Abstract
This study aimed to determine whether E prostanoid receptor-3 (EP3) is involved in prostacyclin (PGI2)-evoked vasoconstrictor activity of resistance arteries and if so, how it changes under hypertensive conditions. Mesenteric resistance arteries from Wistar-Kyoto rats (WKYs) and spontaneously hypertensive rats (SHRs) were isolated for functional and biochemical studies. Here we show that in vessels from WKYs, PGI2 or the endothelial muscarinic agonist ACh (which stimulates in vitro PGI2 synthesis) evoked vasoconstrictor activity, which increased in SHRs. The thromboxane-prostanoid receptor (TP) antagonist SQ29548 partially removed the vasoconstrictor activity, and an increased contractile activity of PGI2 resistant to SQ29548 was observed in SHRs. Interestingly, L798106, an antagonist of EP3 (whose expression was higher in SHRs than in WKYs), not only added to the effect of SQ29548 but also caused relaxation to PGI2 more than that obtained with SQ29548. In accordance, EP3 deletion, which reduced PGI2–evoked contraction, together with SQ29548 resulted in relaxation evoked by the agonist in mouse aortas. These results thus demonstrate an explicit involvement of EP3 in PGI2-evoked vasoconstrictor activity in rat mesenteric resistance arteries and suggest that up-regulation of the receptor contributes significantly to the increased contractile activity evoked by PGI2 under hypertensive conditions.
Similar content being viewed by others
Introduction
Metabolism of arachidonic acid via cyclooxygenase (COX), which includes COX-1 and -2, produces vasoactive prostanoids. Among them, thromboxane A2 (TxA2) is the major product produced in platelets, and it acts on the Tx prostanoid receptor (TP) to mediate vasoconstrictor and platelet-aggregating effects. In contrast, prostacyclin (prostaglandin I2; PGI2) is mainly produced in vascular endothelium and is proposed to act on the I prostanoid receptor (IP) that mediates vasodilatation and opposes the effects of TP1,2,3,4,5. However, in some vascular beds, including those of humans, PGI2 or endothelial COX-derived metabolites evoke vasoconstrictor response6,7,8,9,10,11,12,13,14,15,16,17,18,19,20. This has been explained by a concurrent modulation of PGI2’s vasomotor reaction via IP and TP; an endothelium-derived contracting factor (EDCF)-like action of PGI2 or a vasoconstrictor response evoked by endothelial COX metabolites (which consist mainly of PGI2) can reflect limited expression or function of IP, which leads to uncovering of the vasoconstrictor activity derived from concurrently activated TP21,22,23,24,25,26.
More importantly, endothelial COX-derived vasoconstrictor activity or EDCF-like action of PGI2 plays an important role in the development of endothelial dysfunction under disease conditions, including hypertension27,28,29,30,31,32,33. It has been suggested that in hypertension or under prehypertensive conditions, endothelial PGI2 synthesis may increase25, 34. Also, under the disease condition, IP is suggested to become dysfunctional24, 25. These findings explain why an increased vasoconstrictor response and/or a conversion of dilator responses evoked by endothelial PGI2 synthesis into contractile responses were observed in prehypertensive or hypertensive conditions24, 25, 27, 34. As a result, TP antagonism could be used as an effective remedy for endothelial dysfunction developed under disease conditions24, 27. At the same time, our recent studies suggest that PGI2 also activates the E prostanoid receptor-3 (EP3; a vasoconstrictor receptor of PGE2), which along with TP accounts for the EDCF-like action of PGI2 35. Interestingly, the EDCF activity or PGI2-evoked contractile activity in Wistar-Kyoto (WKY) or spontaneously hypertensive rat (SHR) vessels has been recognized to contain a component of TP-independent mechanism18, 29, 34, which favors an involvement of EP3 that should also be targeted under hypertensive conditions. Indeed, EP3 knockout (EP3−/−) has been found to attenuate ANG II pressor response36. However, the role of EP3 in the EDCF-like action of PGI2 in resistance arteries and how it changes under hypertensive conditions still remain to be elucidated.
Therefore, in this study WKY and SHR mesenteric resistance arteries were isolated for biochemical and/or functional analyses. In addition, a strain of EP3−/− mice was designed and used to explicitly elucidate the role of the receptor in the vasoconstrictor activity of PGI2 under in vitro conditions.
Results
Response evoked by PGI2 and effect of TP and/or EP3 antagonism
The responses to PGI2 were first examined in mesenteric resistance arteries treated with the NO synthase (NOS) inhibitor Nω-nitro-L-arginine methyl ester (L-NAME) under baseline conditions12, 25. As shown in Fig. 1A, in WKY vessels, PGI2 caused a slight contractile response only at concentrations ≥10 μM. However, in those of SHRs, not only the initial concentration of PGI2 to evoke contractile response was lower, but also the extent of contraction evoked by the agonist was significantly increased compared to that in WKYs (Fig. 1A). The IP antagonist CAY10441 (1 μM) significantly increased the contraction evoked by PGI2 both in WKYs and in SHRs (Fig. 1A). In addition, under such conditions, the response evoked by PGI2 in SHRs was still greater than that in WKYs (Fig. 1A).
