity analyses
The sensitivity of number of root, root length, root percentage, and fresh and DW against the investigating media nutrients was determined using the criteria60,61 as follows:
-
The variable sensitivity error (VSE) value: demonstrates the performance of the developed ANN model if that variable is unavailable,
-
The value of variable sensitivity ratio (VSR): is the relative ratio between the VSE and the error of the ANN model when all variables are available. A variable which is more important has a higher VSR value. Therefore, according to the obtained VSR value, the input variables may be ranked in the order of importance.
Validation experiments
In this experiment different culture media were employed: MS medium53, ½ MS, EM (Specific media), LS, and modified LS medium containing predicted-optimized mineral nutrient based on ANN-GA. The media were supplemented with 1 mg/ l IBA, 20 g /l sucrose, 100 mg L−1 myo-inositol (Sigma). The pH of all media was adjusted to pH 5.7–5.8 prior to the addition of a gelling agent (7.0 g L−1 agar).
Optimizing thiamin and Fe-EDDHA for the ANN-GA optimized LS medium
In this experiment, the micro-shoots were cultured in modified LS medium supplemented with different concentrations of thiamine (0.4, 1.6, 2.8 and 4 mg L−1) and Fe-EDDHA at various concentrations (100, 150 and 200 mg L−1). All media were supplemented with 1 mg L−1 IBA as Auxin resource.
Experiments design and data collection
All experiments were conducted by a factorial experiment based on a completely randomized design with five (first, fourth, fifth and sixth experiment) to six (second and third experiment) replicates and each replication included 4 explants. At the end of in vitro rooting stage, five parameters (outputs) were recorded to analyze the effects of the variables (inputs) on rooting: (1) total root produced (number of new roots per explant: 1, 2, 3, …); (2) Length of roots; (3) R%; (4) FW and (5) DW. Commercially available software, Matlab® R2010a21, was used to write the mathematical codes for developing and evaluating the ANN model. The developed program is actually a modified source code of an ANN algorithm which was previously applied by Ahmadi and Golian57. In the sixth experiment to evaluate the ANN-GA efficiency in the prediction and optimization of the new medium, MS, ½ MS, EM and LS basal salts and new formulated medium (Modified LS) were compared. Sixth experiment data were subjected to a one-way analysis of variance. Statistical significance was determined by analysis of variance and significance (P ≤ 0.05) differences between mean values were estimated using LSD test. SAS version 9.1 was used for statistical analyses and a value of P < 0.05 was considered significant.
The mathematical code for developing and evaluating the ANN-GA model was written by Matlab R2010a21 software. In fact, the developed program is a modified source code of an ANN algorithm which was previously applied by57.
References
Arab, M. M., Yadollahi, A., Shojaeiyan, A., Shokri, S. & Maleki Ghojah, S. Effects of nutrient media, different cytokinin types and their concentrations on in vitro multiplication of G×N15 (hybrid of almond×peach) vegetative rootstock. J. Gen. Eng. Biotechnol. 12, 81–87, https://doi.org/10.1016/j.jgeb.2014.10.001 (2014b).
Felipe, A. J. ‘Felinem’,‘Garnem’, and ‘Monegro’almond×each hybrid rootstocks. HortScience. 44, 196–197 (2009).
Arab, M. M., Yadollahi, A., Hosseini-Mazinani, M. & Bagheri, S. Effects of antimicrobial activity of silver nanoparticles on in vitro establishment of G×N15 (hybrid of almond×peach rootstock). J. Gen. Eng. Biotechnol. 12, 103–11 (2014a).
De la Guardia, M. D., Felipe, A. J., Alcántara, E., Fournier, J. M. & Romera, F. J. “Evaluation of experimental peach rootstocks grown in nutrientsolutions for tolerance to iron stress,” in Iron Nutrition in Soils and Plants, ed. J. Abadía (Dordrecht: Kluwer Academic Publishers), 201–205 (1995).
Pinochet, J. et al. Resistance of peach and plum rootstocks from Spain, France, and Italy to root-knot nematode Meloidogyne javanica. HortScience. 34, 1259–1262 (1999).
Beckman, T. & Lang, G. Rootstock breeding for stone fruits, XXVI International Horticultural Congress: Genetics and Breeding of Tree. Fruits and Nuts 622, 531–551 (2002). pp.
