Abstract
Modulation of endothelial calcium-activated K+ channels has been proposed as an approach to restore arterial endothelial cell function in disease. We hypothesized that small-conductance calcium-activated K+ channels (KCa2.3 or SK3) contributes to erectile function. The research was performed in transgenic mice with overexpression (KCa2.3T/T(−Dox)) or down-regulation (KCa2.3T/T(+Dox)) of the KCa2.3 channels and wild-type C57BL/6-mice (WT). QPCR revealed that KCa2.3 and KCa1.1 channels were the most abundant in mouse corpus cavernosum. KCa2.3 channels were found by immunoreactivity and electron microscopy in the apical-lateral membrane of endothelial cells in the corpus cavernosum. Norepinephrine contraction was enhanced in the corpus cavernosum of KCa2.3T/T(+Dox) versus KCa2.3T/T(−Dox) mice, while acetylcholine relaxation was only reduced at 0.3 µM and relaxations in response to the nitric oxide donor sodium nitroprusside were unaltered. An opener of KCa2 channels, NS309 induced concentration-dependent relaxations of corpus cavernosum. Mean arterial pressure was lower in KCa2.3T/T(−Dox) mice compared with WT and KCa2.3T/T(+Dox) mice. In anesthetized mice, cavernous nerve stimulation augmented in frequency/voltage dependent manner erectile function being lower in KCa2.3T/T(+Dox) mice at low frequencies. Our findings suggest that down-regulation of KCa2.3 channels contributes to erectile dysfunction, and that pharmacological activation of KCa2.3 channels may have the potential to restore erectile function.
Similar content being viewed by others
Introduction
Erectile dysfunction (ED) is currently considered as an early clinical manifestation of more generalized cardiovascular disease due to its high prevalence in patients with the major cardiovascular risk factors, diabetes, hypertension, hyperlipidemia, and tobacco abuse1, 2. Moreover, ED is an important cause of decreased quality of life in diabetic male patients. In fact, the prevalence of ED is three times higher in men with type 1 and type 2 diabetes than in the general population3, 4. Intriguingly, about 50% of the patients exhibit suboptimal responses to oral phosphodiesterase type 5 inhibitors4, 5, pointing to a need for alternative treatments that could replace or be adjuvant to current treatments.
Evidence from studies on diabetic patients suggests that endothelial and erectile dysfunction are closely linked to each other6. Moreover, endothelium-dependent flow-mediated dilation of the brachial artery was significantly reduced in ED patients7 and such impairment of flow-mediated dilatation correlated with the severity of ED8. From the pharmacological perspectives, it is therefore worth speculating that an improvement of endothelial function will also improve erectile function.
Impairment of KCa2.3 and KCa3.1 channel activation or gene expression contributes to endothelial dysfunction, and pharmacological activation of these channels has been suggested to improve endothelial function in animal models of cardiovascular disease and diabetes9, 10. In rat penile arteries, we found that KCa2.3 and KCa3.1 channels contribute to flow-induced vasodilation and that these dilatations were impaired in type 2 diabetic Zucker diabetic fatty (ZDF) rats11. Moreover, in mesenteric arteries from ZDF rats an opener of KCa2.1–3 and KCa3.1 channels restored endothelium-derived hyperpolarization (EDH) type relaxations induced by acetylcholine12.
Expression levels of KCa2.3 channels as well as pharmacological activation of the channels may have a strong impact on penile vascular function and thereby erectile function. The major aim of our study was therefore to elucidate the role of KCa2.3 channels in endothelial function in corpus cavernosum and to evaluate whether genetically encoded suppression of KCa2.3 channels contributes to endothelial dysfunction and by this ED. Moreover, we tested whether pharmacological manipulation of KCa2.3 channels could provide a novel endothelium-targeted approach for the treatment of ED.
Results
Expression studies
In corpus cavernosum from WT animals, mRNA expression of the KCa channels was examined and showed that the KCa1.1α subunit followed by KCa2.3 channels and the KCa1.1β1 subunit were the most robustly expressed KCa channel subtypes (Fig. 1A). Q-PCR showed a clear down-regulation of KCa2.3 mRNA in KCa2.3T/T (+Dox) mice (n = 9) as compared to KCa2.3T/T (−Dox) mice (n = 9) (Fig. 1B). Expression of KCa1.1α subunits (coding for the pore forming subunit) were unchanged in corpus cavernosum from the three groups of mice (Fig. 1C), while KCa1.1 β1 subunit expression was upregulated in corpus cavernosum of KCa2.3T/T (+Dox) versus WT mice (Fig. 1D). Immunoblotting was performed for quantification of KCa2.3 in the corpus cavernosum of KCa2.3T/T (−Dox) (n = 5), KCa2.3T/T (+Dox) (n = 5), and WT (n = 5) mice and also aorta samples (n = 3–5). The immunoreactive band for KCa2.3 channels in corpus cavernosum from WT mice is slightly heavier than the band for the general KCa2.3T/T mice because of the loss of the n-terminal poly-glutamate stretch in these animals (Fig. 1E). We observed a linear relation of pan-actin immunoreaction to the amount of protein loaded (results not shown), and therefore immunoblotting results for KCa2.3 channels were normalized to pan-actin (Fig. 1F) and showed that KCa2.3 expression was lower in corpus cavernosum from the KCa2.3T/T (+Dox) mice compared to expression in corpus cavernosum from KCa2.3T/T (−Dox) mice (Fig. 1E,F and Supplemental Figure S1E). The same expression pattern was found in aorta samples from WT (n = 5), KCa2.3T/T (−Dox) (n = 3), and KCa2.3T/T (+Dox) (n = 3) mice (Supplemental Figure S1A and S1E).
