Abstract
Seedless grapes are of considerable importance for the raisin and table grape industries. Previous transcriptome analyses of seed development in grape revealed that genes encoding homeobox transcription factors were differentially regulated in seedless compared with seeded grape during seed development. In the present study, we identified a total of 73 homeobox-like genes in the grapevine genome and analyzed the genomic content and expression profiles of these genes. Based on domain architecture and phylogenetic analyses grape homeobox genes can be classified into eleven subfamilies. An analysis of the exon-intron structures and conserved motifs provided further insight into the evolutionary relationships between these genes. Evaluation of synteny indicated that segmental and tandem duplications have contributed greatly to the expansion of the grape homeobox gene superfamily. Synteny analysis between the grape and Arabidopsis genomes provided a potential functional relevance for these genes. The tissue-specific expression patterns of homeobox genes suggested roles in both vegetative and reproductive tissues. Expression profiling of these genes during the course of ovule development in seeded and seedless cultivars suggested a potential role in ovule abortion associated with seedlessness. This study will facilitate the functional analysis of these genes and provide new resources for molecular breeding of seedless grapes.
Similar content being viewed by others
Introduction
Homeobox (HB) transcription factors often act as master regulators of organ identity and are encoded by a large and conserved gene family. These were originally characterized as regulators of morphogenesis in the fruit fly, Drosophila melanogaster 1. In plants, the founding member of the HB gene family is KNOTTED1, which has a role in meristem function in maize2. Subsequently, numerous HB-encoding genes have been identified from a range of eukaryotes3, including Arabidopsis4, rice5, barley6, and humans7.
The homeodomain (HD) is a conserved, ~60 amino acids long DNA-binding domain, and is encoded by a ~180-nucleotide sequence referred to as the HB8. The characteristic three-dimensional structure of the HD comprises three alpha-helices, with the first and second helices forming a loop structure9. The second and third helices form a helix-turn-helix motif, which can interact with specific DNA sequences, allowing for regulation of expression of target genes10,11. Based on the conserved sequence of the HD along with other characteristic domains and motifs, HB genes are classified into 14 families12, including HD-ZIP I-IV, KNOX, WOX, PHD, NDX, BEL, PLINC, DDT, LD, SAWADEE and PINTOX. Each HB gene family is named according to unique typical domains and motifs outside of the HD, features that may enable functional differences of each subfamily.
HB genes are involved in many aspects of plant growth and development, and participate in various hormone response pathways13,14,15. For example, members of HD-ZIP, the largest HB protein family, play roles in epidermal cell differentiation16, floral organogenesis, fruit ripening15, embryonic shoot meristem formation, embryo patterning and vascular development17. Interestingly, this family is apparently specific to plants18. Members of the KNOX family are known to be required for nuclear localization and homo-dimerization, and suppress target gene expression19,20. Additionally, studies have shown that KNOX genes are involved in cell differentiation and shoot apical meristem maintenance21,22. BEL family members play a critical regulatory role in ovule development23, phyllotactic patterning, and stem cell maintenance and fate24.
In grape (Vitis vinifera), seedlessness is of particular importance for both fresh and dry fruit. Seedlessness in grape results from two distinct mechanisms, parthenocarpy and stenospermocarpy. The formation of stenospermocarpic fruit is associated with progressive ovule abortion following full bloom. Previous transcriptome analyses of seed development in grape hybrids revealed numerous genes, including HB genes, with expression associated with seedlessness25. However, there is little information about the number, organization and regulation of HB genes in grapevine. In this study, we evaluated the genomic content and expression profiles of HB genes in grapevine. We identified 73 grape HB genes, and classified these into 11 families based on phylogenetic analysis with Arabidopsis. Tissue-specific expression analysis in vegetative and reproductive organs suggests that many of the HB genes are developmentally regulated. Furthermore, comparison of ovule-specific expression during the course of ovule development in seeded and seedless grapes suggests that at least a subset of HB genes might be important for ovule development. Taken together, these results will provide a few candidate genes involved in ovule development for future targeted functional characterization, which may be useful in seedlessness-related molecular breeding programs.
Results
Genome-wide identification of HB genes in grape
To identify potential HB genes in the grapevine genome, we first obtained an HMM algorithm (HMMER) for the conserved HD (PFAM PF00046)26, and then used this in conjunction with PSI-BLAST to search a draft grape genome sequence (Grape Genome Website: http://www.genoscope.cns.fr)27. Subsequently, the structural integrity of conserved domains was evaluated, and redundant sequences were eliminated. Using this approach, we identified a total of 73 HB genes. These genes were designated as Vitis vinifera HB (VvHB) 1–73 based on their chromosomal positions (Table 1). The gene locus identifiers, gene accession numbers obtained from NCBI, position and length of the coding sequences, and length of the open reading frames are shown in Table 1.
The result of multiple sequence alignments of the 73 grape HD protein sequences revealed five highly conserved amino acids within the HD (Leu-16, Trp-48, Phe-49, Asn-51, and Arg-53), which may be necessary for the VvHB genes (Supplementary Fig. S1 ).
Phylogenetic analysis of grape and Arabidopsis HB genes
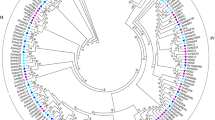
Multiple sequence alignment (Supplementary Fig. S1) of grape HD proteins showed that the most conserved structural feature is the HD, which consists of three alpha-helices. To gain a better understanding about the evolutionary relationships of HB genes among plant species, a total of 183 HB genes, including the 73 genes from grape identified in this study and 110 genes from Arabidopsis, were used to construct a phylogenetic tree based on the HD. Fourteen main clades (HD-ZIP I, HD-ZIP II, HD-ZIP III, HD-ZIP IV, KNOX, WOX, PHD, NDX, BEL, PLINC, DDT, LD, SAWADEE and PINTOX) were identified. Most of the main clades contained representatives from both grape and Arabidopsis, suggesting that a common ancestor of each subfamily must have existed before the divergence of these plant lineages (Fig. 1 and Supplementary Table S1 ). Based on the domain composition and evolutionary relationships of their encoded proteins, the grape HB genes were divided into 11 subfamilies. Interestingly, in grape, no members belonged to the three subgroups PLINC, NDX or LD, which existed in the Arabidopsis genome, suggesting that gene deletions may have occurred during evolution. This was especially striking for the PLINC clade, which contained 14 genes in Arabidopsis but none in grape. The numbers of grape HB genes in most other groups (HD-ZIP I, HD-ZIP II, HD-ZIPIII, KNOX, BEL, DDT, PHD, PINTOX and SAWADEE) were similar to Arabidopsis.
Phylogenetic analysis of HB proteins from grape and Arabidopsis. The phylogenetic tree was constructed with alignments of HD sequences from each gene using MEGA5.0 software. Circles represent Arabidopsis proteins and triangles represent grape proteins. Different colors of branch lines of subtrees indicate different subfamilies. Numbers at the nodes indicate bootstrap values.
