Abstract
Atopic dermatitis (AD) is a chronic inflammatory immune-mediated skin disease characterized by skin colonization by Staphylococcus aureus. Interleukin (IL)-22, in cooperation with IL-17, triggers antimicrobial peptide elaboration and enhances certain immunological responses. In AD, IL-22 is related to epidermal hyperplasia, keratinocyte apoptosis, and inhibition of antimicrobial peptide (AMP) production. We aimed to evaluate the impact of staphylococcal enterotoxins on the Tc22/Th22 induction in the peripheral blood of AD patients and on CD4+/CD8+ T cells expressing IL-22 in AD skin. Our study showed inhibition of the staphylococcal enterotoxins A and B (SEA and SEB) response by Th22 (CD4+IL-22+IL-17A−IFN-γ−) cells in AD patients. In contrast, Tc22 (CD8+IL-22+IL-17A−IFN-γ−) cells were less susceptible to the inhibitory effects of staphylococcal enterotoxins and exhibited an enhanced response to the bacterial stimuli. In AD skin, we detected increased IL-22 transcript expression and T lymphocytes expressing IL-22. Together, our results provide two major findings in response to staphylococcal enterotoxins in adults with AD: dysfunctional CD4+ IL-22 secreting T cells and increased Tc22 cells. Our hypothesis reinforces the relevance of CD8 T cells modulated by staphylococcal enterotoxins as a potential source of IL-22 in adults with AD, which is relevant for the maintenance of immunological imbalance.
Similar content being viewed by others
Introduction
Our skin barrier is a complex network with various functions, such as immunological responses, homeostasis, protection against pathogens, epidermal and adnexal systems with a perfect coordination between immune, neural and endocrine systems1,2,3,4,5. Any imbalance in this milieu, including environmental stressors, leads to several inflammatory skin conditions, mainly in atopic dermatitis6,7.
Atopic dermatitis (AD) is a prevalent, chronic, inflammatory, pruritic, immune-mediated skin disease8. Complex interactions between susceptibility genes encoding skin barrier molecules9, markers of the inflammatory response, environmental factors, and infectious agents, especially Staphylococcus aureus (S. aureus) and herpes virus, with aggravation by altered immunologic responses, are crucial elements for the pathophysiology of AD10,11.
The immune pathogenesis of AD has been described as a representative disease involving dysfunction of the Th1/Th2 biphasic immune response12. However, with the characterization of new subsets of human T helper (Th) cells, including Th17 and Th2213, novel pro-inflammatory and inflammatory components should be considered and better evaluated in AD10,12,14.
IL-17, which is produced by Th17 cells, is able to coordinate local tissue inflammation through the upregulation of pro-inflammatory cytokines and chemokines, including IL-6, TNF-α, IL-1β, CXCL1, CCL2, CXCL2, CCL7, and CCL2014,15. In cooperation with IL-17, IL-22 triggers antimicrobial peptide elaboration and enhances immunological responses16,17. It has been hypothesized that high IL-22 and low IL-17 expression predominate during the chronic phase of AD; therefore, the initial hypothesis of AD as a Th2-driven disease should be modified with the addition of the Th22/Tc22 subsets associated with epidermal changes18.
Th2 and Th22 cytokines are capable of modulating the AD epidermal barrier, including suppression of keratinocyte differentiation and apoptosis, hyperplasia, and antimicrobial peptide (AMP) production19. Furthermore, Th2 (IL-4, IL-13, and IL-31) and Th22 (IL-22) cytokines induce the inhibition of certain skin barrier protein-encoding genes, such as filaggrin, loricrin, and involucrin19,20,21. Th22 cells have demonstrated a capacity to infiltrate AD skin and release IL-22 and TNF-α. Likewise, significant amounts of CD3+CD8+ T cells from inflammatory skin diseases can also stimulate IL-22 secretion22,23. In summary, the major function of IL-22 secreting cells in AD is related to keratinocyte effects via the STAT3 (signal transducer and activator of transcription 3) pathway, enhancing cell proliferation and migration and resulting in hyperplasia, keratinocyte apoptosis, and inhibition of AMP production19,22.
S. aureus colonizes skin in approximately 30–50% of healthy adults, but it is constantly detected in only 10–20% of such individuals24. In AD patients, more than 90% of the skin exhibits an increase in S. aureus colonization as compared with the skin of healthy subjects25. Chronic colonization in AD skin remains a relevant issue, considering the widespread occurrence of antibiotic-resistant strains (methicillin-resistant Staphylococcus aureus; MRSA)26.