KCa channel expression in mouse corpus cavernosum. (A) Q-PCR showing the expression of KCa channel subtypes and in case of KCa1.1 subunit α and β1 in corpus cavernosum of wild type mice (WT). Q-PCR showing expression of (B) KCa2.3 (C) KCa1.1α and (D) KCa1.1β1 channels in corpus cavernosum from upregulated (KCa2.3T/T (−Dox)), WT, and down-regulated (KCa2.3T/T (+Dox)) mice. (E) Immunoblot bands for the KCa2.3 channels in corpus cavernosum from KCa2.3T/T (−Dox), WT, and KCa2.3T/T (+Dox) mice. (F) Immunoblot quantification for the KCa2.3 channels in corpus cavernosum from KCa2.3T/T (−Dox), WT, and KCa2.3T/T (+Dox) mice. Data are means ± SEM of 5–9 animals in each group. P ≤ 0.05 (*) versus KCa2.3T/T (+Dox) mice. Compared with one-way ANOVA followed by a Tukey multiple comparisons test or with a Student’s t-test. ≈Around.
Immunoblotting for the pore-forming alpha-subunit of KCa1.1 showed no differences comparing aorta and corpus cavernosum from all three groups of mice (Supplemental Figure S1B and S1C). Immunoreaction for the regulatory beta-subunit of KCa1.1 channels, KCa1.1β1, was observed at 28 kDa and 110 kDa. A positive and specific band was supported by previous test using the peptide applied to raise the antibody (Supplemental Figure S1D). The KCa1.1β1 showed an expression apparently inverse to up- or down-regulation of KCa2.3 channel expression in corpus cavernosum (Supplemental Figure S1C).
Immunohistochemistry showed expression of KCa2.3 channels in helicine arteries in the corpus cavernosum of WT mice (Fig. 2A,B, panel B shows an enlargement of the inset in panel A). Labelling was also seen in capillaries (arrows, Fig. 2A) between the skeletal muscle fibers surrounding the albuginea layer (asterisks, Fig. 2A) and corpus cavernosum. The KCa2.3 channel was seemly localized in the endothelial cells (arrows, Fig. 2C,D, panel C and D shows enlargements on the insets in panel B).
Immunolocalization for KCa2.3 channels in wild type (WT) mouse corpus cavernosum. (A) Histological image of penile tissue from a WT mouse. The square area represents part of the corpus cavernosum. The corpus cavernosum is surrounded by the albuginea layer (asterisks) and skeletal muscle. Arrows indicate KCa2.3 labeling in capillaries between the skeletal muscle fibers. (B) Histological image showing an enlargement of the square area representing the corpus cavernosum from panel A. KCa2.3 immunoreactivity is seen in blood vessels of the corpus cavernosum (inset C and D). (C) KCa2.3 expression in the apical plasma membrane domains of endothelial cells of a small helicine artery in the corpus cavernosum (arrows, enlargement of inset C). (D) Similar KCa2.3 expression in the apical plasma membrane domains of endothelial cells of a probable larger artery in corpus cavernosum (arrows, enlargement of inset D).
Double labelling for KCa2.3 (green staining, Fig. 3A and C) and smooth muscle actin (red staining, Fig. 3B and C) showed specific KCa2.3 expression in the endothelium of blood vessels and corpus cavernosum (Fig. 3C).
Figure 4 shows immunoelectron microscopical images of endothelial cells lining a sinusoid of the WT corpus cavernosum (Fig. 4A, black asterisk). Gold-particle-labelled KCa2.3 proteins were found on the apical plasma membrane domains of the endothelial cells (Fig. 4B,C). Occasionally, the lateral membrane of the endothelial cells was also labelled with KCa2.3 (Fig. 4D).
Electron microscopical localization of KCa2.3 in endothelial cells of sinusoids in corpus cavernosum from a wild type mouse. (A) Endothelial cells located in a sinusoid of corpus cavernosum (one of the cells is represented by a large black asterisk), small black asterisks represents the basement membrane of the endothelium. White asterisk represents an erythrocyte. (B,C) KCa2.3 immunoreactivity is seen in the apical plasma membrane of the endothelial cells (represented by 10 nm gold particles, arrows, enlargements of inset B and C). (D) Immunoreactivity for KCa2.3 channels is seen occasionally in the lateral membrane of endothelial cells (arrows, enlargement of inset).
Functional studies in isolated corpus cavernosum strips
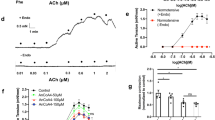
The optimal passive tension for the corpus cavernosum strips examined was similar when comparing preparations from the three groups of mice (Supplemental Figure S2A–F). Therefore, all the experiments were performed with a passive tension of 1.8 mN. Regarding active tension produced by norepinephrine, we found that compared to WT mice, contractions to norepinephrine were enhanced in strips from KCa2.3T/T (+Dox) and reduced in strips from KCa2.3T/T (−Dox) (Fig. 5). Concentration-response curves for acetylcholine (ACh)-induced nitric oxide (NO)-mediated relaxations were unchanged in corpus cavernosum strips from KCa2.3T/T (−Dox), KCa2.3T/T (+Dox) and WT mice, but the slopes of the curves were different for KCa2.3T/T (−Dox) against the WT, and at 0.3 μM ACh relaxation was significantly enhanced in corpus cavernosum strips from KCa2.3T/T (−Dox) mice compared to the WT mice (Fig. 6A).