Sequence and structure analyses of grape HB genes
We used the HD sequences from the 73 grape HB proteins identified here to build a phylogenetic tree, using the neighbor-joining method (Fig. 2a). HD proteins from the same subfamilies within grape clustered together, and the topology was similar to that constructed with HDs from Arabidopsis and grape. In order to further explore the phylogenetic relationship and classification of VvHB genes and the evolutionary relationship between VvHB genes and ATHB genes, we determined the distribution of their conserved motifs using MEME software (http://meme-suite.org/tools/meme) (Fig. 2b and Supplementary Fig. S2). In the current study, a total of 20 conserved motifs were identified within VvHB proteins and ATHB proteins. Nearly all of the HB proteins contain a highly conserved motif 1, corresponding to the alpha-helix III of the HD, which forms a helix-turn-helix structure with the alpha-helix II, and this structure can recognize and bind to specific DNA sequences to regulate the expression of target genes10,11. The motif 2 existing in most subfamilies corresponds to the HD alpha-helix I, which forms a loop structure with alpha-helix II. BEL subgroup members exhibited a motif 8, corresponding to POX domain, and this motif was present only in the BEL subgroup (Fig. 2b and Supplementary Table S2). Members of the HD-ZIP III and HD-ZIP IV subgroups exhibited 13 and 11 motifs, respectively. The two subgroups had four common motifs (motif 3, motif 5 and motif 11 correspond to START domain, motif 18 corresponds to HD-SAD domain). Additionally, there are two characteristic motifs in HD-ZIP III subgroup corresponding to MEKHLA structure. Overall, VvHB proteins within a given subgroup showed a similar distribution of conserved motifs, and this distribution was similar to that in Arabidopsis. For instance, all VvHB proteins from the HD-ZIP III group contained the same 13 conserved motifs (1, 2, 3, 5, 7, 10, 11, 13, 14, 15, 16, 18 and 20), which were also present in members of the ATHB subgroup (Supplementary Fig. S2 and Supplementary Table S2).
The structure analysis of grape HB genes. (a) Phylogenetic analysis and classification of grape genes. The phylogenetic tree was constructed with alignments of HD sequences from each gene using MEGA 5.0 software. Subtree branch lines are colored indicating different subfamilies. Numbers near the tree branches indicate bootstrap values. (b) Motif analysis of grape proteins. Motifs, numbered 1-20, were identified using MEME 4.11.2 software and are distinguished by color. Peptide sequence of each motif is provided in Supplementary Table S2. (c) Exon-intron structures of grape HB genes. Exons are marked as yellow boxes, and introns are represented by black lines connecting two exons. Upstream/downstream sequences are shown as blue boxes. Only exons and upstream/downstream sequences are drawn to scale.
Divergence of exon/intron structure is known to play a vital role in the evolution of gene families. Accordingly, we analyzed the exon/intron structures of VvHB genes to gain insight into evolution of the VvHB superfamily28. As shown in Fig. 2c, the number of exons ranged dramatically, from 1 to 23. Most of VvHB genes showed three to five exons (10 with three exons, 14 with four exons and 12 with five exons). However, VvHB24 has only one exon, VvHB55 has 14 exons, and VvHB42 has 23 exons, suggesting that during the evolution of the VvHB gene family, both exon loss and gain have occurred. This phenomenon may result in functional diversity of closely related genes. In addition, there was an obvious correlation between the phylogeny and exon/intron structure. Within the same family, VvHB genes tended to exhibit similar numbers of exons. For example, the number of exons in HD-ZIP III group was relatively large, ranging from eighteen to nineteen, while genes in WOX family exhibited a relatively small number, ranging from one to four. This similarity of exon pattern may be ascribed to a large number of gene duplications. Particularly, in the DDT group, three of the four members have a larger number: VvHB36 has 19 exons, VvHB71 has 18 exons and VvHB42 has 23 exons. In contrast, VvHB26 has only nine exons, potentially a result of special variations in evolution.
Domain architecture analysis of grape HB proteins
Plant HB proteins can be divided into 14 distinct families with robust (generally 70% or more) bootstrap support12. We found that the VvHB proteins represented 11 of these families. A schematic diagram of the domain architecture of representatives of each of these families was shown in Fig. 3. The category and distribution of these typical structural domains identified in grape were similar to previous studies in other plants such as Arabidopsis, poplar, maize and rice12. This result showed that these structural domains have been highly conserved in plant evolution.
Diagrammatic representation of the characteristic domains of representatives of the 11 subfamilies of grape HB proteins. The following domains and motifs are indicated: HD (homeodomain), LZ (leucine-zipper), ZIBEL motif, CPSCE motif, CESVV motif, START domain, HD-SAD domain (HD-START associated domain), MEKHLA domain, WUS Box, DDT domain, DDT domain, PHD domain, SAWADEE domain, PINTOX domain, POX domain, KNOX domain (1 and 2), ELK motif. Characteristic motifs and domains were identified using SMART and MEME software.
Similar to other plants, the grape HD-ZIP superfamily contains most of the members (33 in our alignment). The HD-ZIP superfamily comprises four individual families, HD-ZIP I, HD-ZIP II, HD-ZIP III, and HD-ZIP IV. All of these are characterized by the presence of a leucine zipper motif, which may mediate protein-protein interactions. The CPSCE motif in HD-ZIP II proteins is found immediately adjacent to the leucine zipper, and plays a role as a redox sensor29. HD-ZIP II proteins also include a ZIBEL motif, which has been shown to be important for interaction between HD-Zip II proteins and BEL HD proteins12. The HD-ZIP III and HD-ZIP IV classes both contain a START (STeroidogenic Acute Regulatory protein–related lipid Transfer)30 domain and a HD-SAD (START associated conserved domain)31,32. HD-ZIP III proteins are distinguished from HD-ZIP IV by virtue of a conserved MEKHLA domain at the carboxyl terminus.
Grape contains 11 members of the WOX class. WOX proteins have an HD of 68 amino acids, which show one or two extra residues between alpha-helices one and two, and four to five extra residues between alpha-helices two and three (Supplementary Fig. S1). WOX class proteins also contain a WUS-box motif at a position carboxyl-terminal to the HD, as well as an acidic amino acid stretch between the HD and WUS-box33.
Four HB proteins belong to the DDT class, which contain a DDT domain located carboxyl-terminal to the HD34. This domain can be divided into eight additional conserved motifs, D-TOX A to H35.
HB proteins containing a PHD domain have been most commonly characterized as pathogenesis-related. Examples include the Pathogenesis-Related HB gene A (PRHA)36 and HAT3.1 genes37 from Arabidopsis. The PHD domain is several hundred amino acids long and is located amino-terminal to the HD. There are two HB genes encoding HB-PHD proteins in both grape and Arabidopsis.
Members of the SAWADEE class, which are represented by only a single protein in grape, contain a 130-140 amino acids, conserved region located carboxyl-terminal to the HD. This class is characterized by the presence of a 10 amino acid insertion between the second and third alpha-helix (Supplementary Fig. S1). The SAWADEE domain includes several conserved cysteine and histidine residues, suggesting that it participates in metal binding12.
PINTOX-class HB proteins, also represented by only a single gene in grape, contain a ~70-amino acids, highly conserved, basic domain located carboxyl-terminal to the HD.
The KNOX and BEL family HB proteins belong to the TALE superfamily, represented by 21 genes in grape. TALE proteins are distinguished by three extra amino acid residues between the first and second helix of the HD38,39 (Supplementary Fig. S1). The KNOX family comprises two subfamilies, KNOX I and KNOX II8. Proteins included in these families exhibit a conserved KNOX domain amino-terminal to the HD39. In addition, they contain a short motif designated ELK immediately adjacent to the HD2.