PGI2-evoked contraction in NOS-inhibited mesenteric resistance arteries and effect of EP3 antagonism. (A) Comparison of responses evoked by PGI2 in L-NAME-treated WKY and SHR vessels with or without the presence of the IP antagonist CAY10441 (1 μM; CAY). N = 5 for each; ** P < 0.01 vs. WKY; ^ P < 0.05 and ^^ P < 0.01 vs. SHR; ++ P < 0.01 vs. WKY/CAY. (B) Comparison of the control contraction evoked by PGI2 (CTL; 10 μM) in WKY or SHR vessels obtained with CAY with that additionally with the TP antagonist SQ29548 (10 μM; +SQ), that with SQ29548 and the EP1 antagonist SC19220 (10 μM; +SQ/SC), or that with SQ29548 and the EP3 antagonist L798106 (1 μM; +SQ/L). (C) Representative traces showing the control contraction evoked by PGI2 (10 μM) in SHR vessels obtained with CAY (top) and that the additionally with the TP antagonist SQ29548 (10 μM; bottom). (D) Summary of control responses evoked by PGE2 (0.1 μM) on the contraction evoked by PE (3 μM) in L-NAME and SQ29548-treated WKY and SHR vessels and those obtained additionally with SC19220 (+SC) or L798106 (1 μM;+SQ/L). In (B) and (D), n = 5 for each; ** P < 0.01 vs. CTL; ^^ P < 0.01 vs. WKY counterparts; ++ P < 0.01 vs. +SQ.
Moreover, we noted that after the treatment with CAY10441, the TP antagonist SQ29548 (10 μM), abolished most of the contraction evoked by 10 μM PGI2 in WKYs, but <50% of that in SHRs (Fig. 1B and C). The EP3 antagonist L798106 (1 μM) but not the EP1 antagonist SC19220 (10 μM) added to the effect of SQ29548, resulted in abolition of the contraction evoked by PGI2 in both WKYs and SHRs (Fig. 1B). In addition, under NOS-inhibited conditions, PGE2 (which evokes only a minor vasoconstrictor activity that peaks at concentrations ≤0.3 μM in TP−/− mouse aortas35) was able to evoke an increase of force at 0.1 μM on the contraction evoked by 3 μM phenylephrine (PE) when SQ29548 was also present (Fig. 1D). Again, this increase of force was more prominent in SHRs than in WKYs and was inhibited by L798106 (1 μM) but not by SC19220 (10 μM; Fig. 1D).
Effect of TP and/or EP3 antagonism on PGI2-response under precontracted conditions
The effect of TP or EP3 antagonism on the response evoked by PGI2 was also determined in L-NAME-treated mesenteric resistance arteries precontracted with PE (3 μM). As shown in Fig. 2, in vessels of either rat strain, PGI2 (1 μM) evoked an initial increase of force, which was followed by a steady relaxation that was to a smaller extent in SHRs than in WKYs (−16.0 ± 4.3% vs. −54.8 ± 6.4%, respectively; P < 0.01).
Effect of TP and/or EP3 antagonism in PE-precontracted, NOS-inhibited or endothelium-denuded rat mesenteric resistance arteries. (A) The control response evoked by PGI2 (1 μM; CTL) in L-NAME-treated WKY vessels precontracted with PE (3 μM) and that obtained with the EP3 antagonist L798106 (1 μM; +L; bottom) or the TP antagonist SQ29548 (+SQ; 10 μM). (B) Representative traces showing the response evoked by PGI2 (top) and that obtained with L798106 (1 μM; +L; bottom) in (A). (C) The control response evoked by PGI2 in PE-precontracted, L-NAME-treated SHR vessels and that obtained with SQ29548 (+SQ), with L798106 (+L), or with L798106 and SQ29548 (+L/SQ). In A and C, n = 5 for each; ** P < 0.01; + P < 0.05 and ++ P < 0.01 vs. the value in +L. (D) Representative traces with summarized values showing the control response evoked by PGI2 (top) in PE-precontracted, endothelium-denuded SHR vessels (top) and that obtained with L798106 (+L; bottom). FC (4 min): the value of force change relative to that of PE 4 min after the application of PGI2. ** P < 0.01.
As expected, the TP antagonist SQ29548 (10 μM) enhanced the relaxation (Fig. 2A and C), with resulting force being smaller in WKYs than in SHRs (−83.3 ± 3.7% vs. −49.9 ± 5.6%, respectively; P < 0.01). Interestingly, the EP3 antagonist L798106 (1 μM) abolished the initial increases of force and yielded greater relaxations than that obtained with SQ29548 (Fig. 2A,B and C) In addition, in SHRs where L798106 (1 μM) did not cause a complete relaxation, SQ29548 (10 μM) added to the effect of L798106 and resulted in a complete relaxation evoked by PGI2 (Fig. 2C).
The effect of L798106 on PGI2-evoked response was also determined in endothelium-denuded, PE (3 μM)-precontracted SHR mesenteric resistance arteries. Under the conditions PGI2 evoked a response similar to that obtained in L-NAME-treated SHR vessels (Fig. 2D). Again, L798106 removed the initial force increase and caused an enhanced relaxation to PGI2, i.e., the force change (relative to the value of PE pre-contraction) 4 min after the application of PGI2 [FC (4 min)] being significantly smaller than that of controls (Fig. 2D).
Expressions and/or functions of EP3, TP and IP
To understand molecular bases for above results, expressions of EP3, TP and IP were examined. Real-time PCR showed that the level of EP3 mRNAs normalized by that of β-actin was higher in SHRs than in WKYs. Also, Western blot revealed that β-actin normalized level of TP proteins was increased, while that of IP was decreased in SHRs than in WKYs.