Original traces showing concentration–response curves for norepinephrine (NE) (0.001–30 μM) in corpus cavernosum from (A) wild type (WT), (B) KCa2.3T/T (−Dox) (upregulated model) or (C) KCa2.3T/T (+Dox) mice (down-regulated model) in mouse corpus cavernosum. (D) Average concentration-response curves for NE in corpus cavernosum from (€) KCa2.3T/T (−Dox) vs KCa2.3T/T (+Dox) or (#) KCa2.3T/T (+Dox) vs WT. Results are means ± S.E.M. P ≤ 0.05 (*), (n = 6–9), two–way ANOVA followed by Tukey post hoc test.
Concentration–response curves for different relaxant compounds (0.001–30 μM) dependent on nitric oxide and KCa2.3 opening in the corpus cavernosum from wild type (WT), KCa2.3T/T (−Dox) (overexpressed model) or KCa2.3T/T (+Dox) (down-regulated model) in the corpus cavernosum. (A) Concentration-response curves for acetylcholine within the KCa2.3 mouse model, (n = 6–9). (B) Concentration-response curves for NS309 within the KCa2.3 mouse model, (n = 6–9). (C) Concentration-response curves for acetylcholine with and without pre-stimulation with 0.5 μM NS309 in WT mice, (n = 6). (D) Concentration-response curves for sodium nitroprusside within the KCa2.3 mouse model. (E) Single concentration administration of 0.01 μM NS309 with and without apamin 0.5 μM in WT mice, (n = 6). Results are means ± S.E.M. P ≤ 0.05 (*), (n = 6–9), Student’s t-test or two-way ANOVA followed by Tukey post hoc test.
In corpus cavernosum, NS309, an opener of KCa2 and KCa3.1 channels, induced concentration-dependent relaxations independent of mouse model (Fig. 6B), where apamin significantly inhibited NS309 relaxation at 0.01 µM (Fig. 6E). Incubation with a combination of inhibitors of NO synthase, nitro-L-arginine (L-NOARG, 100 µM) and of cyclooxygenase, indomethacin (10 µM) significantly inhibited NS309 relaxation (Suppl. Figure S3). Although there was no shift in the concentration-response curves for ACh, pre-treatment in WT corpus cavernosum with NS309 in a concentration (0.5 µM) where it is considered selective for KCa3.1 and KCa2 channels13, enhanced relaxations induced by 0.3 μM ACh (Fig. 6C).
Concentration-response curves for the NO donor sodium nitroprusside (SNP) were similar in corpus cavernosum from all three groups of mice (Fig. 6D). ODQ, a guanylate cyclase inhibitor, inhibited SNP relaxation to the same degree in corpus cavernosum from all three groups of mice (Supplementary Figure S4A–C).
In vivo erectile function
Mean arterial pressure (MAP) was decreased in KCa2.3T/T (−Dox) mice compared to WT and KCa2.3T/T (+Dox) mice (Supplementary Figure S4A). Basal intracavernosal pressure (ICP) was not different among the three groups of animals (Supplementary Figure S5B). Stimulation at 6 V of the cavernous nerve caused frequency-dependent increases in erectile function measured as peak intracavernal pressure (PICP)/MAP. These responses were markedly decreased in KCa2.3T/T (+Dox) mice compared to WT, and also compared to the KCa2.3T/T (−Dox) mice at 8 Hz (Fig. 7A–C). However, at 16 Hz stimulation, the erectile responses in KCa2.3T/T (+Dox) and KCa2.3T/T (−Dox) mice were similar and both reduced compared to those in WT mice (Fig. 7D). At lower frequencies there was an enhancement of erectile function in the KCa2.3T/T (−Dox) mice (Fig. 7A and D). Stimulations at 1.5 and 3 V showed the same pattern of responses (Results not shown).
In vivo erectile measurements comparing the KCa2.3 mouse models. Mean arterial pressure and intracavernous pressure during cavernous nerve stimulation at 6 V with different frequencies (2, 4, 8, 16 Hz) in (A) KCa2.3T/T (−Dox) mice with KCa2.3-over-expression. (B) wild type (WT) mice. (C) KCa2.3T/T (+Dox), mice with KCa2.3-down-expression. (D) PICP at 6 V with different stimulation frequencies (2, 4, 8, 16 Hz) stimulations from WT vs KCa2.3T/T (−Dox) ($)/(#) KCa2.3T/T (+Dox) mice. Comparisons are made between groups and the same stimulation frequencies. Results are means ± SEM, P ≤ 0.05 (*), (n = 4–8), Two-way ANOVA followed by Tukey post hoc test.
Discussion
The main findings of the present study are that 1) Genetically encoded down-regulation of the KCa2.3 channel in mice results in erectile dysfunction measured as lowered ICP/MAP at 4 and 8 Hz. 2) Electron microscopy revealed that KCa2.3 channels are located primarily on the luminal plasma membrane and occasionally on the lateral plasma membrane of endothelial cells in the corpus cavernosum, and that modulating the expression of these channels (up- or down-regulation) changes norepinephrine contraction in corpus cavernosum strips. 3) A non-selective opener of KCa2 and KCa3.1 channels, NS309, induced concentration-dependent relaxations and enhanced the response to 0.3 µM acetylcholine in corpus cavernosum strips of WT mice. Therefore, suggesting that modulation of these channels may hold the potential for developing a novel approach for treatment of erectile dysfunction.
Previous studies have shown mRNA expression of KCa2.3 channels in rat and human corpus cavernosum14, 15. In the present study, Q-PCR showed expression of preferentially the KCa2.3 channel subtype, which suggests that KCa2.3 is the major KCa2 subtype in murine corpus cavernosum.