The BEL class is characterized by a conserved, 10-amino acids motif designated ZIBEL, located carboxyl-terminal and N-terminal of the BEL class, as well as in the HD-ZIP II class. Furthermore, we identified a POX domain, located amino-terminal to the HD.
Expansion patterns of HB genes in grape
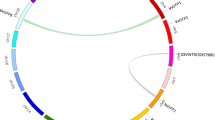
Tandem (two or more genes located on the same chromosome) and segmental duplications (duplicated genes present on different chromosomes), identified based on chromosomal locations, lead to expansion of gene families40. In order to gain a better understanding about the evolutionary relationships among members of the grape HB superfamily, the chromosomal locations of the 73 VvHB genes were determined. VvHB genes are distributed unevenly over 19 of the 20 grape chromosomes (none exists on chromosome 5). For example, 10 VvHB genes are located on chromosome 4, whereas only a single VvHB gene presents on chromosome 7 (Fig. 4). A chromosomal region within 200 kb containing two or more genes implicates a tandem duplication event40. Using this definition, a total of six tandem duplication events were identified (VvHB1/VvHB2, VvHB20/VvHHB21, VvHB36/VvHB37, VvHB63/VvHB64, VvHB67/VvHB68, and VvHB69/VvHB70), on chromosomes 1, 4, 10, 18, 18 and 19, respectively (Supplementary Table S3). Interestingly, on chromosomes 1, 10, 18 and 19, the VvHB genes occur in tandem duplications, however belong to different subfamilies, suggesting that following tandem duplications, sequences of these genes have been altered to a large extent, and protein diversification can be obtained through the reorganization of domains. Therefore, these genes may have lost their original functions or gained new ones after tandem duplications.
Synteny analysis and chromosomal distribution of grape HB genes. Locations of the grape genes are indicated by orange lines on the grape chromosomes. Colored bars connecting two chromosomal regions denote syntenic regions and the corresponding genes on two chromosomes were regarded as segmental duplications. Chr, chromosomes.
Furthermore, as shown in Supplementary Table S4, we also identified 15 pairs of VvHB genes associated with segmental duplications (VvHB48/VvHB32, VvHB59/VvHB3, VvHB58/VvHB4, VvHB57/VvHB2, VvHB66/VvHB17, VvHB67/VvHB10, VvHB55/VvHB53, VvHB51/VvHB5, VvHB40/VvHB15, VvHB47/VvHB23, VvHB33/VvHB13, VvHB7/VvHB54, VvHB7/VvHB56, VvHB42/VvHB36 and VvHB54/VvHB56). In addition, each pair of VvHB genes involved in segmental duplications belongs to the same subfamily. Combining this observation with the results of phylogenetic analyses, it appears that some of these gene pairs (such as VvHB48/VvHB32, VvHB59/VvHB3, VvHB58/VvHB4, VvHB57/VvHB2 and VvHB66/VvHB17), clustering in the same phylogenetic subclade (Fig. 2a), may have the same origin. In summary, half of the VvHB genes participated in either segmental or tandem duplication events which may provide a reference for the evolutionary relationship and functional prediction of VvHB genes.
Evolutionary relationships between grape and Arabidopsis HB genes
The function of HB genes has been most extensively studied in Arabidopsis. To further clarify the origin, evolutionary process and potential function of grape HB genes we created a comparative synteny map between grape and Arabidopsis HB genes. A total of sixty-four pairs of syntenic relationships were identified, including 54 AtHB genes and 39 VvHB genes (Fig. 5). The large number of syntenic events suggested that a large-scale expansion have occurred before the divergence of Arabidopsis and grape. Among these pairs, 17 were single, grape/Arabidopsis HB gene correspondences, such as VvHB61-AT1G20700, VvHB43-AT1G62360, VvHB30-AT5G02030 and VvHB19-AT2G17950. This indicates these genes should have been in the genome of the last common ancestor of grape and Arabidopsis. There were 19 pairs where a single grape gene corresponded to more than one Arabidopsis gene, such as VvHB62-AT4G40060/AT5G65310, VvHB52-AT3G60390/AT4G16780/AT4G17460/AT2G44910/AT5G47370, VvHB18-AT4G36740/AT5G66700 and VvHB55-AT4G25530/AT5G52170. In contrast, nine pairs of grape HB genes were found that corresponded to only one Arabidopsis HB gene, such as VvHB7/VvHB54/VvHB56-AT2G46680, VvHB40/VvHB15-AT4G32040, VvHB51/VvHB5-AT1G26960 and VvHB62/VvHB27-AT5G65310 (Supplementary Table S5).
Additionally, relating these syntenies to the phylogenetic analysis (Fig. 1), we discovered each pair of genes from grape and Arabidopsis belongs to the same subfamily (Supplementary Table S5), some of these gene pairs (such as VvHB36-AT1G28420, VvHB8-AT1G62990, VvHB1-AT2G01430, VvHB19-AT2G17950, VvHB27-AT2G22430, VvHB64-AT2G22800, VvHB17-AT2G23760 and VvHB22-AT2G27990) even cluster in the same phylogenetic subclade, suggesting that many VvHB genes share a common ancestor with their Arabidopsis counterpart, and that they may have the closest genetic relationship. These results could provide a reference for possible functional relevance of VvHB genes.
Expression patterns of grape HB genes in different tissues
The known functions of HB genes in other plants suggest that HB genes may play important roles in grape growth and development. Previous transcriptome analysis of grape ovules/seeds at three developmental stages, comparing seedless and seeded grape varieties, identified a large number of differentially expressed genes25. Based on transcriptome data (Supplementary Table S6), many grape HB genes showed higher expression levels in seedless grape cultivars relative to seeded cultivars. On this basis, we chose 33 differentially expressed VvHB genes for futher analysis. To gain insight into the putative functions of VvHB genes in grape growth and development, semi-quantitative RT-PCR was carried out to evaluate expression patterns of these 33 grape HB genes, in six distinct grape tissues (root, stem, leaf, flower, tendril and fruit) of the seedless cultivar ‘Thompson Seedless’ and seeded cultivar ‘Red Globe’ (Fig. 6a and Supplementary Fig. S3). Additionally, a total of 12 VvHB genes (9 differentially expressed genes and 3 randomly selected genes) were selected to corroborate their expression levels through quantitative real-time RT-PCR analysis (Fig. 6b).
Tissue-specific expression analysis of grape HB genes. Seedless grape cultivar ‘Thompson Seedless’ is denoted as ‘T.S’ and seeded grape cultivar ‘Red Globe’ is denoted as ‘R.G’. (a) Semi-quantitative RT-PCR analysis. For each gene, yellow and blue color scale indicates high and low expression levels respectively. Transcripts were normalized to the expression of the ACTIN1 gene and EF1-α gene, and results are shown in Supplementary Figs S3 and S5. Semi-quantitative RT-PCR was analyzed using GeneTools software, and expression values were normalized based on the mean expression value of each gene in all tissues. The heat map was analyzed using MeV 4.8 software. (b) Real-time PCR validation of twelve genes (9 differentially expressed genes and 3 randomly selected genes) expressed in different tissues. Transcripts were normalized to the expression of the ACTIN1 gene; the mean ±SD of three biological replicates is presented.