In AD patients, cutaneous S. aureus is capable of inducing myeloid-derived suppressor cells, leading to in vivo immune suppression of the T cell activation in the skin; in mice exposed to wild-type S. aureus on skin, there are reduced numbers of CD4+ and CD8+ T cells in the spleen27. Moreover, evidence of decreased peripheral blood mononuclear cell (PBMC) proliferation response to staphylococcal enterotoxin A (SEA) and other recall antigens and mitogens (TT, CMA, and PHA) suggests a compromised immune profile in adults with AD28.
Effector memory T cells directed against antigens derived from skin pathogens such as S. aureus are important for providing instant protection against viral and/or bacterial infections29. CD4 T cell functions include cytokine production to control infections, in addition to optimizing and maintaining CD8 T cell memory30,31. In this study, we aimed to evaluate the potential role of staphylococcal enterotoxins (SEA and SEB) in modulating IL-22 producing CD4+/CD8+ T cells in adults with atopic dermatitis.
Results
Staphylococcal enterotoxins inhibit CD4+ T cells secreting IL-22
In a previous study, our group demonstrated a decreased PBMC proliferation response to SEA compared with SEB stimulation, recall antigens, and mitogens in adults with AD28. Considering that IL-22 is beneficial to the host in many infectious and inflammatory disorders, with inherent pro-inflammatory properties32, we analyzed the effects of staphylococcal enterotoxins on IL-22 secretion by PBMCs in AD patients.
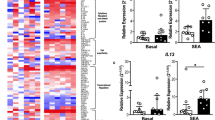
First, evaluating whether IL-22 levels changed in AD patients at the systemic level, we detected a significant increase of IL-22 serum levels in AD patients compared with the healthy control (HC) group (Fig. 1A). Next, IL-22 secretion induced by SEA and SEB stimulation in PBMC from AD patients and controls was assessed. Figure 1B shows a marked increase of IL-22 levels in AD patients with both SEA and SEB stimulation compared with the HC group (Fig. 1B).
IL-22 levels in sera and PBMCs induced by SEA and SEB in AD. (A) Determination of IL-22 serum levels from healthy controls (HC, n = 40) and atopic dermatitis patients (AD, n = 38), assessed by ELISA. (B) IL-22 levels in PBMC culture supernatants from AD patients (AD, n = 26) and healthy controls (HC, n = 23), evaluated by ELISA at baseline and with SEA and SEB stimulation after 48 hours of incubation. (C) Representative gating strategy for the selection of populations containing CD3, CD4, and CD8 T cells. Each subsequent panel shows only the population of interest producing IL-22 after SEA and SEB stimulation. Frequencies of CD4 T cells and (D) CD8 T cells (E) secreting IL-22 after SEA and SEB stimulation from healthy controls (HC, n = 10) and adults with atopic dermatitis (AD, n = 15), evaluated by flow citometry. Data represent the medians with interquartile ranges. *p ≤ 0.05 and ***p ≤ 0.001.
The gating strategy for CD4+/CD8+ T cells producing IL-22, as analyzed by flow cytometry, is shown in Fig. 1C. The frequency of these cells was evaluated after stimulation of PBMCs with SEA and SEB. The frequency of CD4+IL-22+ T cells was decreased when compared with the controls (Fig. 1D). A different profile from that in CD4+ T cells was seen in CD8+ T cells, where IL-22 was significantly increased following SEA stimulation (Fig. 1E). Moreover, no changes in the frequencies of CD4+ or CD8+ T cells secreting IL-17 under SEA and SEB stimulation were detected in DA individuals compared with the HC group (data not shown). It is possible that IL-17-secreting cells are less refractory to the inhibitory effects of SEA/SEB. There was no relationship between disease severity and cytokine frequency (data not shown).
Differential staphylococcal enterotoxin effects in circulating Th22 and Tc22 cells in AD patients
Although Th22 and Tc22 cells have been described as relevant participants in AD etiopathogenesis, more evidence is needed concerning the effects of staphylococcal enterotoxins on these T cell subsets18,33. Th22 cells produce large amounts of IL-22 but do not secrete IL-17A or IFN-γ17. To evaluate Th22 cells by flow cytometry, we excluded IL-17A+ and IFN-γ+ cells in the gating strategy, defining Th22 cells as CD3+CD4+IL-17A−IFN-γ−IL-22+ and Tc22 cells as CD3+CD8+IL-17A−IFN-γ−IL-22+ (Fig. 2A).
Staphylococcal antigens induce opposite effects in circulating Th22 and Tc22 cells in AD. (A) Gating strategy for the analysis of Th22 and Tc22 cells in PBMCs by flow cytometry, excluding IL-17A and IFN-γ secretion. (B) The frequencies of Th22+ cells represented after SEA and SEB stimulation from healthy controls (HC, n = 10) and adults with atopic dermatitis (AD, n = 15). (C) The frequencies of Tc22+ cells represented after SEA and SEB stimulation from healthy controls (HC, n = 10) and adults with atopic dermatitis (AD, n = 15). Data represent the medians with interquartile ranges. *p ≤ 0.05.