The KCa2.3 mouse model used in the present study is a conditional model, in which doxycycline treatment causes suppression of the overexpressed channel resulting in expression levels below WT levels16, 17. Only few studies had compared against the WT17, 18 and biometrical vascular changes are expected on overexpression of KCa2.3 channels19. Consequently KCa2.3 currents in endothelial cells were found to be significantly lower than in the WT cells18. This is further confirmed by the immunoblotting for KCa2.3 channels in corpus cavernosum from transgenic animals showing upregulation in the vehicle-treated and down-regulation in the doxycycline-treated animals. Certainly, further research in complete KCa2.3 channels deficient models are advised in newer studies.
Immunohistochemical studies have suggested that KCa2.3 and KCa3.1 channels are expressed in the endothelium of rat and human penile arteries20. In the present study on murine penile tissue, immunohistochemical staining’s confirmed expression of KCa2.3 channels in endothelial cells of penile arteries and corpus cavernosum. To the contrary, we did not see staining of smooth muscle, suggesting that KCa2.3 channels are mainly expressed in the endothelium of erectile tissue.
KCa2.3 channels have been suggested to be compartmentalized within the endothelial cells of the systemic circulation, and to co-precipitate with caveolin-1, endothelial NO synthase and transient receptor potential channels21, 22, suggesting that these proteins interact physically with each other perhaps in caveolae. Other studies have suggested that the KCa2.3 channels are connexin-37 associated and are localized close to endothelial-endothelial cell gap junctions23. In the present study, the electron microscopical examinations revealed that in endothelium of corpus cavernosum, there is expression of KCa2.3 channels on the apical or luminal plasma membrane of the endothelial cells and occasionally on lateral membranes of inter-endothelial junctions. In contrast, we found no or only few KCa2.3 channels on basal membranes of the endothelial cells in corpus cavernosum. This localization of the KCa2.3 channels on the endothelial cells in erectile tissue suggests that they may be physically coupled to calcium influx pathways (e.g. calcium-permeable TRPV4 or Piezo-1 channels) on the luminal membrane. On the lateral membrane KCa2.3 channels may be closely coupled to endothelial-endothelial cell communication, but other approaches e.g. measurements of endothelial cell calcium in situ and co-staining of channels and myoendothelial gap junctions will be required to clarify this issue.
Suppression of KCa2.3 channels and KCa3.1 channels either by genetic knockdown or their inhibition by the peptide blockers, apamin and charybdotoxin, respectively, have previously been reported to enhance the responses to vasoconstrictors in rat mesenteric arteries18, 24, 25, lamb coronary arteries26, and neurogenic contractions in rat penile arteries27. In the present study, down-regulation of the KCa2.3 channel in mouse corpus cavernosum, markedly enhanced norepinephrine contraction, while the norepinephrine contraction was reduced in corpus cavernosum from mice with up-regulation of KCa2.3. These results suggest that activation and presence of the KCa2.3 channels counterbalances the vasocontraction elicited by the sympathetic neurotransmitter, norepinephrine in corpus cavernosum, that may perhaps provide an important negative feedback on tone and thus favour erectile function.
In systemic arteries, KCa2.3 and KCa3.1 are involved in endothelium-dependent relaxations18, 28. The channels also contribute to relaxation in rat penile arteries27, 29. Experiments, in which only KCa2 channels were blocked, showed attenuation of acetylcholine relaxations in horse penile arteries as well as in rat corpus cavernosum30, 31, suggesting major roles of KCa2.3 channels in relaxation of corpus cavernosum. However, often combined inhibition of both KCa2 and KCa3.1 channels is needed to effectively reduce acetylcholine relaxation28, 32, suggesting important roles for both endothelial KCa channel subtypes in endothelium-dependent relaxation in many vessels. Our present results, provide the first evidence that this may also be true for corpus cavernosum and penile arteries because in the present study down-regulation of KCa2.3 channel expression did not alter relaxation to acetylcholine or to the opener of KCa2/3.1 channels, NS309 in corpus cavernosum from KCa2.3T/T (+Dox) mice. Interestingly, we found potentiating effects of NS309 and of upregulation of KCa2.3 channels on ACh-induced relaxation suggesting that KCa2.3 channels could add to acetylcholine relaxation under normal conditions. This further suggests that pharmacological modulation of KCa2.3 channels holds the potential for developing a novel approach for treatment of erectile dysfunction.
In contrast to the effect of apamin on NS309 relaxation, genetic modulation of the KCa2.3 channel failed to cause marked changes in NS309 relaxation. Apart from the KCa2.3 channel we cannot exclude contribution from other apamin-sensitive channels to NS309 relaxation in corpus cavernosum. However, the expression of the KCa2.2 and KCa2.1 channels is markedly lower than of the KCa2.3 channels (Fig. 1A). Therefore, another possibility is that there is an upregulation of the KCa1.1 channel current due to upregulation of the KCa1.1β1 subunit. NS309 can lead to release of endothelium-derived NO and prostaglandins followed by activation of smooth muscle KCa1.1 channels10, 13. The marked expression of the KCa1.1 channels and our observation that combined inhibition of NO synthase, L-NOARG and cyclooxygenase inhibited NS309 relaxation (Suppl. Fig. S3) support that upregulation of KCa1.1 activity may counteract the effect of downregulation of the KCa2.3 channel on relaxations induced by acetylcholine in corpus cavernosum.