We found that all the 33 VvHB genes were expressed in at least one of the six grape tissues, and the expression levels of most VvHB genes (like VvHB40 and VvHB50) varied among the tissues tested, or varied strikingly between ‘Thompson Seedless’ and ‘Red Globe’. Specially, VvHB37 showed low expression levels in all tissues of ‘Thompson Seedless’ but a moderate level in fruits of ‘Red Globe’. On the contrary, VvHB23 and VvHB21 showed high expression levels in most of the tissues. VvHB61 exhibited predominant expression in reproductive tissues of ‘Red Globe’. In addition, several genes showed distinct, tissue-specific expression, including VvHB49 which showed a low expression level in leaves in both ‘Thompson Seedless’ and ‘Red Globe’, and VvHB63 which was not expressed in leaves and fruits in both cultivars. This phenomenon may be due to some species-specific variations. Several genes (such as VvHB27, VvHB40, VvHB58 and VvHB59) exhibited similar expression levels in both ‘Thompson Seedless’ and ‘Red Globe’. This may showed that some genes exhibited conserved expression patterns between the two cultivars. Taken together, these results revealed both similarities and differences in expression patterns between two cultivars.
Combining these results with syntenic blocks and phylogeny reconstructions, we found that some homologous and segmental duplication genes (such as VvHB40 and VvHB15) showed a similar expression pattern, a relatively weak expression level in all tissues. However, other segmental duplication genes showed converse expression patterns. For instance, for the gene pair VvHB53/VvHB55, VvHB53 showed relatively strong expression in most organs, while its homolog VvHB55 exhibited low expression in almost all tissues. For the gene pair VvHB54/VvHB7, VvHB54 was expressed with obviously higher levels in most tissues. The divergences in expression patterns between homologous and segmental duplication genes indicated that some of them may lose function or obtain new function after polyploidy and duplication in evolutionary process.
Expression patterns of grape HB genes during ovule/seed development
To further identify potential roles for VvHB genes in ovule development, semi-quantitative RT-PCR was carried out to analyze the expression of these 33 selected VvHB genes during ovule development at 27, 30, 33, 36, 39 and 42 days after flowering (DAF), in ‘Red Globe’, another seeded cultivar, ‘Kyoho’, ‘Thompson Seedless’, and additional seedless cultivar, ‘Flame Seedless’ (Fig. 7a and Supplementary Fig. S4). A subset of 12 VvHB genes (9 differentially expressed genes and 3 randomly selected VvHB genes) were selected to corroborate the results of the semi-quantitative RT-PCR through quantitative real-time RT-PCR analysis (Fig. 7b).
Expression profiles of grape HB genes during ovule development in four grape cultivars. Two seedless grape cultivars, ‘Thompson Seedless’ and ‘Flame Seedless’, are denoted as ‘T.S’ and ‘F’ respectively. Two seeded grape cultivars, ‘Red Globe’ and ‘Kyoho’, are denoted as ‘R.G’ and ‘K’ respectively. (a) Semi-quantitative RT-PCR analysis. For each gene, yellow and blue color scale indicates high and low expression levels, respectively. Numbers indicate the number of days after full bloom (DAF). Transcripts were normalized to the expression of the ACTIN1 gene and EF1-α gene and the results are shown in Supplementary Figs S4 and S5. Semi-quantitative RT-PCR measurements were analyzed using GeneTools software, and data was normalized based on the mean expression value of each gene in all development stages. The heat map was analyzed using MeV 4.8 software. (b) Real-time PCR validation of twelve genes (9 differentially expressed genes and 3 randomly selected genes) expressed during ovule development in four grape cultivars. Transcripts were normalized to the expression of the ACTIN1 gene; the mean ± SD of three biological replicates is presented.
As shown in Fig. 7, several genes (such as VvHB59, VvHB40, VvHB50 and VvHB58) showed similar expression levels in different grape cultivars and displayed no obvious changes in different ovule developmental stages, suggesting that these genes are functionally conserved in all grape cultivars and may be crucial for ovule development. However, most of the VvHB genes, including VvHB62, VvHB63, VvHB25, VvHB7, VvHB53, VvHB49, VvHB54, VvHB32, VvHB18, VvHB37 and VvHB56, exhibited higher expression levels in seedless cultivars relative to seeded cultivars. In particular, VvHB62, VvHB56 and VvHB54 showed remarkable differences in the expression patterns during ovule development between seeded and seedless grape cultivars, with expression almost undetectable in seeded cultivars. These results indicated that these genes may participate in ovule abortion. In contrast, two genes, VvHB38 and VvHB17, showed higher expression levels in seeded cultivars relative to seedless cultivars, suggesting that they may be related to the normal ovule development process. Taken together, we propose that there is a close relationship between VvHB genes and grape stenospermocarpy.
Discussion
HB gene family members play varied and important roles in plants including in embryogenesis17, response to ABA41 and abiotic stress42,43. Many HB TFs have been analyzed in a variety of plants, such as Arabidopsis12, poplar12, rice44 and legumes35. However, there have been few studies of HB genes in grape. In the present study, we identified a total of 73 grape HB genes and analyzed their evolutionary relationships, domain architectures and potential functions. Furthermore, we analyzed the expression profiles of 33 key VvHB genes in various grape organs and ovule developmental stages to clarify the importance of the VvHB genes in the growth and development of grapes.
HB genes can be found in diverse plant species, including angiosperms, bryophyta and lycophytes12. The HD consists of an alpha- helix I which is 12 residues length, a connecting loop of 6 amino acid residues leading to helix II (11 aa length), a tight turn of three residues, a helix III (11 aa length) and a flexible and disordered 7 aa length helix IV45. We found that almost all of the 73 grape HB genes encode proteins with a complete HD, with the exception of VvHB54, in which the first alpha-helix is absent. Previous studies have indicated that helices II and III form a helix-turn-helix motif that is required for regulatory function of the proteins46. This means that the second and the third helices may be the most important and necessary, and may similarly influence the functions of eukaryotic genes. Additionally, we found that VvHB54 showed substantial expression levels in various organs, suggesting that it is not a pseudogene.
During the evolution process of land plants, gene duplication, including tandem duplications, segmental duplications and whole genome duplications (WGDs), has played an important role in genomic expansions and rearrangements47,48. Whole genome duplications have recurred in many lineages of the angiosperms49, leading to remarkable differences in genome sizes. Synteny and collinearity analyses of plant genomes indicated that an ancient genome triplication (γ-triplication) event occurred in the common ancestor of grape and Arabidopsis, leading to a paleohexaploid50. Following the γ-triplication event, two recent paleopolyploidy events, β-duplications and α-duplications occurred in Arabidopsis. However in grape, there was only the common γ-triplication event and no subsequent WGDs27. The ancient polyploidization events could influence the number of genes in multiple gene families, through gene loss or expansions51,52. These evolutionary relationships might lead to the expansion of ancestral genes in Arabidopsis. Therefore the number of HB genes between Arabidopsis and grape showed a relatively large difference. Because the grapevine genome had not undergone the recent whole genome duplications, tandem duplications and segmental duplications would be the main causes of gene family expansions in grape. In this study, a total of 21 gene pairs from grape HB genes were identified as products of tandem or segmental duplication events (Fig. 4 and Supplementary Tables S3 and S4), consistent with findings in soybean whereby a total of 246 (89.1%) HB genes were found located on duplicated chromosomal blocks35, suggesting that tandem and segmental duplications likely played a crucial role in the expansion of the HB gene family in plants. Combining synteny analysis with phylogenetic analysis, we found most of the grape HB genes that clustered in the same phylogenetic subclade were segmental or tandem duplications. Some orthologous gene pairs, such as VvHB56/VvHB7 and VvHB40/VvHB15, resulting from segmental duplications, showed similar expression patterns during ovule development stages. Additionally, expression patterns of these orthologous gene pairs were more similar than other orthologous pairs that only clustered together in the phylogenetic tree or were syntenic orthologs. However, regarding other segmental duplication orthologous gene pairs, such as VvHB55/VvHB53, showed the distinctly different transcript levels during ovule development stages. It seems possible that high sequence similarity is not necessarily correlated with similar transcript levels, because they may perform similar biochemical functions in different tissues or periods during plant growth and development. Differences in gene expression pattern were considered to arise through duplication in the evolutionary process. Functional diversification of these segmental duplication orthologous genes was also considered a major feature of the long-term evolution of polyploids53. Following gene duplication, functional differentiation may result from pseudogenization, conservation of gene function, non-functionalization and sub-functionalization54. Additionally, we also identified 64 gene pairs involved in segmental duplications between grape and Arabidopsis HB genes, suggesting they might have a common ancestor. Based on the reported function of their Arabidopsis homologs, we can speculate the possible functions of these orthologous pairs in grape. Taken together, these results enable further analyses of evolution and potential functions among these genes, and are useful for further study on the functions of homologous genes in different plant species.