Custódio, L., Martins-Loucao, M. & Romano, A. Influence of sugars on in vitro rooting and acclimatization of carob tree. Biol. Plant. 48, 469–472 (2004).
Ying-Ning, Z. Micropropagation of Chinese plum (Prunus salicina Lind l.) using mature stem segments. Not Bot Horti Agrobot Cluj Napoca. 38, 214 (2010).
Sadeghi, F., Yadollahi, A., Kermani, M. J. & Eftekhari, M. Optimizing culture media for in vitro proliferation and rooting of Tetra (Prunus empyrean 3) rootstock. J. Gen. Eng. Biotechnol. 13, 19–23 (2015).
Dobránszki, J. et al. Influence of aromatic cytokinins on shoot multiplication and their after-effects on rooting of apple cv. Húsvéti rozmaring. Int. J. Hortic. Sci. 6, 84–87 (2000).
Magyar-Tábori, K. et al. Post-effects of cytokinins and auxin levels of proliferation media on rooting ability of in vitro apple shoots (Malus domestica Borkh.)‘Red Fuji’. Int. J Hortic Sci. 7, 26–29 (2001).
Dobránszki, J. & Teixeira da Silva, J. A. Micropropagation of apple—a review. Biotechnol. Adv. 28, 462–488, https://doi.org/10.1016/j.biotechadv.2010.02.008 (2010).
Harbage, J. F. & Stimart, D. P. Effect of pH and 1H-indole-3-butyric acid (IBA) on rooting of apple microcuttings. J. Am. Soc. 121, 1049–1053 (1996).
de Klerk, G. J., van der Krieken, W. & de Jong, J. C. Review the formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev Biol Plant. 35, 189–199 (1999).
Sharma, M., Modgil, M. & Sharma, D. Successful propagation in vitro of apple rootstock MM106 and influence of phloroglucinol. Indian J Exp Biol. 38, 1236–1240 (2000).
Ricci, A. et al. Cytokinin-like activity of N, N′-diphenylureas. N, N′-bis-(2, 3-methylenedioxyphenyl) urea and N, N′-bis-(3, 4-methylenedioxyphenyl) urea enhance adventitious root formation in apple rootstock M26 (Malus pumila Mill. Plant Science. 160, 1055–1065 (2001).
Ruzic, D. & Vujovic, T. The Q18 protocol for rapid propagation of plum (Prunus domestica L.) by in vitro micropropagation. Vocarstvo 41 (2007).
Molassiotis, A., Dimassi, K., Therios, I. & Diamantidis, G. Fe-EDDHA promotes rooting of rootstock GF-677 (Prunus amygdalus×P. persica) explants in vitro. Biol. Plant. 47, 141–144 (2003).
Antonopoulou, C., Dimassi, K., Therios, I., Chatzissavvidis, C. & Papadakis, I. The effect of Fe-EDDHA and of ascorbic acid on in vitro rooting of the peach rootstock GF-677 explants. Acta Physiol Plant. 29, 559–56 (2007).
Arab, M. M., Yadollahi, A., Shojaeiyan, A. & Ahmadi, H. Artificial neural network genetic algorithm as powerful tool to predict and optimize in vitro proliferation mineral medium for G×N15 rootstock. Front. Plant Sci. 7, 1526, https://doi.org/10.3389/fpls.2016.01526 (2016).
Matlab. Matlab R. Version 7.1. The Math Works Inc., Natick, MA. 2010.
Prasad, V. & Gupta, S.D. Applications and potentials of artificial neural networks in plant tissue culture, Plan Tissue Culture Engineering. Springer, pp. 47-67 (2008).
Arab, M.M., Yadollahi, A., Ahmadi, H., Eftekhari, M. & Maleki, M. Mathematical modeling and optimizing of in vitro hormonal combination for G×N15 vegetative rootstock proliferation using Artificial Neural Network-Genetic Algorithm (ANN-GA). Front. Plant Sci. 8 (2017).
Kosiński, W., Prokopowicz, P. & Ślȩzak, D. Fuzzy Reals with Algebraic Operations: Algorithmic Approach, Intelligent Information Systems. Springer, pp. 311-320 (2002).
Albiol, J., Campmajo, C., Casas, C. & Poch, M. Biomass estimation in plant cell cultures: a neural network approach. Biotechnol Prog. 11, 88–92 (1995).