There was a remarkable difference between the Th22 and Tc22 cell responses in AD patients. CD4+IL-22+ T cells showed a diminished response to SEA/SEB stimuli when comparing AD patients with HC subjects (Fig. 2B). In contrast, when evaluating CD8+ T cells, we found augmented levels of IL-22 after stimulation in AD patients compared with those in HC subjects (Fig. 2C). These findings may indicate that staphylococcal enterotoxins have opposite effects on T cell subsets, suppressing Th22 cells and enhancing Tc22 cells in AD.
IL-22 expression profile in skin lesions from adults with AD
Considering that there are IL-22-secreting keratinocytes and epidermal thickening with impaired barrier function in AD20,34,35, we then evaluated the IL-22 expression profiles in skin samples of AD patients.
Figure 3A–C show the IL-22 expression profile in skin specimens from HCs, and Fig. 3D–F show the IL-22 expression profile in skin specimens from AD individuals. The profile of IL-22 expression of AD patients was similar to that of the controls at the epidermal level (Fig. 3G), in contrast to the enhanced dermal IL-22 expression observed in AD patients when compared with the controls (Fig. 3H).
Expression of IL-22 in skin specimens from adults with AD tested by immunohistochemistry. (A) Photograph of a skin specimen from a healthy control: IL-22 expression. (B) Photograph of epidermis from a healthy control: IL-22 expression. (C) Photograph of dermis from a healthy control: IL-22 expression. (D) Photograph of a skin specimen from an AD patient: enhanced IL-22 expression. (E) Photograph of epidermis from an AD patient: enhanced IL-22 expression. (F) Photograph of dermis from an AD patient: enhanced IL-22 expression. IL-22 expression levels (μm2/μm2) in the epidermis (G) and dermis (H) of the healthy control group (HC, n = 20) compared with AD patients (AD, n = 31), evaluated by immunohistochemistry. Lines represent medians with interquartile ranges of cytokines in skin specimens. *p ≤ 0.05 and **p ≤ 0.01.
We then evaluated whether IL-22 transcript levels could be altered in AD. Figure 4 shows increased expression levels of IL-22 (Fig. 4A) and IL-4 (Fig. 4B) in AD samples, with similar levels of IL-10 (Fig. 4C) and TNF-α (Fig. 4D) gene expression in the two groups. To investigate the presence of IL-22-secreting T cells, we analyzed CD4+/CD8+ T cells in skin sections of AD and HC samples (Fig. 5A,B). The enhanced IL-22 expression within the dermis in AD may have a relevant role in the immune response, considering that dermal leukocyte clusters are essential structures for eliciting acquired cutaneous immunity36.
Cytokine gene expression profiles in AD skin. Expression profiles of IL-22, IL-4, IL-10, and TNF-α in skin of the healthy control group (HC, n = 5) compared with AD patients (AD, n = 10), assessed by real-time PCR. Lines represent medians with interquartile ranges of cytokines in skin specimens. *p ≤ 0.05 and **p ≤ 0.01.
Discussion
Our study showed compromised responses of Th22 cells to SEA and SEB in the peripheral blood of AD patients, with an increase in Tc22 cells, which appear to be less susceptible to the enterotoxins’ effects. Moreover, we demonstrated the presence of CD4+/CD8+ T cells secreting IL-22 within the dermis of AD skin.
The present group of atopic patients had long-lasting AD (with a mean of 19.45 years), with increased circulating IL-22 levels and high levels secreted by PBMCs after stimulation with SEA and SEB. The main source of IL-22, a cytokine often released by inflamed tissue and detected in this study in both serum and in PBMCs, may include CD4+ T cells (e.g., Th17, Th22, Th1, and smaller numbers of Th2 cells)22,34,35, CD8+ T cells, natural killer cells, and dendritic cells22,37,38.
However, when we evaluated intracellular IL-22 expression in CD4+ T cells, a diminished frequency was detected, in contrast to the increased frequency of CD8+ T cells secreting IL-22, induced by SEA and SEB stimulation in AD patients. Interestingly, the decreased IL-22 secretion by CD4+ T cells is reinforced by previous data that showed inhibited statuses in both the antigen-specific proliferative response and polyclonal activators28 in AD, suggesting a suppressed profile of CD4+ T cells in AD patients, corroborated by reduced cytokine secretion after stimulation.