In line with specific roles of KCa2.3 channels in endothelium-dependent vasodilation and norepinephrine-induced tone, KCa2.3 channels have an impact on blood pressure regulation. Indeed, mice with down-regulation of KCa2.3 channel expression have a higher blood pressure17, 18, 33. In contrast, up-regulation of KCa2.3 channels has been found to have no effect on systemic blood pressure18. This normal blood pressure seems to be caused by higher levels of circulating norepinephrine34, which likely counterbalance the tonic vasodilator input provided by KCa2.3 overexpression as observed in vitro. Concerning pharmacological experiments, administration of another selective opener of KCa2 and KCa3.1 channels, SKA31, reduces blood pressure over several hours in mice35. In conscious dogs, intravenous infusion of SKA-31 produced a strong but short-lived depressor response36. In the present study, we also provide evidence that upregulation of KCa2.3 channel expression leads to significantly reduced blood pressure in the systemic circulation, this was evident in the anesthetized mice. Concerning down-regulation of KCa2.3 our study revealed a trend towards systemically elevated pressure, which is in line with previous reports on elevated pressures in the KCa2.3T/T (+Dox) mice.
In contrast to the changes observed in the systemic blood pressure, we here found that the basal intracavernosal pressure were similar in the mice with either up- or down-regulation of KCa2.3 channel expression. The basal intracavernosal pressures were low, due to the pronounced activity of the sympathetic nerves to the erectile tissue, when the penis is in the flaccid state37, 38. Down-regulation of KCa channels has also been found to up-regulate sympathetic norepinephrine activity34. Although our in vitro studies suggest that overexpression of the KCa2.3 channels can inhibit norepinephrine contraction in corpus cavernosum strips, this effect is not reflected in vivo, probably due to a low basal pressure in corpus cavernosum in vivo.
Drugs currently used to treat erectile function e.g. sildenafil or vardenafil, are phosphodiesterase type 5 inhibitors, which causes relaxation in corpus cavernosum, and penile arteries through increased cyclic GMP also involving activation of KCa1.1 channels39. Although the precise mechanism of action needs to be clarified, other drugs, such as calcium dobesilate, can enhance EDH type relaxation in human erectile tissue and restore erectile function in a diabetic rat model by activation of KCa2.3 and KCa3.1 channels40, 41. Knockout mice of KCa1.1 channels have reduced erectile function42, and openers of KCa1.1 channels can enhance rat erectile function43, 44, but so far this is the first study reporting that down-regulation of KCa2.3 channels causes erectile dysfunction in mice.
The expression of KCa2.3 channels in corpus cavernosum from KCa2.3T/T (+Dox) mice is downregulated compared to the KCa2.3T/T (−Dox) mice, but the downregulation compared to WT mice does not reach significance. In previous studies of KCa2.3T/T mice with and without doxycycline-treatment, only few studies have compared the results with wild type mice17, 18, 45, and it can be discussed whether the wild type or the upregulated KCa2.3T/T (−Dox) mice are the correct controls animals to compare the responses with in animals with downregulation of the KCa2.3 channels. The KCa2.3 T/T channel mice are born with an overexpression of the KCa2.3 channel and that affects probably also the vascular structure and heart during the growth of the animals45. At 16 Hz stimulation of the cavernous nerve, the erectile responses are less compared to the WT both in the KCa2.3T/T (−Dox) mice and the KCa2.3T/T (+Dox) mice. The latter observation may suggest that structural changes influence the erectile response in KCa2.3T/T mice compared to control animals. However, compared to the upregulated KCa2.3T/T (−Dox) mice, the KCa2.3T/T (+Dox) mice have lower erectile responses at lower frequencies of stimulation of the cavernous nerve. Although an instantaneous discharge frequency can reach 35 Hz, the pulse frequency in autonomic nerves rarely exceed 10 Hz46, 47, and therefore the findings of lower erectile responses at these frequencies seem relevant. The in vivo measurement in the present study were performed in anaesthetized animals and that may also influence the erectile responses, and consequently further investigation in conscious animals using other approaches will be required to confirm that KCa2.3 downregulation and/or pharmacological modulation of KCa2.3 channels play a role for erectile function.
KCa1.1 channels consist of pore forming alpha-subunits and regulatory beta-subunits sensitive to calcium and membrane potential, respectively48, 49. Post-transcriptional modulation e.g. sex hormones play a role in regulation of KCa1.1 expression50. As far as we understand this is the first time it is reported that a KCa1.1 beta-subunit can be up-regulated by a down-regulated gen and protein expression of KCa2.3 channels. It would be interesting in future studies to examine whether drugs targeting KCa1.1 channels may restore erectile function in mice with down-regulation of KCa2.3 channels.
In contrast to down-regulation of KCa2.3 channels, up-regulation of the channels gave normal intracavernosal pressure responses to low frequency stimulation of the cavernous nerve, while the maximal responses at 16 Hz were reduced compared to the responses in wild-type mice. However, the present study has been performed in healthy animals. Further studies in animal models for cardiovascular disease would be interesting to examine whether erectile function can be restored in diabetes by selective openers of KCa2.3 channels, once they become available.
KCa2.3 channels are also expressed in the brain and in the conduction system of the heart. The effect on the brain can be limited by development of drugs with hydrophilic groups preventing them from crossing the blood brain barrier. In the heart, blockers of KCa2.3 channels have been shown to prevent atrial fibrillation51, 52, but currently it is unknown whether specific openers of KCa2.3 channels will per se have pro-arrhythmic effects. So far results from experimentation using a non-selective KCa2/3-opener with moderate selectivity for KCa3.1 over KCa2 channels, SKA-31 and SKA-121, in mice and dogs53 did not show pro-arrhythmic action of these openers. Regarding KCa2/3 negative gating modulators54, a recent study showed that the combined KCa2/3 negative gating modulator, RA-2, has no gross blood pressure elevating effects. However it is worth mentioning that the compound produced mild bradycardia in mice that may reflect a baroreceptor response or prolongation of cardiac action potential duration and thus the cardiac cycle.