Also, we noted that the TP agonist U46619 was found to evoke a greater contraction in SHR than in WKY vessels (Fig. 3C). The TP antagonist SQ29548 (10 μM) completely abolished the contraction in SHRs. Notably, although the EP3 antagonist L798106 (1 μM) also inhibited the response, its effect was to a smaller extent than that of SQ29548 (Fig. 3C). In addition, after vessels had been treated with L798106 and SQ29548, the relaxation evoked by PGI2 on contractions induced by PE (3 μM) was smaller in SHRs than that in WKYs (Fig. 3D).
Expressions and/or functions of EP3, TP and IP. (A) β-actin normalized EP3 mRNA level detected by real-time PCR in WKY and SHR vessels. N = 6 for each. (B) Representative Western blots of TP and IP (left lane: WKY; right lane: SHR) and summary of results (from 5 replicates) showing the band density of TP or IP normalized by that β-actin in WKY and SHR mesenteric resistance arteries. Full length representative Western blots of TP and IP bands and that of β-actin were presented in the supplementary file. In (A) and (B), the normalized EP3 mRNA level, TP and IP band densities were expressed relative to the average of WKY vessels (which was assumed a value of 1.0). * P < 0.05 and ** P < 0.01 vs. the value in WKY. (C) Contractions evoked by the TP agonist U46619 in L-NAME-treated WKY and SHR vessels and that of SHR vessels obtained with TP antagonist SQ29548 (10 μM; SHR/SQ) or the EP3 antagonist L798106 (1 μM; SHR/L). (D) PGI2-evoked relaxation in L-NAME-treated WKY and SHR vessels precontracted with PE (3 μM) after both EP3 and TP were antagonized (with L798106 and SQ29548). In (C) and (D), n = 5 for each; ** P < 0.01 vs. WKY; ++ P < 0.01 vs. SHR; ^^P < 0.01 vs. SHR/L.
ACh-evoked responses in mesenteric resistance arteries
The muscarinic agonist ACh stimulates endothelial COX to produce PGI2 12, 34. Therefore, the production of native PGI2 in WKY and SHR mesenteric resistance arteries was examined. As shown in Fig. 4A, in WKY and SHR vessels, ACh (10 μM) stimulated an increase in the production of the PGI2 metabolite 6-keto-PGF1α that was comparable between the two rat strains. HPLC-MS further revealed that 6-keto-PGF1α was the only detected COX-derived product in ACh-stimulated SHR vessels (Fig. 4B).
ACh-evoked production of 6-keto-PGF1α in rat mesenteric resistance arteries. (A) production of 6-keto-PGF1α measured with EIA in WKY and SHR vessels under the basal (un-stimulated in PSS) and ACh-stimulated conditions. N = 7 for each; ** P < 0.01; NS: not significant. (B) HPLC-MS showing peaks of 6-keto-PGF1α and other products in basal (middle) or ACh-stimulated SHR vessels (bottom). The top shows peaks obtained with solution of mixed standard compounds with amount indicated (ng/ml).
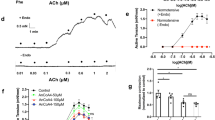
Vasomotor reactions evoked by ACh (10 μM) were also determined. Vessels were again treated with L-NAME12, 25, 34. As shown in Fig. 5, when vessels were precontracted with PE (3 μM), ACh (which was unable to evoke any response under baseline conditions) evoked relaxation, which was blunted by a biphasic force that was more prominent in SHRs than in WKYs (peak of the force was 5.6 ± 9.7% above PE-evoked response vs. −77.9 ± 2.6%, respectively; P < 0.01; Fig. 5). The COX-1 inhibitor FR122047 (1 μM) and the EP3 antagonist L798106 (1 μM) similarly abolished the biphasic force (Fig. 5). In contrast, the TP antagonists SQ29548 (10 μM), although it abolished the biphasic force in WKYs (Fig. 5B), showed an effect in SHRs less than that of L798106 (Fig. 5D). Also, the COX-2 inhibitor rofecoxib (1 μM) did not show an effect in SHR vessels (Fig. 5D).
ACh-evoked responses in NOS-inhibited rat mesenteric resistance arteries precontracted with PE. (A) Representative traces showing the control response (CTL) evoked by ACh (10 μM; top) in L-NAME-treated WKY vessels precontracted with PE (3 μM) and that obtained with the EP antagonist L798106 (1 μM; +L; bottom). (B) Summary of results from 5 replicates in (A), and those obtained with the TP antagonist SQ29548 (10 μM; +SQ) or the COX-1 inhibitor FR122047 (1 μM; +FR). (C) Representative traces showing the control response (CTL) evoked by ACh (top) in PE-precontracted, L-NAME-treated SHR vessels and that obtained with L798106 (+L; bottom). (D) Summary of results from 5 replicates in (C) and those obtained with SQ29548 (+SQ), FR122047 (+FR), or the COX-2 inhibitor rofecoxib (1 μM; +RO). In (B) and (D), ** P < 0.01 vs. CTL while ++ P < 0.01 vs. +SQ.
Effect of EP3−/− on contractile response to PGI2
Finally, abdominal aortas (which normally show contraction to PGI2 12, 35) from EP3−/− mice were used to verify the role of EP3 in PGI2-evoked contractile activity. As shown in Fig. 6A and B, in L-NAME-treated abdominal aortas of EP3−/− mice, the response evoked by U46619 was unaltered (Fig. 6A); however, that of PGI2 was reduced compared to WT mice (Fig. 6B). Moreover, in such vessels pre-contracted with PE (2 μM), treatment with the TP antagonist SQ29548 (10 μM) resulted in relaxation evoked by PGI2, which was in contrast to an enhancement of contraction evoked by the agonist in WT controls (Fig. 6C). Also, genotyping showed that in EP3 locus of EP3−/− mice, a DNA fragment of 1146 bp was deleted as expected (Fig. 6D).