Other studies have shown enhanced IL-22 levels in the CD4+ T cells of children and adults with AD following stimulation with α-toxin from S. aureus and SEB39,40. Our cohort of adults may display a different profile compared with children due to chronic stimulation with staphylococcal enterotoxins, driving them toward a chronic activation and nonresponsive status.
Interestingly, our data indicated that in vitro, staphylococcal enterotoxins exert opposite effects on T cell subsets, suppressing Th22 cells and enhancing Tc22 cells after SEA and SEB stimulation in the AD group. CD4+IL22+ T cells appeared to be more susceptible than CD8+IL-22+ T cells to SEA/SEB effects, regardless disease severity. However, similar frequencies of circulating Th22 and Tc22 cells after stimulation with PMA/Ionomycin have been described in AD and psoriasis23. Moreover, in severe AD, selective expansion of circulating Th2/Tc2 and Th22/Tc22 cells has been described in CLA+ T cells induced by PMA/ionomycin33,41. Our results suggest that circulating Tc22 cells producing IL-22 contribute to the perpetuation of inflammation in AD patients, despite the presence of IL-17 and IFN-γ, reinforcing the relevance of CD8+ T cells modulated by staphylococcal enterotoxins in AD pathogenesis.
In AD dermal lesions, we detected enhanced expression of IL-22; additionally, we identified both CD4 and CD8 T cells expressing IL-22 in AD skin, in accordance with previous findings42,43. These authors found dermal cellular infiltrates of mainly CD4+ and CD8+ T cells, with a CD4:CD8 ratio similar to the profile detected in the peripheral blood in AD subjects42,43. Furthermore, high percentages of CD8+ T cells isolated from AD skin explant cultures are related to IL-22, IFN-γ, IL-13, and IL-17 production44, reinforcing the important role of these cells in barrier dysfunction and as a significant source of inflammatory cytokines in AD23.
We also described increased IL-22 and IL-4 transcript expression, emphasizing the Th2/T22 deviation in AD patients. Epicutaneous sensitization to house dust mites induces upregulation of IL-22 in an AD-like skin mouse model, leading to pruritus, intensified dermal and systemic Th2 immunity, downregulation of epidermal differentiation complex genes, and enhanced epidermal colonization of S. aureus45. In AD, decreases in the skin barrier function and Th2 cytokine release favor S. aureus penetration of the skin, with diminished in vitro expression of filaggrin and human β-defensin (HBD) 346,47.
In conclusion, staphylococcal enterotoxins may play a role as a modulating factor on T lymphocyte–IL-22 secreting cells in AD patients, as evidenced by the presence of CD4/CD8 T cells expressing IL-22 in skin lesions, dysfunctional circulating Th22, and Tc22 cell upregulation.
Methods
Subjects
Thirty-eight patients with AD (aged between 19–48 years; mean age: 27.68 ± 8.45; 26 males and 12 females) and 40 healthy non-AD volunteers (aged between 19–53 years; mean age: 31.10 ± 9.05; 18 males and 22 females) were enrolled in this study. AD was diagnosed according to the Hanifin & Rajka criteria48. Disease severity was evaluated by the EASI (Eczema Area and Severity Index)49, and AD patients were classified as mild (n = 9), moderate (n = 15), or severe (n = 14). IgE serum levels ranged from 3,120 to 119,000 IU/mL (average of 29,681). None of the patients were using oral steroids or immunosuppressants. All patients were interviewed about any associations with respiratory symptoms and the age of AD onset. This study was approved by the Ethics Committee of the University of Sao Paulo School of Medicine, and informed consent was obtained from all subjects. All methods were performed in accordance with the relevant guidelines and regulations of this institution. Demographic data are shown in Tables 1 and S1 (supplementary file).
ELISA
PBMCs were isolated from heparinized venous blood via Ficoll-Hypaque gradient centrifugation (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) and resuspended in RPMI 1640 medium supplemented with gentamicin (40 µg/mL) and 10% pooled AB normal human serum (Sigma-Aldrich, St. Louis, MO, USA). PBMCs (2 × 106/well) were cultured in 96-well microplates (Costar, Cambridge, MA, USA) at 2 × 105 cells/well with SEA (0.04 µg/mL; Sigma) and SEB (1 µg/mL; Sigma) for 48 hours at 37 °C in 5% CO2. Serum samples were also obtained. Cell-free supernatants and serum samples were stored at −70 °C until use in cytokine assays. IL-22 measurements were performed by ELISA in both culture supernatants and sera, following the manufacturer’s recommendations (DY782, R&D Systems, Minneapolis, MN, USA). The detection limit of IL-22 was 31.2 pg/mL.