Interestingly, we found highly variable numbers of genes on different chromosomes. Chromosome 4 contained 10 VvHB genes while chromosome 5 had none. These results indicated that duplication of VvHB genes likely occurred in chromosome 4 during the evolution of gene families, which might be associated with gene functions.
During the evolutionary process, various organisms had been participated in gene duplication events, after which gene loss and gene sub-functionalization can occur frequently. Gene loss is a common phenomenon during the evolution of the multigene families. Following gene loss, neo-functionalization and sub-functionalization contributed to the retention of new genes. In this study, gene loss observed in the PLINC, NDX and LD clades is expected to decrease functional redundancy and define the key VvHB genes55. Alternatively, our data is not inconsistent with these three groups representing Brassicaceae-specific HB gene lineages.
In this study, in order to further define the evolutionary relationship within grape HB genes, we identified 20 highly conversed motifs among the 73 HB proteins. Different motifs were conserved within every group, and motifs within each subgroup tended to exhibit a similar distribution pattern. These results supported the phylogenetic relationship between different HB genes in grape (Fig. 2a). In addition, these motifs were also detected in Arabidopsis, and the distribution pattern was similar between grape and Arabidopsis, suggesting that these motifs were highly conserved during evolution, which could provide a possible functional relevance. The evolution of gene family members could also be analyzed by exon/intron diversification, which includes exon/intron gain/loss, insertion/deletion and exonization/pseudoexonization56. In this study, the 73 VvHB genes exhibited between 0 and 22 introns. A total of 62 of the genes (85%) have zero to ten introns, whereas only 11 genes have more than 10 introns. Overall, genes within the same group had similar numbers of introns. However, there were exceptions: VvHB17, which had twelve exons, is orthologous to VvHB66 which has only five exons. In general, the gain/loss of the exons or introns may be the main reason for the chromosomal rearrangement and fusion25 and the differences of the functions of the grape HB proteins, contributing to functional diversification of these genes. Furthermore, divergence in exon/intron length could also potentially lead to the generation of functionally distinct paralogs or orthologous. Taken together, the grape HB genes show highly similar structures of exons/introns and conserved motifs distribution in the same subgroup.
The HD-ZIP subfamily is the largest group of HB proteins in grape, and contains HD-ZIP I, HD-ZIP II, HD-ZIP III, and HD-ZIP IV. The number of genes in HD-ZIP I (13) is similar to that in Arabidopsis (17) (Supplementary Table S1). Interestingly, genes in HD-ZIP III are longer and contain more exons (Fig. 2c). The number of genes in HD-ZIP IV is smaller than that in Arabidopsis, suggesting that genes included in the HD-ZIP IV subfamily may not be strongly conserved. Arabidopsis AtHB8 belongs to HD-ZIP III, was expressed in procambial cells of the embryo and developing organs57, and played a role in vascular development and differentiation58. AtHB8 is clearly homologous to grape VvHB13, suggesting that VvHB13 may have the similar function as its Arabidopsis counterpart. In addition, VvHB23, which belongs to this subgroup, showed differential expression during ovule development between seedless and seeded grape varieties. Other subfamilies of HB genes also have been demonstrated to be important. Genes in the WOX subfamily have been reported to play an important role in early embryogenesis in Arabidopsis33. Moreover, WOX family genes have been shown to play a key role in coordinating gene transcription involved in the early phases of embryogenesis in grape. In a previous study, the VvWOX genes showed different expression profiles during somatic embryogenesis59. During somatic embryogenesis, VvWOX2 and VvWOX9 were the most important WOX genes, while VvWOX3 and VvWOX11 showed strong expression in the torpedo and cotyledonary stages, but weak expression in the earlier developmental stages. In this study, VvHB37, which belongs to the WOX subfamily, showed dramatically higher expression levels during different development stages of ovules in seedless grape cultivars (Fig. 7b). Consistent with previous research, these results suggest that this subfamily may play an important role in embryogenesis. In addition, the WOX family genes in grape also showed various expression profiles in different grapevine tissues60, which has also been found in this study. The TALE family includes two families, KNOX and BELL, and the KNOX family is composed of the smaller KNOX I and KNOX II families, which are highly conserved between monocots and dicots61. Additionally, members of the small PHD family have been shown to participate in chromatin-mediated transcriptional regulation62.
HB genes have been widely studied in legumes35 and tomato15, and their expression profiles and potential functions during development have been documented. In a recent study, a tomato HD-ZIP I family gene designated LeHB1 was shown to play key roles in carpel development and fruit maturation15. MdHB1, the homologous gene of LeHB1 in apple, has been reported to be involved in the regulation of anthocyanin accumulation63. In our study, some VvHB genes (VvHB62, VvHB54 and VvHB7) belonging to the HD-ZIP I subfamily showed markedly differential expression during ovule development between seedless and seeded grape. Compared to climacteric fruit, such as tomato and apple, there are relatively few studies of non-climacteric fruits like grape. Moreover, the characterization of Arabidopsis HB genes allows for prediction of potential functions of the VvHB genes. Besides HB genes, there are many other genes associated with seed morphogenesis in grapevine. For example, VviAGL11, a class D MADS-box transcription factor, exhibited relatively high expression levels in seeds at 2, 4 and 6 weeks after fruit set, relative to seedless grape at any developmental stage64. The metacaspase gene family of V. vinifera, containing six genes, also showed differential expression profiles during ovule abortion in stenospermocarpic seedless grapes65. Moreover, there were some other genes in different plant species also related to ovule development. In petunia, the floral binding protein 11 (FBP11) MADS box gene was found only expressed in ovule primordia and subsequently showed a role in ovule formation66.
We documented the expression of 33 VvHB genes in six organs and six developmental stages of the ovule in distinct grape cultivars, revealing their potential functions in grape growth and development. For example, VvHB37, VvHB62 and VvHB63 showed extremely low expression levels in nearly all ovule developmental stages in seeded grape cultivars, but were expressed to relatively high levels in seedless grape cultivars (Fig. 7b), suggesting a role in ovule abortion of seedless grape cultivars. In addition, as demonstrated in a previous study25, a substantial number of the 33 selected genes, such as VvHB25, VvHB7, VvHB53, VvHB49, VvHB54, VvHB32 and VvHB18, showed higher expression levels in seedless grape cultivars relative to seeded grape cultivars. We also identified a subset of genes, such as VvHB38 and VvHB17, which showed opposite expression profiles in ovules between seeded and seedless grape cultivars, being expressed more strongly in ovules of seeded grape cultivars (Fig. 7a). This suggests that these two genes may have a function in normal seed development. These results will facilitate the functional analysis of these genes and provide new resources for molecular breeding of seedless grapes. Of course, future studies may address how these HB genes may contribute to the seedless trait.