In summary, the present study shows that KCa2.3 channels are located in the apical plasma membrane of endothelial cells, and occasionally at inter-endothelial junctions of the corpus cavernosum. We found that down-regulation of these channels increases norepinephrine contraction in corpus cavernosum strips, and it seems associated with erectile dysfunction. Moreover, pharmacological activation of KCa2 channels enhances acetylcholine-induced relaxations in corpus cavernosum, suggesting that modulation of these channels holds the perspectives for developing new drugs and a novel strategy to treat erectile dysfunction.
Materials and Methods
Animals and Tissues
Mice were breed at the animal facility of Aarhus University, and animal experiments were performed conform to the guidelines from the Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes. The Animal Experiments Inspectorate from the Ministry of Environment and Food of Denmark approved the study protocol (permissions (2011/561–2011 and 2014-15-2934-01059).
The modified genetically mice used in the present study have a tetracycline-base genetic insertion in a 5′ untranslated region that can be activated by addition of doxycycline (Dox) in the water intake of the animals. For a minimum of 7 days before experimentation Dox (0.5 mg/mL) and sucrose (2%) was administered in dark bottles to the mice. Homozygous KCa2.3 targeted mice (KCa2.3T/T) with addition (KCa2.3T/T (+Dox)) or not (KCa2.3T/T (−Dox)) of Dox, together with their wild-type (WT) littermates were used for experiments. Dox in the water intake was maintained until the research protocols were performed.
For in vivo measurements, the mice were anesthetized with intraperitoneal pentobarbital (50 mg/Kg). Pain was assessed regularly during surgery and pressure measurements by pressing a needle against the paw. In case of reaction additional anesthesia (17 mg/Kg pentobarbital) was administered. The mice were cervical-dislocated-euthanized followed by exsanguination after in vivo studies or for isolation of tissues and in vitro studies.
Corpus cavernosum was isolated from mice with KCa2.3 overexpression (KCa2.3T/T (−Dox), n = 25), down-regulation (KCa2.3T/T (+Dox), n = 23), and control wild type mice (WT, n = 24)16. Genotyping was performed as previously described18, 19. The manuscript followed ARRIVE guidelines55.
Immunoblotting
Corpus cavernosum and aorta from KCa2.3T/T (−Dox) mice, KCa2.3T/T (+Dox) mice and WT mice were snap frozen and kept at −80 °C in different quantities for KCa evaluation. Protein was extracted, quantified and mixed in sample buffer before samples were separated by SDS-PAGE and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad). The following protein amounts were used for detection: aorta 12 μg for KCa2.3; corpus cavernosum 12 μg for KCa2.3 (sc-28621), 10 μg for KCa3.1 or 8 μg for KCa1.1α (-alpha-subunit) (Alomone 1184–1200) or KCa1.1β1 (-beta-1-subunit) (ab3587); cerebellum and liver 8 μg for KCa1.1β1. Samples were incubated with the indicated antibodies, all raised in rabbits: KCa2.3 (1:200), KCa1.1α (1:400), KCa1.1β1 (1:500); and housekeeping proteins: pan-actin (1:1000) and beta-tubulin (1:200). Membranes were then incubated with a secondary anti-rabbit IgG (1:4000) and processed with an ECL-Plus kit (General Electric “GE” Health care). Bands were visualized with a luminescence camera (Image Quant LAS 4000 mini from GE) and intensity was quantified by Image Quant TL software (Amersham Biosciences).
PCR and Q-PCR
Corpus cavernosum tissue was stored in RNA later (Sigma-Aldrich) until extraction and purification of total RNA was performed using the RNeasy Mini Plus Kit (Qiagen). cDNA was synthetized using SuperScript III Reverse Transcriptase (Life Technologies).
The Q-PCR was performed in a MX3005 Q-PCR system (Agilent Technologies). The samples were run for a 40 cycles protocol. Ct-values for the gene of interest were normalised against Ct values for the housekeeping gene (GAPDH), after quantification with the program MxPro v.4.10. (Stratagene, Agilent Technologies). Values are expressed as a ratio of GAPDH. For genotyping of the mice, conventional PCR was performed in a Peqstar thermal cycler (Peqlab). The protocol followed a ‘hot-start’ procedure and thermal cycling conditions.
Immunohistochemistry
Penile tissue was fixed in 2.5–3% paraformaldehyde overnight and paraffin embedded using standard protocols56. Sections were cut, fixed, target retrieve activated and labeled as described in the supplemental protocol for single labeling: primary rabbit anti-KCa2.3 antibody (1:400, Santa Cruz Biotechnology) and secondary goat anti-rabbit peroxidase–conjugated antibody (1:200) were used. Detection was done with 3,3′-diaminobenzidine (DAB) and images were taken using a light microscope (Leica DMRE). For double labeling, sections were incubated with rabbit anti-KCa2.3 antibody (1:400) and mouse anti-smooth muscle actin antibody (1:800, Dako). Visualization was performed with donkey anti-rabbit Alexa Fluor 488-conjugated and donkey anti-mouse Alexa Fluor 555-conjugated secondary antibodies (1:1000, Molecular Probes, Life Technologies). Imaging was obtained with a Leica TCS SL laser scanning confocal microscope and Leica confocal software (Leica).