Flow cytometry
PBMCs (2 × 106/well) were cultivated in 96-well microplates (Costar) at 37 °C and 5% CO2 in the presence of SEA (0.04 µg/mL; Sigma) and SEB (1 µg/mL; Sigma), for 6 hours. Brefeldin A (10 µg/mL; Sigma) was added 2 hours after the culture was started. Next, the cells were stained with the viability marker LIVE/DEAD PE-Texas Red (Invitrogen, Carlsbad, CA, USA) for 30 minutes at room temperature. Cells were then incubated with Cytofix/Cytoperm (BD Biosciences, San Jose, CA, USA) and stained with the following antibodies: CD3-Qdot 605 (Invitrogen), CD4-Horizon V500 (BD), CD8-PerCP-Cy5.5 (BD), IL-17A-Alexa 488 (eBioscience, San Diego, CA, USA), IL-22-PE (eBioscience), and IFN-γ-Horizon V450 (BD Biosciences). Cells were then fixed with 1% paraformaldehyde, and 400,000 events were acquired on an LSR Fortessa flow cytometer (BD Biosciences). Fluorescence minus one (FMO) controls were performed for all antibody panels to check for proper compensation and to define positive signals. Data analysis was performed using FlowJo v10 software (Tree Star, Ashland, OR, USA).
Immunohistochemistry
Immunohistochemistry was performed on 4-μm paraffin-embedded samples, as described elsewhere50. The primary antibody IL-22 (ab134035, Abcam, Cambridge, UK) was utilized at 1:200 dilution, and the detection system was a Novolink Max Polymer Detection System (RE7280-K, Leica Biosystems, Newcastle Upon Tine, UK), and the chromogen used was DAB (3,3′ diamibenzidine, D5637, Sigma). Total tissue distribution of IL-22 was calculated by dividing the stained area by the total area measured in the epidermis or dermis. Immunohistochemically stained specimens were scanned using an Aperio Scan-scope Cs (Aperio Technologies, Vista, CA, USA). Photographs were analyzed utilizing Image-Pro Plus version 4.5.0.29 (Media Cybernetics Inc., Bethesda, Maryland, USA)51.
Immunofluorescence assay
Immunofluorescence was performed on 4-μm paraffin-embedded samples, as described elsewhere50. After dewaxing, samples were fixed with paraformaldehyde 4% and blocked with phosphate-buffered saline with bovine serum albumin (PBS-BSA) 2% for 30 minutes. The primary antibodies IL-22 (ab134035, Abcam), CD4 (104R-16, Cell Marque, Rocklin, CA, USA), or CD8 (ab101500, Abcam) were incubated overnight at 4 °C. On the following day, the secondary antibodies, donkey anti-mouse Alexa 488 fluorescein (A21202, ThermoFisher Scientific, Waltham, MA, USA) (for IL-22 staining) and goat anti-rabbit Alexa 546 fluorescein (A11035, ThermoFisher Scientific) (for CD4 or CD8 staining), and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI, D1306, ThermoFisher Scientific) for nuclear identification were diluted and incubated for 90 minutes at room temperature. Images were acquired utilizing the appropriate filters of an Axiovert 200 inverted immunofluorescence microscope (Zeiss, Oberkochen, Germany).
Real-time PCR
Six-millimeter punch samples were taken from the skin lesions of AD patients with local anesthesia. Normal skin samples as controls were obtained after plastic surgery. All of the specimens were stored in RNAlater solution (Sigma), at −20 °C. Frozen samples were cut, and the tissue was homogenized with a Tissue Ruptor (Qiagen, Valencia, CA, USA) after debris removal by centrifugation, and supernatants were used for RNA extraction. Total RNA was extracted from each skin biopsy using an RNeasy Plus Mini Kit (Qiagen), including an extra step for separation of genomic DNA (gDNA eliminator column). The samples were then treated with a DNase set (Qiagen). Reverse transcription was performed with a Reverse Transcriptase Kit (Bio-Rad, Hercules, California, USA). For PCR amplification, TNF-α gene: forward primer (5′-CCCAGGCAGTCAGATCATCTTC-3′) and reverse primer (5′-GCTTGAGGGTTTGCTACAACAT-3′); IL-4 gene: forward primer (5′-CCAACGTACTCTGGTTGGCT-3′) and reverse primer (5′-GCACCGAGTTGACCGTAACA-3′); IL-10 gene: forward primer (5′- CACATGCGCCTTGATGTCTG-3′) and reverse primer (5′-CAGGGCACCCAGTCTGAGA-3′); IL-22 gene: forward primer (5′-GAGAAACTGTTCCACGGAG-3′) and reverse primer (5′-TGCTTAGCCTGTTGCTGAG-3′); and GAPDH gene: forward primer (5′-GAAGGTGAAGGTCGGAGT-3′) and reverse primer (5′-GAAGATGGTGATGGGATTTC-3′) were utilized.