Effect of EP3−/− on responses evoked by PGI2 in NOS-inhibited mouse abdominal aortas. (A) and (B) Responses evoked by the TP agonist U46619 (A) and PGI2 (B) in WT and EP3−/− abdominal aortas. (A) representative traces with summarized values showing the response evoked by PGI2 in WT (top) or EP3−/− abdominal aortas (bottom) with the presence of TP antagonist SQ29548 (10 μM; SQ). In A–C, n = 5 for each; ** P < 0.01 vs. WT. (D) Genotyping showing the difference in the PCR product of EP3 gene between WT and EP3−/− mice. M: size marker.
Discussion
In this study, we show that in WKY mesenteric resistance arteries PGI2 evokes a vasoconstrictor activity that increases in SHRs. The TP antagonist SQ29548, which completely abolishes the response evoked by U46619 (a TxA2 analogue and TP agonist), only partially reduces the vasoconstrictor activity of PGI2. In contrast, the EP3 antagonist L798106, which also appears as a partial TP antagonist, not only adds to the effect of SQ29548, but reduces PGI2’s contractile activity more than SQ29548. In addition, EP3 expression level is higher in SHRs than in WKYs. These results may suggest an intimate link between EP3 and the increased vasoconstrictor activity of PGI2 in SHR mesenteric resistance arteries.
The responses obtained under baseline or PE-precontracted conditions clearly show that in mesenteric resistance arteries of either rat strain, PGI2 evokes both dilator and contractile activities, which can produce a contractile response that increases under hypertensive conditions. Interestingly, after IP (which mediates the dilator activity of PGI2) was blocked, 10 μM of the TP antagonist SQ29548, which has been shown to have an effect comparable to that of TP−/− 35, and was able to completely abolish the response to U46619 here in SHR vessels, only partially inhibited the contractile response of PGI2. In addition, the part of response resistant to SQ29548 was more prominent (>50%) in SHRs than in WKYs. These results together with the antagonistic effect of L798106 (EP3 antagonist) may point to an involvement of EP3 in PGI2-evoked contractile activity that increases under hypertensive conditions. In its consistency, a smaller relaxation was evoked by PGI2 in SHR vessels than in WKY counterparts after TP had been blocked. Moreover, mRNA levels and extents of TP-independent vasoconstrictor activity of PGE2 sensitive to L798106 suggest that the function or expression of EP3 is up-regulated in SHR mesenteric resistance arteries.
Meanwhile, in SHR vessels the expression of TP was also increased, while that of IP was reduced compared to WKY counterparts. Accordingly, U46619 (TP agonist) evoked greater contraction, while the relaxation to PGI2 after both TP and EP3 had been antagonized was smaller in SHRs than in WKYs. Indeed, an increased contraction to the TP agonist and/or reduced or absent relaxation to PGI2 in SHR vessels has been reported previously by other groups24, 25, 30. Thus, TP is also up-regulated, while IP is down-regulated in SHR mesenteric resistance arteries. Such findings would not contradict with the involvement of EP3 in PGI2’s response. This is because results from EP3−/− mice not only explicitly verify the ability of PGI2 to activate EP3, but along with those in TP−/− mice we reported previously suggest that the agonist can activate EP3 along with TP and IP35. In addition, the fact that L798106 partially antagonized the contraction evoked by U46619 would not undermine its concurrent effect on EP3 as well, since its effects on both TP and EP3 explain why the antagonist resulted in relaxation to PGI2 more than SQ29648, yet the later added to its effect. As a result, the increased contraction to PGI2 observed in SHR mesenteric resistance arteries could possibly result from a synergy of effects from up-regulation of both EP3 and TP together with down-regulation of IP.
Also, under NOS inhibited conditions, the relation evoked by ACh (which concurrently stimulates release of endothelium-derived hyperpolarizing factor; EDHF37) was blunted by a biphasic force sensitive to COX-1 inhibition but increased in SHRs. Interestingly, PGI2 was found to be the major COX product evoked by ACh and as reported previously, its amount was similar between the two rat strains30. Thus, the vasoconstrictor activity of ACh blunting EDHF activity that increases in SHR vessels mainly results from natively produced PGI2 via mechanisms of the above mentioned for extraneously applied PGI2. In support of this idea, L798106 (EP3 antagonist), which only partially antagonized TP, removed the biphasic force as COX-1 inhibition. The fact that under PE-precontracted conditions, SQ29548 (TP antagonist) was more effective on the vasoconstrictor activity of ACh than on the response evoked by PGI2 could be due to the concurrent EDHF activity, which might mask any smaller contractile activity derived from native PGI2 resulting from one of the vasoconstrictor receptors being antagonized. Also, the concurrent EDHF activity could have possibly prevented ACh from evoking contraction under baseline conditions as we previously noted in mouse renal arteries38.