Interestingly, we found that three genes, VvHB62, VvHB63 and VvHB56, showed low expression levels in fruits of ‘Thompson Seedless’, but high expression levels in ovules. In order to illustrate this phenomenon, the sarcocarp was separated from fruits (at 42 days post anthesis) and ovules (42 days after full bloom) of ‘Thompson Seedless’ and analyzed by real-time RT-PCR (Supplementary Fig. S6). The results showed that these three genes were expressed to lower levels in the sarcocarp than in ovules, consistent with our previous results (Fig. 6 and Fig. 7). Further study can be taken to clarify the reasons that cause these situations.
Materials and Methods
Identification and annotation of the grape HB gene family
HB genes in Arabidopsis were as identified in a previous study12. Homology with those identified Arabidopsis HB genes was assessed using the NCBI non-redundant protein database. Protein sequence was analyzed for domain structure using PFAM. HB genes in grape were identified by using Hidden Markov Model (HMM) profiles and BLAST-P to search the Grape Genome Database (http://www.genoscope.cns.fr). Sequence integrity of the HD (PF00046) was analyzed using SMART (http://smart.embl-heidelberg.de), and only domains with Expect (e)-values less than ±1e-05 were retained for further analysis35. Protein sequences and coding sequences were retrieved from the Grape Genome Database, and SMART software was used to analyze domain structure.
Multiple sequence alignment and phylogenetic analysis of the grape HB gene
Multiple sequence alignments of a total of 73 grape HD sequences and 110 Arabidopsis HD sequences12 were performed using ClustalX 2.1 with default parameters67. The phylogenetic tree including sequences from grape and Arabidopsis was constructed using the neighbour-joining (NJ) method68,69 and MEGA 5.0 software. Bootstrap analysis was performed using 1000 replicates70 with the following parameters: “p-distance”, “Complete Deletion” and gap setting.
Exon-intron structure analysis, distribution of conserved motifs and characteristic domain architecture in grape HB genes
The exon-intron structures of the grape HB genes were determined based on alignments of transcribed sequences and corresponding genomic sequences, and diagrams were created using the online Gene Structure Display Server 2.071, which showed upstream/downstream sequence, exon/intron position. Conserved motifs and domains of grape HB genes other than the HD were identified using MEME 4.11.2 (http://meme-suite.org/tools/meme) and SMART (http://smart.embl-heidelberg.de) software.
Synteny analysis of grape HB genes
Tandem and segmental duplications of HB genes in the grape genome were identified based on chromosomal locations. For synteny analysis, adjacent homologous grape HB genes on a single chromosome, without the presence of intervening genes, were defined as tandem duplications. Gene duplication events occuring on different chromosomes were defined as segmental duplications47. The list of grape HB genes in duplicated genomic regions, and a comparison of grape and Arabidopsis genomes, were obtained from the Plant Genome Duplication Database72. Diagrams were generated using the program Circos version 0.63 (http://circos.ca/).
Plant material
Grapevine cultivars used in this study included two seeded grapevines, Red Globe (Vitis vinifera) and Kyoho (V. vinifera×V. labrusca), and two seedless grapevines, Thompson Seedless (V. vinifera) and Flame Seedless (V. vinifera). Plants were maintained in the grape germplasm resource orchard of Northwest A&F University, Yangling, China (34°20′N 108°24′E). The plant parts collected were young roots, stems, leaves, flowers (at full bloom), tendrils, fruits (42 days after full bloom (DAF). All tissues samples were obtained from 3-year-old ‘Thompson Seedless’ and ‘Red Globe’ plants under natural conditions. Previous studies have reported that seed weight of seedless grapes usually begin to decrease at 27 ~ 33 days after full bloom (DAF)25,73. Therefore, ovules samples were collected from berries at 27 DAF, 30 DAF, 33 DAF, 36 DAF, 39 DAF and 42 DAF from two seeded grape cultivars, ‘Kyoho’ and ‘Red Globe’, and two seedless grape cultivars, ‘Thompson Seedless’ and ‘Flame Seedless’. Additionally, all grape samples were immediately frozen in liquid nitrogen and stored at −80 °C for RNA extraction and expression analysis.
Expression analysis of grape HB genes
Total RNA was extracted from grape samples using an EZNA Plant RNA Kit, according to the manufacturer’s instructions (R6827-01, OMEGA Biotek, USA). A total of 500 ng DNase-treated RNA was subjected to reverse transcription to generate cDNA, using PrimeScript RTase (TransGen Biotech, Beijing, China). Resulting cDNA products were diluted six-fold and stored at −40 °C prior to analysis. We used the grape ACTIN1 gene (GenBank Accession number NC_012010) with the forward primer (5′-GAT TCT GGT GAT GGT GTG AGT-3′) and reverse primer (5′-GAC AAT TTC CCG TTC AGC AGT-3′), and the grape EF1-α gene (GenBank Accession number NC_012012) with the forward primer (5′-AGG AGG CAG CCA ACT TCA CC-3′) and reverse primer (5′-CAA ACC CTG CAT CAC CAT TC-3′) as internal controls.
Gene-specific primers were designed for 33 grape HB genes (Supplementary Table S7) using Primer Premier 6.0. Semi-quantitative RT-PCR was carried out in a volume of 20 μl per reaction containing 1 μl of cDNA template, 1.6 μl of gene-specific primers (1.0 μM), 7.6 μl sterile distilled water and 9.8 μl PCR Master Mix (BIOSCI BIOTHCH CO. LTD, Hangzhou, China). The profile was 94 °C for 2 min, 29–38 cycles of 94 °C for 30 s, 52–63 °C for 30 s and 72 °C for 30 s, with a final extension of 72 °C for 2 min. In each case, 5 μl of the resulting semi-quantitative RT-PCR product was resolved on a 1.5% (w/v) agarose gel, dyed with ethidium bromide, and then photographed under ultraviolet light using GeneSnap software. Each assay was performed with three biological replicates. RT-PCR expression data was visualized using GeneTools software, and the normalized data based on the mean expression value of each gene73 in all tissues or ovule developmental stages in all cultivars was log2-transformed to generate heat-maps using MultiExperiment Viewer software (Mev 4.8.1)72.
Quantitative real-time PCR analysis was conducted using SYBR Green (TransGen Biotech, Beijing, China) on an IQ5 real time-PCR machine (Bio-Rad, Hercules, CA, USA). The grape ACTIN1 gene (GenBank Accession number NC_012010) was used as the internal reference genes and each reaction was carried out in triplicate with a volume of 20 μl containing 1 μl of cDNA template, 0.8 μl each primer (1.0 μM), 7.4μl sterile distilled water and 10 μl of SYBR green. PCR was performed following the parameters: 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Melting-curve analyses were performed with an intial incubation at 95 °C for 15 s and then a constant increase from 60 °C to 95 °C. For each assay, three independent biological replications were performed. Relative expression levels were analyzed using the IQ5 software and the normalized expression method73, visualized using SigmaPlot 10.0.