Real-time PCR was performed in an Applied Biosystems 7500 system using specific primers and SYBR Green (Applied Biosystems, Carlsbad, CA, USA), as described by Pereira et al.,52. Primers for qPCR (Life Technologies) were only accepted if their efficiency reached 100 ± 10%. Corrections were made for primer efficiency. The specificity of the reaction was examined by dissociation curve. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels of the samples in the same plate were analyzed to normalize the mRNA contents among the tested samples. The cycling protocol consisted of 10 minutes at 95 °C, followed by 40 cycles of 15 seconds at 95 °C and 60 seconds at 60 °C. The amplification results were analyzed using Sequence Detection System (SDS) software (Applied Biosystems). Normalized expression was calculated as previously described by Livak53.
Statistical analysis
The Mann–Whitney test or Kruskal–Wallis with Dunn’s post-test were utilized to compare 2 or 3 sets of data, respectively. Correlations were established using the Spearman non-parametric correlation test. Differences between groups were considered statistically significant when p ≤ 0.05.
References
Levin, J., Friedlander, S. F. & Del Rosso, J. Q. Atopic dermatitis and the stratum corneum: part 1: the role of filaggrin in the stratum corneum barrier and atopic skin. The Journal of clinical and aesthetic dermatology 6, 16–22 (2013).
Slominski, A. T. et al. Sensing the environment: regulation of local and global homeostasis by the skin’s neuroendocrine system. Adv Anat Embryol Cell Biol 212, v, vii, 1–115 (2012).
Slominski, A. T. et al. Key role of CRF in the skin stress response system. Endocr Rev 34, 827–884, https://doi.org/10.1210/er.2012-1092 (2013).
Slominski, A. T., Manna, P. R. & Tuckey, R. C. On the role of skin in the regulation of local and systemic steroidogenic activities. Steroids 103, 72–88, https://doi.org/10.1016/j.steroids.2015.04.006 (2015).
Slominski, A. T., Brozyna, A. A. & Tuckey, R. C. Cutaneous Glucocorticoidogenesis and Cortisol Signaling Are Defective in Psoriasis. J Invest Dermatol 137, 1609–1611, https://doi.org/10.1016/j.jid.2017.04.004 (2017).
Kuo, I. H., Yoshida, T., De Benedetto, A. & Beck, L. A. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol 131, 266–278, https://doi.org/10.1016/j.jaci.2012.12.1563 (2013).
Slominski, A. T., Zmijewski, M. A., Plonka, P. M., Szaflarski, J. P. & Paus, R. How ultraviolet light touches the brain and endocrine system through skin, and why. Endocrinology, https://doi.org/10.1210/en.2017-03230 (2018).
Leung, D. Y. New insights into atopic dermatitis: role of skin barrier and immune dysregulation. Allergol Int 62, 151–161, https://doi.org/10.2332/allergolint.13-RAI-0564 (2013).
Elias, P. M. & Schmuth, M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Current opinion in allergy and clinical immunology 9, 437–446, https://doi.org/10.1097/ACI.0b013e32832e7d36 (2009).
Hayashida, S., Uchi, H., Moroi, Y. & Furue, M. Decrease in circulating Th17 cells correlates with increased levels of CCL17, IgE and eosinophils in atopic dermatitis. J Dermatol Sci 61, 180–186, https://doi.org/10.1016/j.jdermsci.2010.10.013 (2011).
De Benedetto, A., Agnihothri, R., McGirt, L. Y., Bankova, L. G. & Beck, L. A. Atopic dermatitis: a disease caused by innate immune defects? J Invest Dermatol 129, 14–30, https://doi.org/10.1038/jid.2008.259 (2009).
Eyerich, K. & Novak, N. Immunology of atopic eczema: overcoming the Th1/Th2 paradigm. Allergy 68, 974–982, https://doi.org/10.1111/all.12184 (2013).
Auriemma, M., Vianale, G., Amerio, P. & Reale, M. Cytokines and T cells in atopic dermatitis. European cytokine network 24, 37–44, https://doi.org/10.1684/ecn.2013.0333 (2013).
Koga, C., Kabashima, K., Shiraishi, N., Kobayashi, M. & Tokura, Y. Possible pathogenic role of Th17 cells for atopic dermatitis. J Invest Dermatol 128, 2625–2630, https://doi.org/10.1038/jid.2008.111 (2008).
Orfali, R. L., Zaniboni, M. C. & Aoki, V. Profile of skin barrier proteins and cytokines in adults with atopic dermatitis. G Ital Dermatol Venereol 152, 140–147, https://doi.org/10.23736/S0392-0488.16.05533-4 (2017).