Gago, J., Landín, M. & Gallego, P. P. A neurofuzzy logic approach for modeling plant processes: A practical case of in vitro direct rooting and acclimatization of Vitis vinifera L. Plant sci. 179, 241–9, https://doi.org/10.1016/j.plantsci.2010.05.009 (2010).
Mehrotra, S., Prakash, O., Mishra, B. & Dwevedi, B. Efficiency of neural networks for prediction of in vitro culture conditions and inoculum properties for optimum productivity. Plant Cell Tissue Organ Cult. 95, 29–35 (2008).
Prakash, O., Mehrotra, S., Krishna, A. & Mishra, B. N. A neural network approach for the prediction of in vitro culture parameters for maximum biomass yields in hairy root cultures. J. Theor. Biol. 265, 579–585 (2010).
Mehrotra, S., Prakash, O., Khan, F. & Kukreja, A. Efficiency of neural network-based combinatorial model predicting optimal culture conditions for maximum biomass yields in hairy root cultures. Plant Cell Rep. 32, 309–317 (2013).
Jamshidi, S., Yadollahi, A., Ahmadi, H., Arab, M. M. & Eftekhari, M. Predicting in vitro culture medium macro-nutrients composition for pear rootstocks using regression analysis and neural network models. Front. Plant Sci. 7, 274, https://doi.org/10.3389/fpls.2016.00274 (2016).
Nezami-Alanagh, E., Garoosi, G. A., Maleki, S., Landín, M. & Gallego, P. P. Predicting optimal in vitro culture medium for Pistacia vera micropropagation using neural networks models. Plant Cell Tissue Organ Cult. 129(1), 19–33 (2017).
Gago, J., Landín, M. & Gallego, P. P. Artificial neural networks modeling the in vitro rhizogenesis and acclimatization of Vitis vinifera L. J. Plant Physiol. 167, 1226–1231, https://doi.org/10.1016/j.jplph.2010.04.008 (2010a).
Kleinfeld, D. Sequential state generation by model neural networks. Proceedings of the National Academy of Sciences of the United States of America 83, 9469–9473 (1986).
Dimassi-Theriou, K. In vitro rooting of rootstock GF-677 (Prunus amygdalus×P. persica) as influenced by mineral concentration of the nutrient medium and type of culture-tube sealing material. J. Hortic. Sci. 70, 105–108 (1995).
Driver, J. & Suttle, G. Nursery handling of propagules, Cell and tissue culture in forestry. Springer, pp. 320-335 (1987).
Marino, G. & Bertazza, G. Micropropagation of Actinidia deliciosa cvs.‘Hayward’and ‘Tomuri’. Sci Hort. 45, 65–74 (1990).
Quorin, M. & Lepoivre, P. Improved media for in vitro culture of Prunus spp. ActaHortic. 78(437), 442 (1977).
Strange, K. Cellular and molecular physiology of cell volume regulation. CRC Press (1993).
Handa, S., Bressan, R. A., Handa, A. K., Carpita, N. C. & Hasegawa, P. M. Solutes contributing to osmotic adjustment in cultured plant cells adapted to water stress. Plant Physiol. 73(3), 834–843 (1983).
Gürel, S. & Gülşen, Y. The effects of different sucrose, agar and pH levels on in vitro shoot production of almond (Amygdalus communis L.). Turk J Bot. 22, 363–374 (1998).
Nag, S., Saha, K. & Choudhuri, M. Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. J Plant Growth Regul. 20, 182–194 (2001).
Ludwig-Müller, J. Indole-3-butyric acid in plant growth and development. Plant Growth Regul. 32, 219–230 (2000).
Vaez-Livari, B. & Salehi-Soghadi, Z. In vitro rooting of hybrid GF677 (prunus dulcis × Prunus persica), IV International Symposium on Pistachios and Almonds 726, pp. 171-178 (2005).
Berardi, G., Neri, D., Maiorino, A. & Adversi, R. In vitro rooting of Pyrus calleryana. In Vitro. Culture, XXIII IHC 300, 181–188 (1990).
Antonopoulou, C., Dimassi, K., Therios, I. & Chatzissavvidis, C. The influence of radiation quality on the in vitro rooting and nutrient concentrations of peach rootstock. Biol. Plant. 48, 549–553 (2004).
Channuntapipat, C., Sedgley, M. & Collins, G. Micropropagation of almond cultivars Nonpareil and Ne Plus Ultra and the hybrid rootstock Titan×Nemaguard. Sci Hort. 98, 473–484 (2003).