Change history
17 November 2017
A correction to this article has been published and is linked from the HTML version of this paper. The error has been fixed in the paper.
References
Garber, R. L., Kuroiwa, A. & Gehring, W. J. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. EMBO J. 2, 2027–2036 (1983).
Vollbrecht, E., Veit, B., Sinha, N. & Hake, S. The developmental gene Knotted-1 is a member of a maize HB gene family. Nature. 350, 241–243 (1991).
Derelle, R., Lopez, P., Le Guyader, H. & Manuel, M. Homeodomain proteins belong to the ancestral molecular toolkit of eukaryotes. Evol Dev. 9, 212–219 (2007).
Ruberti, I., Sessa, G., Lucchetti, S. & Morelli, G. A novel class of plant proteins containing a homeodomain with a closely linked leucine zipper motif. EMBO J. 10, 1787–1791 (1991).
Matsuoka, M. et al. Expression of a rice homeobox gene causes altered morphology of transgenic plants. Plant Cell. 5, 1039–1048 (1993).
Müller, K. J. et al. The barley Hooded mutation caused by a duplication in a homeobox gene intron. Nature. 374, 727–730 (1995).
Nam, J. & Nei, M. Evolutionary change of the numbers of homeobox genes in bilateral animals. Mol Biol Evol. 22, 2386–2394 (2005).
Kerstetter, R. et al. Sequence analysis and expression patterns devide the maize knotted-like homeobox gene into two classes. Plant Cell. 6, 1877–1887 (1994).
Gehring, W. J., Affolter, M. & Burglin, T. Homeodomain proteins. Annu Rev Biochem. 63, 487–526 (1994).
Desplan, C., Theis, J. & O’Farrell, P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 54, 1081–1090 (1988).
Otting, G. et al. Protein-DNA contacts in the structure of a homeodomain-DNA complex determined by nuclear magnetic resonance spectroscopy in solution. EMBO J. 9, 3085–3092 (1990).
Mukherjee, K., Brocchieri, L. & Bürglin, T. R. A comprehensive classification and evolutionary analysis of plant homeobox genes. Mol Biol Evol. 26, 2775–2794 (2009).
Sawa, S. et al. The HAT2 gene, a member of the HD-Zip gene family, isolated as an auxin inducible gene by DNA microarray screening, affects auxin response in Arabidopsis. Plant J. 32, 1011–1022 (2002).
Henriksson, E. et al. Homeodomain leucine zipper class I genes in Arabidopsis. Expression patterns and phylogenetic relationships. Plant Physiol. 139, 509–518 (2005).
Lin, Z. et al. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J. 55, 301–310 (2008).
Abe, M., Katsumata, H., Komeda, Y. & Takahashi, T. Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development. 130, 635–643 (2003).
Prigge, M. J. et al. Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell. 17, 61–76 (2005).
Schena, M. & Davis, R. W. Structure of homeobox leucine zipper genes suggests a model for the evolution of gene families. Proc Natl Acad Sci USA 91, 8393–8397 (1994).
Hofer, J., Gourlay, C., Michael, A. & Ellis, T. H. Expression of a class I knotted1-like homeobox gene is down-regulated in pea compound leaf primordia. Plant Mol Biol. 45, 387–398 (2001).
Nagasaki, H., Sakamoto, T., Sato, Y. & Matsuoka, M. Functional analysis of the conserved domains of a rice KNOX homeodomain protein, OSH15. Plant Cell. 13, 2085–2098 (2001).
Belles-Boix, E. et al. KNAT6: an Arabidopsis homeobox gene involved in meristem activity and organ separation. Plant Cell. 18, 1900–1907 (2006).
Mele, G., Ori, N., Sato, Y. & Hake, S. The knotted 1-like homeobox gene BREVIPEDICELLUS regulates cell differentiation by modulating metabolic pathways. Genes Dev. 17, 2088–2093 (2003).
Reiser, L. et al. The BELL1 gene encodes a homeodomain protein involved in pattern formation in the Arabidopsis ovule primordium. Cell. 83, 735–742 (1995).
Byrne, M. E., Groover, A. T., Fontana, J. R. & Martienssen, R. A. Phyllotactic pattern and stem cell fate are determined by the Arabidopsis homeobox gene BELL-RINGER. Development. 130, 3941–3950 (2003).
Wang, L. et al. Transcriptome analyses of seed development in grape hybrids reveals a possible mechanism influencing seed size. BMC Genomics. 17, 898 (2016).
Finn, R. D. et al. The Pfam protein families database. Nucleic Acids Res. 38, D211–D222 (2010).
Jaillon, O. et al. The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature. 449, 463–467 (2007).
Zhang, Y. et al. Genome-wide identification and analysis of grape aldehyde dehydrogenase (ALDH) gene superfamily. PLoS ONE. 7, e32153 (2012).
Chan, R. L., Gago, G. M., Palena, C. M. & Gonzalez, D. H. Homeoboxes in plant development. Biochim Biophys Acta. 1442, 1–19 (1998).
Ponting, C. P. & Aravind, L. START: a lipid-binding domain in StAR, HD-ZIP and signalling proteins. Trends Biochem Sci. 24, 130–132 (1999).
Schrick, K., Nguyen, D., Karlowski, W. M. & Mayer, K. F. START lipid/sterol-binding domains are amplified in plants and are predominantly associated with homeodomain transcription factors. Genome Biol. 5, R41 (2004).
Mukherjee, K. & Bürglin, T. R. MEKHLA, a novel domain with similarity to PAS domains, is fused to plant homeodomain-leucine zipper III proteins. Plant Physiol. 140, 1142–1150 (2006).
Haecker, A. et al. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development. 131, 657–668 (2004).
Doerks, T., Copley, R. & Bork, P. DDT-a novel domain in different transcription and chromosome remodeling factors. Trends Biochem Sci. 26, 145–146 (2001).
Bhattacharjee, A., Ghangal, R., Garg, R. & Jain, M. Genome-Wide Analysis of Homeobox Gene Family in Legumes: Identification, Gene Duplication and Expression Profiling. PLoS One. 10, e01191982015 (2015).
Plesch, G., Stormann, K., Torres, J. T., Walden, R. & Somssich, I. E. Developmental and auxin-induced expression of the Arabidopsis prha homeobox gene. Plant J. 12, 635–647 (1997).
Schindler, U., Beckmann, H. & Cashmore, A. R. HAT3.1, a novel Arabidopsis homeodomain protein containing a conserved cysteine-rich region. Plant J. 4, 137–150 (1993).
Bertolino, E., Reimund, B., Wildt-Perinic, D. & Clerc, R. G. A novel homeobox protein which recognizes a TGT core and functionally interferes with a retinoid-responsive motif. J Biol Chem. 270, 31178–31188 (1995).
Bürglin, T. R. Analysis of TALE superclass homeobox genes (MEIS, PBC, KNOX, Iroquois, TGIF) reveals a novel domain conserved between plants and animals. Nucleic Acids Res. 25, 4173–4180 (1997).
Guo, R. et al. Genome-wide identification, evolutionary and expression analysis of the aspartic protease gene superfamily in grape. BMC Genomics. 14, 1–18 (2013).