Liang, S. C. et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med 203, 2271–2279, https://doi.org/10.1084/jem.20061308 (2006).
Duhen, T., Geiger, R., Jarrossay, D., Lanzavecchia, A. & Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat Immunol 10, 857–863, https://doi.org/10.1038/ni.1767 (2009).
Fujita, H. The role of IL-22 and Th22 cells in human skin diseases. J Dermatol Sci 72, 3–8, https://doi.org/10.1016/j.jdermsci.2013.04.028 (2013).
Leung, D. Y. & Guttman-Yassky, E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol 134, 769–779, https://doi.org/10.1016/j.jaci.2014.08.008 (2014).
Suárez-Fariñas, M. et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol 127(954-964), e951–954, https://doi.org/10.1016/j.jaci.2010.12.1124 (2011).
Batista, D. I. et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol 29, 1091–1095, https://doi.org/10.1111/jdv.12753 (2015).
Cavani, A., Pennino, D. & Eyerich, K. Th17 and Th22 in skin allergy. Chem Immunol Allergy 96, 39–44, https://doi.org/10.1159/000331870 (2012).
Nograles, K. E. et al. IL-22-producing “T22” T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. J Allergy Clin Immunol 123, 1244–1252 e1242, https://doi.org/10.1016/j.jaci.2009.03.041 (2009).
Lowy, F. D. Staphylococcus aureus infections. N Engl J Med 339, 520–532, https://doi.org/10.1056/NEJM199808203390806 (1998).
Leung, D. Y. & Bieber, T. Atopic dermatitis. Lancet 361, 151–160, https://doi.org/10.1016/S0140-6736(03)12193-9 (2003).
Saeed, K., Marsh, P. & Ahmad, N. Cryptic resistance in Staphylococcus aureus: a risk for the treatment of skin infection? Curr Opin Infect Dis 27, 130–136, https://doi.org/10.1097/QCO.0000000000000046 (2014).
Skabytska, Y. et al. Cutaneous innate immune sensing of Toll-like receptor 2-6 ligands suppresses T cell immunity by inducing myeloid-derived suppressor cells. Immunity 41, 762–775, https://doi.org/10.1016/j.immuni.2014.10.009 (2014).
Orfali, R. L. et al. Atopic dermatitis in adults: evaluation of peripheral blood mononuclear cells proliferation response to Staphylococcus aureus enterotoxins A and B and analysis of interleukin-18 secretion. Exp Dermatol 18, 628–633, https://doi.org/10.1111/j.1600-0625.2009.00842.x (2009).
Laborel-Préneron, E. et al. Effects of the Staphylococcus aureus and Staphylococcus epidermidis Secretomes Isolated from the Skin Microbiota of Atopic Children on CD4+ T Cell Activation. PLoS One 10, e0141067, https://doi.org/10.1371/journal.pone.0141067 (2015).
Roetynck, S. et al. Phenotypic and functional profiling of CD4 T cell compartment in distinct populations of healthy adults with different antigenic exposure. PLoS One 8, e55195, https://doi.org/10.1371/journal.pone.0055195 (2013).
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856, https://doi.org/10.1038/nature01441 (2003).
Rutz, S., Eidenschenk, C. & Ouyang, W. IL-22, not simply a Th17 cytokine. Immunol Rev 252, 116–132, https://doi.org/10.1111/imr.12027 (2013).
Czarnowicki, T. et al. Severe atopic dermatitis is characterized by selective expansion of circulating T2/T2 and T22/T22, but not T17/T17, cells within the skin-homing T-cell population. J Allergy Clin Immunol, https://doi.org/10.1016/j.jaci.2015.01.020 (2015).
Akdis, M., Palomares, O., van de Veen, W., van Splunter, M. & Akdis, C. A. TH17 and TH22 cells: a confusion of antimicrobial response with tissue inflammation versus protection. J Allergy Clin Immunol 129, 1438–1449, quiz1450-1431, https://doi.org/10.1016/j.jaci.2012.05.003 (2012).
Eyerich, S. et al. Th22 cells represent a distinct human T cell subset involved in epidermal immunity and remodeling. J Clin Invest 119, 3573–3585, https://doi.org/10.1172/JCI40202 (2009).
Natsuaki, Y. et al. Perivascular leukocyte clusters are essential for efficient activation of effector T cells in the skin. Nat Immunol 15, 1064–1069, https://doi.org/10.1038/ni.2992 (2014).
Michalak-Stoma, A. et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers 35, 625–631, https://doi.org/10.1155/2013/856056 (2013).
Sonnenberg, G. F., Fouser, L. A. & Artis, D. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat Immunol 12, 383–390, https://doi.org/10.1038/ni.2025 (2011).