Therefore, our above results demonstrate an explicit involvement of EP3 in PGI2-evoked contractile activity and suggest that up-regulation of the receptor contributes to the increased EDCF-like action of PGI2 under hypertensive conditions. Interestingly, the extent of contraction evoked by PGI2 or endothelial COX metabolites in WKY mesenteric resistance arteries is similar to that reported in human vessels6, and although subtle, it can be remarkably increased under hypertensive conditions. Also, compared to that of NO, the synthesis of PGI2 is suggested to be less vulnerable to impairment caused by vascular pathology39. In addition, the amount of PGI2 released by endothelial stimuli such as ACh, could be higher than its minimal level to evoke vasoconstrictor activity (1 ng/mg 6-keto-PGF1α could be translated into 2.7 μmol/kg PGI2/tissue). These facts together underscore a pathogenic role of the EDCF action of PGI2, especially under disease conditions. Indeed, COX-1−/−, which abolishes endothelial PGI2 synthesis although along with that of TxA2 in platelets, not only alleviates renovascular and diabetic hypertension, but also reduces atherosclerotic lesions39,40,41. Similar results have been obtained with COX-1 or non-selective COX inhibition when started from prehypertensive stage34, 42. Thus, EP3 could be an important target of pharmacological intervention along with TP for the improvement of endothelial function under disease conditions.
Similar to our present findings, an increased vasoconstrictor activity caused by PGI2 or endothelial COX metabolites had been reported in SHR mesenteric resistance arteries previously29, 30. A contraction to PGI2 was also reported in similar vessels of normal Sprague-Dawley rats, although the response could also be inhibited by the EP1 antagonist SC19220 (which also functions as a partial TP antagonist17, 43). Moreover, an increased level of EP3 or TP-independent vasoconstrictor activity of PGE2 had been previously found in SHR aortas43. However, the present study further suggest a novel mechanism, which involves the up-regulation of EP3, for the increased vasoconstrictor activity or EDCF-like function of PGI2 in rat resistance arteries developed under hypertensive conditions25, 27, 34. It should be noted that PGI2 effectively activating EP3 along with TP to mediate vasoconstrictor activity, an idea we recently put forward based on results obtained in vessels from TP−/− mice35, was further verified in the present study by the effect EP3−/− on PGI2’s in vitro response. Indeed, the idea of PGI2 being able to effectively act on EP3 explains why many of clinically used PGI2 analogues, such as iloprost, are also EP3 agonists44.
One might have also noted that the EP1 antagonist SC19220 (at 100 μM) was previously found to abolish the part of increased vasoconstrictor activity of PGE2 resistant to TP antagonism in SHR aortas43, arousing a concern that the absence of effect by the antagonist in our present study might be due to an inadequate amount used. However, in SHR aortas the level of EP1 was down-regulated and in fact, such an effect of SC19220 was not considered to result from EP1 antagonism43. In addition, SC19220 is known to antagonize EP1 from 1 μM45, 46. As such, the absence of effect by 10 μM SC19220 in the present study could be reasonably considered to result from little, if any functional involvement of EP146. Also, in one of prior reports COX-2 inhibition abolished EDCF activity in SHR mesenteric resistance arteries17, which contradicts with the results obtained in our present study. Again, one must also note that the COX-2 inhibitor used in the prior report even inhibits EDCF activity in COX-2−/− mice47. In contrast, our current results were not only consistent with findings obtained in COX-1−/− or -2−/− mice, but also those in other vessels of WKYs or SHRs12, 21, 25, 34, 48, 49.
In summary, in this study our results not only explicitly demonstrate that EP3 is involved in PGI2-evoked vasoconstrictor activity, but also suggest that its up-regulation could, in synergy with that of TP and down-regulation of IP account for the increased contractile activity of PGI2 in SHR mesenteric arteries.
Material and Methods
Chemicals and solution
L-NAME, ACh, PE, the EP3 antagonist L798106, and the EP1 antagonist SC19220 were purchased from Sigma (St Louis, MO, USA). The COX-1 selective inhibitor FR122047, the IP antagonist CAY10441, the TP antagonist SQ29548, TP agonist U46619, PGI2 and standard compounds of COX products were bought from Cayman Chemical (Ann Arbor, MI, USA). The selective COX-2 inhibitor rofecoxib was purchased from US Biological (Salem, MA, USA). L-NAME, PE, ACh, and FR122047 were dissolved in distilled water, while PGI2 was dissolved in carbonate buffer (50 mM, pH 10.0). SQ29548, L798106, SC19220, CAY10441, and rofecoxib were dissolved in DMSO at 2,000-fold working concentration (the final concentration of DMSO was 0.05/100, v/v). The concentration of an inhibitor or antagonist used was based on previous reports, in which a selective inhibition of the effect of its intended target was considered to be achieved23, 35, 45, 46, 50, 51.
The composition of physiological salt solution (PSS; pH 7.4 with 95%O2-5% CO2) was as follows (in mM): NaCl 123, KCl 4.7, NaHCO3 15.5, KH2PO4 1.2, MgCl2 1.2, CaCl2 1.25, and D-glucose 11.5. The 60 mM K+-PSS (K+) was prepared by replacing an equal molar of NaCl with KCl.
Animals and tissue preparation
All procedures were in conformance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996), and approved by The Institutional Animal Research and Use Committee of Shantou University.
Male WKYs or SHRs (10–14 wk) were purchased from Vital River (Beijing, China). Prior to each experiment, systemic blood pressure (BP) was measured in each rat, using a computerized noninvasive BP system (Kent Scientific Corporation, Torrington, CT, USA). WKYs with BP < 120/90 mmHg or SHRs with systolic BP > 170 mmHg were included in this study.
The breeder male and female EP3−/− mice (on a C57BL/6 background) were custom produced by View Solid Biotech (Beijing, China), using a CRISPR-Cas9 method that targeted introns flanking exon 1 of the EP3 locus and resulted in deletion of a DNA fragment of 1,146 bp, which contains the whole exon 1 (coding for the 1–276th of 362 amino acids). C57BL/6 wild-type (WT) mice were purchased from Vital River (Beijing, China). The deletion of EP3 in each EP3−/− mouse was further confirmed by genotyping with tail biopsy. PCR primers were 5′-TCC CAG ATG TGA GTA TCA TAT G-3′ (sense), and 5′-TAG CTA CCT GAG AAC CTT TAG TG-3′ (anti-sense). The expected PCR product sizes are 1,749 bp in WT, while 603 in EP3−/− mice. Male WT and EP3−/− mice (8–12 wk) were used for experimental purpose.