Dezar, C. A., Gago, G. M., Gonzalez, D. H. & Chan, R. L. Hahb-4, a sunflower homeobox-leucine zipper gene, is a developmental regulator and confers drought tolerance to Arabidopsis thaliana plants. Transgenic Res. 14, 429–40 (2005).
Agalou, A. et al. A genome-wide survey of HD-Zip genes in rice and analysis of drought-responsive family members. Plant Mol Biol. 66, 87–103 (2008).
Olsson, A. S., Engström, P. & Söderman, E. The homeobox genes ATHB12 and ATHB7 encode potential regulators of growth in response to water deficit in Arabidopsis. Plant Mol Biol. 55, 663–77 (2004).
Jain, M., Tyagi, A. K. & Khurana, J. P. Genome-wide identification, classification, evolutionary expansion and expression analyses of homeobox genes in rice. FEBS J. 275, 2845–61 (2008).
Gehring, W. J., Affolter, M. & Burglin, T. Homeodomain Proteins. Annu Rev Biochem. 63, 487–526 (1994).
Pabo, C. O. & Sauer, R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 61, 1053–95 (1992).
Liu, Y. et al. Genome-wide analysis of the auxin response factor (ARF) gene family in maize (Zea mays). Plant Growth Regul. 63, 225–234 (2011).
Vision, T. J., Brown, D. G. & Tanksley, S. D. The origins of genomic duplications in Arabidopsis. Science. 290, 2114–2117 (2000).
Bowers, J. E., Chapman, B. A., Rong, J. & Paterson, A. H. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature. 422, 433–438 (2003).
Tang, H. et al. Synteny and collinearity in plant genomes. Science. 320, 486–488 (2008).
Adams, K. L., Percifield, R. & Wendel, J. F. Organ-specific silencing of duplicated genes in a newly synthesized cotton allotetraploid. Genetic. 168, 2217–2226 (2004).
Blanc, G. & Wolfe, K. H. Widespread paleopolyploidy in model plant species inferred from age distributions of duplicate genes. Plant Cell. 16, 1667–1678 (2004).
Blanc, G. & Wolfe, K. H. Functional divergence of duplicated genes formed by polyploidy during Arabidopsis evolution. Plant Cell. 16, 1679–1691 (2004).
Zhang, J. Z. Evolution by gene duplication: an update. Trends Ecol Evol. 18, 292–298 (2003).
Wang, M. et al. Genome-wide identification, phylogeny and expressional profiles of mitogen activated protein kinase kinase kinase (MAPKKK) gene family in bread wheat (Triticum aestivum L.). BMC Genomics. 17, 668 (2016).
Xu, G., Guo, C., Shan, H. & Kong, H. Divergence of duplicate genes in exon-intron structure. Proc Natl Acad Sci. 109, 1187–1192 (2012).
Baima, S., Nobili, F., Sessa, G., Lucchetti, S. & Ruberti, I. & MorelliG. The expression of the ATHB-8 homeobox gene is restricted to provascular cells in Arabidopsis thaliana. Development. 121, 4171–4182 (1995).
Baima, S. et al. The Arabidopsis ATHB-8 HD-Zip Protein Acts as a differentiation-Promoting Transcription Factor of the Vascular Meristems. Plant Physiology. 126, 643–655 (2001).
Gambino, G. et al. Characterization of expression dynamics of WOX homeodomain transcription factors during somatic embryogenesis in Vitis vinifera. J Exp Bot. 62, 1089–1101 (2011).
Boccacci P. et al. Cultivar-specific gene modulation in Vitis vinifera: analysis of the promoters regulating the expression of WOX transcription factors. Sci Rep. 7, 45670 (2017).
Bharathan, G., Janssen, B. J., Kellogg, E. A. & Sinha, N. Phylogenetic relationships and evolution of the KNOTTED class of plant homeodomain proteins. Mol Biol Evol. 16, 553–63 (1999).
Aasland, R., Gibson, T. J. & Stewart, A. F. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem Sci. 20, 56–9 (1995).
Jiang, Y. et al. MdHB1 down-regulation activates anthocyanin biosynthesis in the white-fleshed apple cultivar ‘Granny Smith’. J Exp Bot. 68, 1055–1069 (2017).
Malabarba, J. et al. The MADS-box gene Agamous-like 11 is essential for seed morphogenesis in grapevine. J Exp Bot. 68, 1493–1506 (2017).
Zhang, C. et al. The metacaspase gene family of Vitis vinifera L.: Characterization and differential expression during ovule abortion in stenospermocarpic seedless grapes. Gene. 528, 267–276 (2013).
Colombo, L. et al. The Petunia MADS Box Gene FBPI7 Determines Ovule ldentity. Plant Cell. 7, 1859–1868 (1995).
Larkin, M. A. et al. Clustal W and Clustal X version 2.0. Bioinformatics 23, 2947–2948 (2007).
Saitou, N. & Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 4, 406–425 (1987).
Tamura, K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28, 2731–2739 (2011).
Tamura, K. et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Bio Evol. 28, 2731–2739 (2011).
Zhang, Y. et al. Genome-wide identification and analysis of the Tify gene family in grape. PLoS One. 7, e44465 (2012).
Guo, C. et al. Evolution and expression analysis of the grape (Vitis vinifera L.) WRKY gene family. J Exp Bot. 65, 1513–1528 (2014).
Wang, L. et al. Evolutionary and expression analysis of a MADS-box gene superfamily involved in ovule development of seeded and seedless grapevines. Mol Genet Genomics. 290, 825–46 (2015).
Acknowledgements
This work was supported by the Joint Funds of National Natural Science Foundation of China (U1603234), and the Program for Innovative Research Team of Grape Germplasm Resources and Breeding (2013KCT-25). We thank Yi-Min Zhang (Sagene Biotech Co, Ltd, Guangzhou, China) for providing editorial support for the synteny analysis.
Author information
Authors and Affiliations
Contributions
X.W. and Y.L. designed the study. X.W. and L.W. provided guidance for the experiment. Y.L. performed most of the experiments and data analysis, and L.W. provided technical assistance. C.G. and J.Y. contributed to the synteny analysis. S.Z. and J.Y. helped with the sample collecting and RNA extraction. Y.L. and Y.Z. wrote the manuscript with contributions of all the authors. X.W. and S.V.N. revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Change History: A correction to this article has been published and is linked from the HTML version of this paper. The error has been fixed in the paper.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A correction to this article is available online at https://doi.org/10.1038/s41598-017-16207-6.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, Y., Zhu, Y., Yao, J. et al. Genome-wide identification and expression analyses of the homeobox transcription factor family during ovule development in seedless and seeded grapes. Sci Rep 7, 12638 (2017). https://doi.org/10.1038/s41598-017-12988-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-12988-y
- Springer Nature Limited
This article is cited by
-
WUSCHEL-related homeobox (WOX) transcription factors: key regulators in combating abiotic stresses in plants
Horticulture Advances (2024)
-
Identification and Transcriptional Analysis of Zinc Finger-Homeodomain (ZF-HD) Family Genes in Cucumber
Biochemical Genetics (2021)
-
Genome-Wide Identification and Comprehensive Analyses of TCP Gene Family in Banana (Musa L.)
Tropical Plant Biology (2021)
-
Genome-wide identification and transcript profiles of walnut heat stress transcription factor involved in abiotic stress
BMC Genomics (2020)
-
The grapevine homeobox gene VvHB58 influences seed and fruit development through multiple hormonal signaling pathways
BMC Plant Biology (2019)