Niebuhr, M. et al. Staphylococcal alpha-toxin is a strong inducer of interleukin-17 in humans. Infect Immun 79, 1615–1622, https://doi.org/10.1128/IAI.00958-10 (2011).
Niebuhr, M., Scharonow, H., Gathmann, M., Mamerow, D. & Werfel, T. Staphylococcal exotoxins are strong inducers of IL-22: A potential role in atopic dermatitis. J Allergy Clin Immunol 126, 1176–1183 e1174, https://doi.org/10.1016/j.jaci.2010.07.041 (2010).
Brandt, E. B. & Sivaprasad, U. Th2 Cytokines and Atopic Dermatitis. J Clin Cell Immunol 2, https://doi.org/10.4172/2155-9899.1000110 (2011).
Luckheeram, R. V., Zhou, R., Verma, A. D. & Xia, B. CD4(+)T cells: differentiation and functions. Clin Dev Immunol 2012, 925135, https://doi.org/10.1155/2012/925135 (2012).
Akdis, C. A., Akdis, M., Trautmann, A. & Blaser, K. Immune regulation in atopic dermatitis. Curr Opin Immunol 12, 641–646 (2000).
Hijnen, D. et al. CD8(+) T cells in the lesional skin of atopic dermatitis and psoriasis patients are an important source of IFN-gamma, IL-13, IL-17, and IL-22. J Invest Dermatol 133, 973–979, https://doi.org/10.1038/jid.2012.456 (2013).
Lou, H. et al. Expression of IL-22 in the Skin Causes Th2-Biased Immunity, Epidermal Barrier Dysfunction, and Pruritus via Stimulating Epithelial Th2 Cytokines and the GRP Pathway. J Immunol 198, 2543–2555, https://doi.org/10.4049/jimmunol.1600126 (2017).
Howell, M. D. et al. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol 120, 150–155, https://doi.org/10.1016/j.jaci.2007.04.031 (2007).
Nakatsuji, T. et al. Staphylococcus aureus Exploits Epidermal Barrier Defects in Atopic Dermatitis to Trigger Cytokine Expression. J Invest Dermatol 136, 2192–2200, https://doi.org/10.1016/j.jid.2016.05.127 (2016).
Hanifin, J. M. & Rajka, G. Diagnostic Features of Atopic-Dermatitis. Acta Derm-Venereol, 44–47 (1980).
Hanifin, J. M. et al. The eczema area and severity index (EASI): assessment of reliability in atopic dermatitis. EASI Evaluator Group. Exp Dermatol 10, 11–18 (2001).
Pagliari, C. et al. Paracoccidioidomycosis: cells expressing IL17 and Foxp3 in cutaneous and mucosal lesions. Microbial pathogenesis 50, 263–267, https://doi.org/10.1016/j.micpath.2010.12.008 (2011).
Prasad, K. & Prabhu, G. K. Image analysis tools for evaluation of microscopic views of immunohistochemically stained specimen in medical research-a review. Journal of medical systems 36, 2621–2631, https://doi.org/10.1007/s10916-011-9737-7 (2012).
Pereira, N. Z. et al. Upregulation of innate antiviral restricting factor expression in the cord blood and decidual tissue of HIV-infected mothers. PLoS One 8, e84917, https://doi.org/10.1371/journal.pone.0084917 (2013).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, https://doi.org/10.1006/meth.2001.1262 (2001).
Acknowledgements
We are grateful to all the individuals who participated in the study. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo - FAPESP (Grants # 2014/25645-7 and 2016/24161-1) and the Laboratory of Dermatology and Immunodeficiencies (LIM-56), Department of Dermatology, University of Sao Paulo Medical School.
Author information
Authors and Affiliations
Contributions
R.L.O. designed and conducted the experiments, analyzed the results and wrote the manuscript. R.L.O., M.N.S. and V.A. conceived the ideas for the project. M.C.Z., J.F.L., N.Z.P., L.M.S.O. and A.J.S.D. participated in the design and the troubleshooting of experiments; data interpretation and discussion. N.Z.P. designed the primers and ran transcriptome datasets. J.F.L. and Y.A.L.R. ran the flow cytometry experiments and analyzed the data. G.C.C, N.P.V., Y.A.L.R., and M.N.S. ran and analyzed immunohistochemistry and immunofluorescence data. All authors contributed to discussion and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Orfali, R.L., da Silva Oliveira, L.M., de Lima, J.F. et al. Staphylococcus aureus enterotoxins modulate IL-22-secreting cells in adults with atopic dermatitis. Sci Rep 8, 6665 (2018). https://doi.org/10.1038/s41598-018-25125-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25125-0
- Springer Nature Limited