Rats or mice were killed by CO2 inhalation. Rat mesenteric branches or mouse abdominal aortas were isolated and dissected free of adherent tissues with the help of a binocular microscope.
Analyses of vasomotor reaction
For analyses of vascular function, 2nd generation branches of rat mesenteric arteries or mouse abdominal aortas were cut into 1 mm rings. Vasomotor reaction was measured as described previously34. Briefly, the vascular ring was mounted between two tungsten wires in an organ bath filled with PSS aerated with 95%O2-5% CO2 and maintained at 37 °C. One wire was stationary, whereas the other was connected to an AE801 force transducer (Kronex, Oakland, USA). For some experiments, the endothelium of rat vessels was denuded as previously described35. Thereafter, vessels were stimulated with 60 mM K+ every 15 minutes, and the resting tension was adjusted stepwise to an optimal level (~250 mg for rat mesenteric resistance arteries, while ~300 mg for mouse abdominal aortas), at which point the response to 60 mM K+ was maximal and reproducible.
To remove the influence of NO, vessels were treated with the NOS inhibitor L-NAME (1 mM), under which the response of arteries appears similar to that of eNOS−/− mice19. Inhibitors or solvents were added 30 min before the vessel was contracted with an agonist and was kept in the solution throughout the experiment. The response elicited by an agonist under the baseline condition was expressed relative to that of 60 mM K+, while that during the contraction evoked by PE (3 μM in endothelium-denuded or L-MAME-treated rat vessels to achieve a sustained contraction of 90–110% that by 60 mM K+, or at concentrations otherwise indicated) was expressed relative to the value immediately prior to the application of the agent.
Assay of 6-keto-PGF1α
The PGI2 metabolite 6-keto-PGF1α was measured with an EIA kit12. Briefly, after rinsed of blood components, mesenteric arterial branches were incubated with PSS at 37 °C for 30 min, followed by exposure to PSS (100 μl) or ACh (10 μM) in 100 μl PSS (37 °C) for 15 min. Thereafter, vessels were taken out, and the reaction solutions were diluted with PSS (1:50), and 100 μl of final solution was used for 6-keto-PGF1α measurement, according to instructions of the manufacturer. The amount of 6-keto-PGF1α was expressed in ng per mg of wet tissue.
In addition, the production of 6-keto-PGF1α in SHR mesenteric branches was verified with HPLC-mass spectroscopy (HPLC-MS), by which signals of other COX-derived products could be simultaneously monitored40. Briefly, after being incubated with PSS at 37 °C for 30 min, SHR mesenteric branches (all branches of a single rat were pooled for one measurement) were additionally treated in 1,000 μl PSS (37 °C) or that containing ACh (10 μM) for 15 min. The extraction of COX-derived products and its HPLC-MS detection were performed as described previously52.
Real-time PCR
The expression of EP3 was detected with real-time PCR. The preparation of total RNA from rat mesenteric arteries and RT reactions were performed as described elsewhere previously12. First-strand cDNA was synthesized using total RNA (250 ng) and oligo(dT)15 primers (TaKaRa; Dalian, China).
The primers for EP3 were: 5′-TCG CCG CTA TTG ATA ATG ATG C-3′ (sense), 5′-GCA CTC CTT CTC CTT TCC CAT CT-3′ (antisense). Those for β-actin (internal control) were 5′-CCG TAA AGA CCT CTA TGC CAA CA-3′ (sense) and 5′-CGG ACT CAT CGT ACT CCT GCT-3′ (antisense).
Western blot
Expressions of TP, IP, and β-actin (internal controls) were detected by Western blot. Anti-TP (polyclonal; rabbit; 1:3,000), and anti-IP (polyclonal; rabbit; 1:2,000) antibodies (polyclonal; rabbit; 1:3,000) were purchased from Cayman Chemical, while the anti-β-actin antibody (polyclonal; rabbit; 1:2,000) was bought from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Immunocomplexes were visualized with reaction solution from an ECL Prime detection kit (GE Healthcare, Shanghai, China), and detected using Kodak X-ray film (XBT-1; Xiamen, China).
Data Analysis
Data were expressed as means ± SEM from n numbers or pools of vessels from different animals. Student’s t-test was used to compare the difference between two means for statistical evaluation. When more than two means were compared, one-way or two-way ANOVA followed by Bonferroni’s post-hoc test was used. P < 0.05 was considered to be statistically significant.
References
Bunting, S., Gryglewski, R., Moncada, S. & Vane, J. R. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac ateries and inhibits platelet aggregation. Prostaglandins 12, 897–913 (1976).
Mitchell, J. A., Ali, F., Bailey, L., Moreno, L. & Harrington, L. S. Role of nitric oxide and prostacyclin as vasoactive hormones released by the endothelium. Exp Physiol 93, 141–147 (2008).
Samuelsson, B. et al. Prostaglandins and thromboxanes. Annu Rev Biochem 47, 997–1029 (1978).
Needleman, P. et al. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature 261, 558–560 (1976).
Davidge, S. T. Prostaglandin H synthase and vascular function. Circ Res 89, 650–660 (2001).