Mencuccini, M. Effect of medium darkening on in vitro rooting capability and rooting seasonality of olive (Olea europaea L.) cultivars. Sci Hort. 97, 129–139 (2003).
Bassuk, N. & Maynard, B. Stock plant etiolation. HortScience (USA) (1987).
Druart, P., Kevers, C., Boxus, P. & Gaspar, T. In vitro promotion of root formation by apple shoots through darkness effect on endogenous phenols and peroxidases. Zeitschrift für Pflanzenphysiologie. 108, 429–436 (1982).
Curie, C. et al. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 103, 1–11 (2009).
Römheld, V. & Marschner, H. Iron deficiency stress induced morphological and physiological changes in root tips of sunflower. Physiol. Plant. 53, 354–360 (1981).
Ahn, I. P., Kim, S. & Lee, Y. H. Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol. 138, 1505–1515 (2005).
Murashige, T. & Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 15, 473–497, https://doi.org/10.1111/j.1399-3054.1962.tb08052.x (1962).
Linsmaier, E. M. & Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant. 18, 100–127 (1965).
Chuang, Y. H., Bell, N. R. & Stacy, R. W. An automaton analysis approach to the study of neural nets. Comput. Biomed. Res. 1, 173–186 (1967).
Demuth, H., Beale, M. & M. Hagan. Neural network toolbox™ 6. User Guide, COPYRIGHT. 1992. (2008).
Ahmadi, H. & Golian, A. Response surface and neural network models for performance of broiler chicks fed diets varying in digestible protein and critical amino acids from 11 to 17 days of age. Poult. Sci. 90, 2085–2096, https://doi.org/10.3382/ps.2011-01367 (2011).
Gulati, T., Chakrabarti, M., Sing, A., Duvuuri, M. & Banerjee, R. Comparative study of response surface methodology, artificial neural network and genetic algorithms for optimization of soybean hydration. Food Technol. Biotechnol. 48, 11–18 (2010).
Haupt, R.L. & Haupt, S.E. Practical genetic algorithms. John Wiley & Sons 30. (2004).
Ahmadi, H. & Golian, A. Growth analysis of chickens fed diets varying in the percentage of metabolizable energy provided by protein, fat, and carbohydrate through artificial neural network. Poult. Sci. 89, 173–179, https://doi.org/10.3382/ps.2009-00125 (2010a).
Ahmadi, H. & Golian, A. The integration of broiler chicken threonine responses data into neural network models. Poult. Sci. 89, 2535–2541, https://doi.org/10.3382/ps.2010-00884 (2010b).
Author information
Authors and Affiliations
Contributions
M.M.A. Designing and performing the experiments, summing up, and writing. A.Y. Designing and leading. M.E. interpreting experiments and revising the manuscript. H.A. Statistical analyzing and writing. M.A. Designing and drafting the manuscript. S.S.K. performing the experiments and revising manuscript. All authors listed, have made substantial, direct and intellectual contribution to the work, and approved it for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arab, M.M., Yadollahi, A., Eftekhari, M. et al. Modeling and Optimizing a New Culture Medium for In Vitro Rooting of G×N15 Prunus Rootstock using Artificial Neural Network-Genetic Algorithm. Sci Rep 8, 9977 (2018). https://doi.org/10.1038/s41598-018-27858-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27858-4
- Springer Nature Limited
This article is cited by
-
Optimized medium composition in Physalis alkekengi callus culture altered nitric oxide level for inducing antioxidant enzyme activities and secondary metabolites
Scientific Reports (2024)
-
Identification of Drought-Tolerant Tomato Genotypes Using Multi-trait Index at Early Growth Stage
Journal of Soil Science and Plant Nutrition (2024)
-
Prediction and optimization of indirect shoot regeneration of Passiflora caerulea using machine learning and optimization algorithms
BMC Biotechnology (2023)
-
Artificial neural network and decision tree–based models for prediction and validation of in vitro organogenesis of two hydrophytes—Hemianthus callitrichoides and Riccia fluitans
In Vitro Cellular & Developmental Biology - Plant (2023)
-
Artificial neural network and decision tree facilitated prediction and validation of cytokinin-auxin induced in vitro organogenesis of sorghum (Sorghum bicolor L.)
Plant Cell, Tissue and Organ Culture (PCTOC) (2023)