Electron microscopy
Penile tissue was maintained in 4% paraformaldehyde in a 0.1 M sodium cacodylate buffer overnight, followed by incubation in 2.3 M Sucrose for 2 hours and snap frozen. Ultrathin cryosections were obtained (Reichert Ultracut S, Leica) and incubated with rabbit anti-KCa2.3 antibody 1:1200) followed by incubation with goat-anti rabbit antibody conjugated to 10 nm gold particles (1:50). The sections were stained for 5 min in a 1.8% methylcellulose/0.4% uranyl acetate solution and observed with an electron microscope (Morgagni 268 from FEI Phillips Electron Optics).
Isometric tension recording in isolated corpus cavernosum
After dissection of corpus cavernosum as previously reported43, 57, the strips were mounted between two wire clamps with one clamp connected to an isometric transducer (Danish Myo Technology), and immersed in 10 ml of physiological salt solution (PSS), bubbled with a gas mix (95% O2 and 5% CO2) while kept at 37 °C during the whole experiment27.
For strips from WT, KCa2.3T/T (−Dox) or KCa2.3T/T (+Dox) mice length–contraction curves were constructed with norepinephrine (3 μM) and then acetylcholine ((ACh)−1 μM) to obtain an optimal contraction and relaxation length.
After stable basal tension, corpus cavernosum strips were activated with high potassium salt solution (125 mM KPSS). Afterwards, endothelial function was assessed with norepinephrine (3 μM), and ACh (1 μM). Concentration-response curves (0.001–0.3 μM) were constructed for norepinephrine. The preparations were contracted with norepinephrine to 80% of the maximum response and concentration-response curves constructed for ACh a muscarinic activator, NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime) a KCa2 and KCa3.1 opener, and sodium nitroprusside (SNP) a NO donor (0.001–0.3 μM). SNP concentration-response curves were constructed in the absence or in the presence of a guanylate cyclase inhibitor (ODQ 3 × 10−6M).
To investigate whether opening of KCa2.1-3 and KCa3.1 channels enhances acetylcholine relaxation, the preparations were incubated with NS309 (5 × 10−7 M) prior to construction of concentration-response curves for acetylcholine.
In vivo pressure measurements of intracavernous pressure
Mean arterial blood pressure (MAP) and intracavernous blood pressure (ICP) was measured using catheters placed, respectively, in the carotid artery and corpus cavernosum as previously described11. Maximal stimulation (6 V, 1ms, 16 Hz, 60 s) was applied to check maximal erectile function at the beginning of each experiment, before incremental frequencies (2, 4, 8 and 16 Hz) were applied at 1.5, 3, and 6 V. At the end of the experiment the maximal response was repeated to ensure that the cavernous nerve was intact and erectile function maintained.
Statistical analysis
Statistical comparisons were performed using Graphpad Prism-5.1 (GraphPad Software). Values are presented as means ± S.E.M. QPCR and immunoblotting results were compared with Student’s t-test or in case of three groups with one-way ANOVA followed by Tukey test for multiple comparisons. Norepinephrine-induced-contractions were expressed as mili Newton (mN) of contraction over milligram (mg) of corpus cavernosum dry weight (mN/mg). The responses to ACh, NS309, or SNP were expressed as percentage of relaxation of norepinephrine-(3 μM)-contracted strips. Concentration-response curves were compared using two-way ANOVA followed by a Tukey test or a t-test, when a single concentration between two groups was compared. When the response of a single concentration was examined, with more than two groups, one-way ANOVA followed by Tukey test for multiple comparisons was used. Erectile function was analyzed as the ratio of peak ICP (PICP)(mmHg)/MAP (mmHg) × 100. For each frequency, two-way ANOVA with a Tukey test for multiple comparisons were used. Significance was accepted at P ≤ 0.05.
References
Montorsi, F. et al. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur. Urol. 44, 360–365 (2003).
Kirby, M., Jackson, G. & Simonsen, U. Endothelial dysfunction links erectile dysfunction to heart disease. Int. J. Clin. Pract 59, 225–229 (2005).
Hakim, L. & Goldstein, I. Diabetic sexual dysfunction. Endocrinol Metab Clin North Am 25, 379–400 (1996).
Vickers, M. a. & Wright, E. a. Erectile dysfunction in the patient with diabetes mellitus. Am. J. Manag. Care 10, S3–11–6 (2004).
Vardi, Y. Microvascular complications in diabetic erectile dysfunction: do we need other alternatives? Diabetes Care 32(Suppl 2), 420–422 (2009).
Kaiser, D. R. et al. Impaired brachial artery endothelium-dependent and -independent vasodilation in men with erectile dysfunction and no other clinical cardiovascular disease. J. Am. Coll. Cardiol. 43, 179–184 (2004).
Yavuzgil, O. et al. Endothelial function in patients with vasculogenic erectile dysfunction. Int. J. Cardiol. 103, 19–26 (2005).
Kovacs, I. et al. Correlation between flow-mediated dilation and erectile dysfunction. J Cardiovasc Pharmacol 51, 148–153 (2008).
Grgic, I. et al. Renal fibrosis is attenuated by targeted disruption of KCa3.1 potassium channels. PNAS 106, 14518–14523 (2009).
Dalsgaard, T., Kroigaard, C. & Simonsen, U. Calcium-activated potassium channels - a therapeutic target for modulating nitric oxide in cardiovascular disease? Expert Opin. Ther. Targets 14, 825–37 (2010).