Baxter, G. S. et al. Characterization of the prostanoid receptors mediating constriction and relaxation of human isolated uterine artery. Br J Pharmacol 116, 1692–1696 (1995).
Tiritilli, A., El Habach, T., Haury, L. & Duret, J. F. BK2 but not BK1 receptors mediating contractile response in human umbilical arteries: role of thromboxane A2. Methods Find Exp Clin Pharmacol 26, 247–252 (2004).
Hoenicka, M. et al. Endothelium-dependent vasoconstriction in isolated vessel grafts: a novel mechanism of vasospasm? Ann Thorac Surg 92, 1299–1306 (2011).
Dusting, G. J., Moncada, S. & Vane, J. R. Prostacyclin (PGI2) is a weak contractor of coronary arteries of the pig. Eur J Pharmacol 45, 301–304 (1977).
Levy, J. V. Prostacyclin-induced contraction of isolated aortic strips from normal and spontaneously hypertensive rats (SHR). Prostaglandins 19, 517–525 (1980).
Zhao, Y. J., Wang, J., Tod, M. L., Rubin, L. J. & Yuan, X. J. Pulmonary vasoconstrictor effects of prostacyclin in rats: potential role of thromboxane receptors. J Appl Physiol (1985) 81, 2595–2603 (1996).
Liu, B. et al. Involvement of cyclo-oxygenase-1-mediated prostacyclin synthesis in the vasoconstrictor activity evoked by ACh in mouse arteries. Exp Physiol 97, 277–289 (2012).
Liu, B. et al. Role of Cyclooxygenase-1-Mediated Prostacyclin Synthesis in Endothelium-Dependent Vasoconstrictor Activity of Porcine Interlobular Renal Arteries. Am J Physiol Renal Physiol 302, F1133–1140 (2012).
Katusic, Z. S. & Shepherd, J. T. Endothelium-derived vasoactive factors: II. Endothelium-dependent contraction. Hypertension 18, III86–92 (1991).
Yu, H., Carretero, O. A., Juncos, L. A. & Garvin, J. L. Biphasic effect of bradykinin on rabbit afferent arterioles. Hypertension 32, 287–292 (1998).
De Mey, J. G. & Vanhoutte, P. M. Heterogeneous behavior of the canine arterial and venous wall. Importance of the endothelium. Circ Res 51, 439–447 (1982).
Xavier, F. E., Blanco-Rivero, J., Ferrer, M. & Balfagon, G. Endothelium modulates vasoconstrictor response to prostaglandin I2 in rat mesenteric resistance arteries: interaction between EP1 and TP receptors. Br J Pharmacol 158, 1787–1795 (2009).
Rapoport, R. M. & Williams, S. P. Role of prostaglandins in acetylcholine-induced contraction of aorta from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension 28, 64–75 (1996).
Zhou, Y. et al. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol 289, H1027–1032 (2005).
Okon, E. B., Golbabaie, A. & van Breemen, C. In the presence of L-NAME SERCA blockade induces endothelium-dependent contraction of mouse aorta through activation of smooth muscle prostaglandin H2/thromboxane A2 receptors. Br J Pharmacol 137, 545–553 (2002).
Luo, W., Liu, B. & Zhou, Y. The endothelial cyclooxygenase pathway: Insights from mouse arteries. Eur J Pharmacol 780, 148–158 (2016).
Williams, S. P., Dorn, G. Wn. & Rapoport, R. M. Prostaglandin I2 mediates contraction and relaxation of vascular smooth muscle. Am J Physiol 267, H796–803 (1994).
Liu, B. et al. Concomitant activation of functionally opposing prostacyclin and thromboxane prostanoid receptors by cyclo-oxygenase-1-mediated prostacyclin synthesis in mouse arteries. Exp Physiol 97, 895–904 (2012).
Feletou, M., Verbeuren, T. J. & Vanhoutte, P. M. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. Br J Pharmacol 156, 563–574 (2009).
Gluais, P., Lonchampt, M., Morrow, J. D., Vanhoutte, P. M. & Feletou, M. Acetylcholine-induced endothelium-dependent contractions in the SHR aorta: the Janus face of prostacyclin. Br J Pharmacol 146, 834–845 (2005).
Liu, B. & Zhou, Y. Emerging challenges to the existing paradigm of cyclo-oxygenase pathways in regulating vascular function. Exp Physiol 99, 1–2 (2014).
Vanhoutte, P. M. Endothelium-dependent contractions in hypertension: when prostacyclin becomes ugly. Hypertension 57, 526–531 (2011).
Dai, F. X., Skopec, J., Diederich, A. & Diederich, D. Prostaglandin H2 and thromboxane A2 are contractile factors in intrarenal arteries of spontaneously hypertensive rats. Hypertension 19, 795–798 (1992).
Jameson, M. et al. Endothelium-derived contracting factors in resistance arteries of young spontaneously hypertensive rats before development of overt hypertension. Hypertension 21, 280–288 (1993).
Xavier, F. E. et al. Aldosterone induces endothelial dysfunction in resistance arteries from normotensive and hypertensive rats by increasing thromboxane A2 and prostacyclin. Br J Pharmacol 154, 1225–1235 (2008).
Feletou, M., Huang, Y. & Vanhoutte, P. M. Endothelium-mediated control of vascular tone: COX-1 and COX-2 products. Br J Pharmacol 164, 894–912 (2011).
Koga, T. et al. Age and hypertension promote endothelium-dependent contractions to acetylcholine in the aorta of the rat. Hypertension 14, 542–548 (1989).
Luscher, T. F. & Vanhoutte, P. M. Endothelium-dependent contractions to acetylcholine in the aorta of the spontaneously hypertensive rat. Hypertension 8, 344–348 (1986).
Liu, D. et al. A vasoconstrictor response to COX-1-mediated prostacyclin synthesis in young rat renal arteries that increases in prehypertensive conditions. Am J Physiol Heart Circ Physiol 309, H804–811 (2015).
Li, Z. et al. Role of E-type prostaglandin receptor EP3 in the vasoconstrictor activity evoked by prostacyclin in thromboxane-prostanoid receptor deficient mice. Sci Rep 7, doi:10.1038/srep42167 (2017).
Chen, L. et al. Inactivation of the E-prostanoid 3 receptor attenuates the angiotensin II pressor response via decreasing arterial contractility. Arterioscler Thromb Vasc Biol 32, 3024–3032 (2012).
Garland, C. J. & McPherson, G. A. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol 105, 429–435 (1992).
Liu, B. et al. A vasoconstrictor role for cyclooxygenase-1-mediated prostacyclin synthesis in mouse renal arteries. Am J Physiol Renal Physiol 305, F1315–1322 (2013).
Li, S. et al. Role of cyclooxygenae-1 and -2 in endothelium-dependent contraction of atherosclerotic mouse abdominal aortas. Clin Exp Pharmacol Physiol 43, 67–74 (2016).
Liu, B. et al. Vasomotor Reaction to Cyclooxygenase-1-Mediated Prostacyclin Synthesis in Carotid Arteries from Two-Kidney-One-Clip Hypertensive Mice. PLoS One 10, e0136738 (2015).
Zhu, N. et al. Vasoconstrictor role of cyclooxygenase-1-mediated prostacyclin synthesis in non-insulin-dependent diabetic mice induced by high-fat diet and streptozotocin. Am J Physiol Heart Circ Physiol 307, H319–327 (2014).
Asirvatham-Jeyaraj, N., King, A. J., Northcott, C. A., Madan, S. & Fink, G. D. Cyclooxygenase-1 inhibition attenuates angiotensin II-salt hypertension and neurogenic pressor activity in the rat. Am J Physiol Heart Circ Physiol 305, H1462–1470 (2013).
Tang, E. H. et al. The role of prostaglandin E and thromboxane-prostanoid receptors in the response to prostaglandin E2 in the aorta of Wistar Kyoto rats and spontaneously hypertensive rats. Cardiovasc Res 78, 130–138 (2008).
Morrison, K., Ernst, R., Hess, P., Studer, R. & Clozel, M. Selexipag: a selective prostacyclin receptor agonist that does not affect rat gastric function. J Pharmacol Exp Ther 335, 249–255 (2010).
Rakovska, A. & Milenov, K. Antagonistic effect of SC-19220 on the responses of guinea-pig gastric muscles to prostaglandins E1, E2 and F2 alpha. Arch Int Pharmacodyn Ther 268(1), 59–69 (1984).
Smid, S. D. & Svensson, K. M. Inhibition of cyclooxygenase-2 and EP1 receptor antagonism reduces human colonic longitudinal muscle contractility in vitro. Prostaglandins Other Lipid Mediat 88, 117–121 (2009).
Liu, B. et al. Effect of celecoxib on cyclooxygenase-1-mediated prostacyclin synthesis and endothelium-dependent contraction in mouse arteries. Eur J Pharmacol 698, 354–361 (2013).
Ahmetaj-Shala, B. et al. Evidence That Links Loss of Cyclo-Oxygenase-2 With Increased Asymmetric Dimethylarginine: Novel Explanation of Cardiovascular Side Effects Associated With Anti-inflammatory Drugs. Circulation 131, 633–642 (2015).
Kirkby, N. S. et al. Cyclooxygenase-1, not cyclooxygenase-2, is responsible for physiological production of prostacyclin in the cardiovascular system. Proc Natl Acad Sci U S A 109, 17597–17602 (2012).
Botella, A., Delvaux, M., Fioramonti, J., Frexinos, J. & Bueno, L. Receptor subtypes involved in dual effects induced by prostaglandin E2 in circular smooth muscle from dog colon. J Pharmacol Exp Ther 273, 1008–1014 (1995).
Fairbrother, S. E., Smith, J. E., Borman, R. A. & Cox, H. M. Characterization of the EP receptor types that mediate longitudinal smooth muscle contraction of human colon, mouse colon and mouse ileum. Neurogastroenterol Motil 23, 782–e336 (2011).
Zhou, Y. et al. Cyclo-oxygenase-1 or -2-mediated metabolism of arachidonic acid in endothelium-dependent contraction of mouse arteries. Exp Physiol 98, 1225–1234 (2013).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (81470572 to Y Zhou and 81370384 to BL), and by the Department of Education, Guangdong Government under the Top-tier University Development Scheme for Research and Control of Infectious Diseases (2015052 to BL).
Author information
Authors and Affiliations
Contributions
Conception and design of the experiments: Y. Zhou, B.L. Collection, analysis and interpretation of data: B.L., M.Z., Y. Zhang, H.L., X.W., F.Z., W.L. Drafting the article: Y. Zhou. All authors approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, B., Zhan, M., Zhang, Y. et al. Increased role of E prostanoid receptor-3 in prostacyclin-evoked contractile activity of spontaneously hypertensive rat mesenteric resistance arteries. Sci Rep 7, 8927 (2017). https://doi.org/10.1038/s41598-017-09288-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-09288-w
- Springer Nature Limited