Schjørring, O. et al. Flow‐evoked vasodilation is blunted in penile arteries from zucker diabetic fatty rats. J. Sex. Med. 9, 1789–1800 (2012).
Brøndum, E., Kold-Petersen, H., Simonsen, U. & Aalkjaer, C. NS309 restores EDHF-type relaxation in mesenteric small arteries from type 2 diabetic ZDF rats. Br. J. Pharmacol. 159, 154–65 (2010).
Stankevicius, E. et al. Opening of small and intermediate calcium-activated potassium channels induces relaxation mainly mediated by nitric-oxide release in large arteries and endothelium-derived hyperpolarizing factor in small arteries from rat. J. Pharmacol. Exp. Ther. 339, 842–850 (2011).
Chen, M. X. et al. Small and intermediate conductance Ca 2+ -activated K+ channels confer distinctive patterns of distribution in human tissues and differential cellular localisation in the colon and corpus cavernosum. Naunyn. Schmiedebergs. Arch. Pharmacol. 369, 602–615 (2004).
Zhu, J.-H. et al. Reduced expression of SK3 and IK1 channel proteins in the cavernous tissue of diabetic rats. Asian J. Androl. 12, 599–604 (2010).
Bond, C. T. et al. Respiration and parturition affected by conditional overexpression of the Ca2+ -activated K+ channel subunit, SK3. Science 289, 1942–1946 (2000).
Taylor, M. S. et al. Altered expression of small-conductance Ca2+ -activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ. Res. 93, 124–131 (2003).
Brähler, S. et al. Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119, 2323–2332 (2009).
Wandall-Frostholm, C. et al. Genetic deficit of KCa3.1 channels protects against pulmonary circulatory collapse induced by TRPV4 channel activation. Br. J. Pharmacol. 172, 4493–4505 (2015).
González-Corrochano, R. et al. Ca 2+ -activated K+ channel (KCa) stimulation improves relaxant capacity of PDE5 inhibitors in human penile arteries and recovers the reduced efficacy of PDE5 inhibition in diabetic erectile dysfunction. Br. J. Pharmacol. 169, 449–461 (2013).
Sandow, S. L., Neylon, C. B., Chen, M. X. & Garland, C. J. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (KCa) and connexins: Possible relationship to vasodilator function? J. Anat 209, 689–698 (2006).
Dora, Ka., Gallagher, N. T., McNeish, A. & Garland, C. J. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ. Res. 102, 1247–55 (2008).
Saliez, J. et al. Role of caveolar compartmentation in endothelium-derived hyperpolarizing factor-mediated relaxation: Ca2+ signals and gap junction function are regulated by caveolin in endothelial cells. Circulation 117, 1065–74 (2008).
Liu, M. et al. Alterations in EDHF-mediated hyperpolarization and relaxation in mesenteric arteries of female rats in long-term deficiency of oestrogen and during oestrus cycle. Br. J. Pharmachology 132, 1035–1046 (2001).
Dora, K. A., Hinton, J. M., Walker, S. D. & Garland, C. J. An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery. Br. J. Pharmacol. 129, 381–7 (2000).
Simonsen, U., Garcia-Sacristan, A. & Prieto, D. Apamin-sensitive K+ channels involved in the inhibition of acetylcholine-induced contractions in lamb coronary small arteries. Eur. J. Pharmacol. 329, 153–163 (1997).
Kun, A. et al. Ca 2+ -activated K+ channels in the endothelial cell layer involved in modulation of neurogenic contractions in rat penile arteries. Eur. J. Pharmacol. 474, 103–115 (2003).
Zygmunt, P. M. & Högestätt, E. D. Role of potassium channels in endothelium-dependent relaxation resistant to nitroarginine in the rat hepatic artery. Br. J. Pharmacol. 117, 1600–6 (1996).
Prieto, D. Physiological regulation of penile arteries and veins. Int. J. Impot. Res. 20, 17–29 (2008).
Prieto, D., Simonsen, U., Hernández, M. & García-Sacristán, A. Contribution of K+ channels and ouabain-sensitive mechanisms to the endothelium-dependent relaxations of horse penile small arteries. Br J Pharmacol 123, 1609–20 (1998).
Comerma-Steffensen, S., Dalsgaard, T., Hedegaard, E. & Simonsen, U. Effects of openers of small and intermediate calcium-activated potassium channels on rat erectile function and cardiac rhythm. J. Sex. Med. 13, S94 (2016).
Stankevicius, E. et al. Combination of Ca2+ -activated K+ channel blockers inhibits acetylcholine-evoked nitric oxide release in rat superior mesenteric artery. Br. J. Pharmacol. 149, 560–72 (2006).
Yap, F. C. et al. Endothelial SK3 channel-associated Ca2+ microdomains modulate blood pressure. Am. J. Physiol. - Hear. Circ. Physiol. 310, H1151–H1163 (2016).
Lambertsen, K. L. et al. Genetic KCa3.1-deficiency produces locomotor hyperactivity and alterations in cerebral monoamine levels. PLoS One 7 (2012).
Sankaranarayanan, A. et al. Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol. Pharmacol. 75, 281–95 (2009).
Damkjaer, M. et al. Pharmacological activation of KCa3.1/KCa2.3 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br. J. Pharmacol. 165, 223–234 (2011).
Giuliano, F., Rampin, O., Jardin, A. & Rousseau, J. P. Electrophysiological study of relations between the dorsal nerve of the penis and the lumbar sympathetic chain in the rat. J. Urol. 150, 1960–4 (1993).
Andersson, K.-E. Mechanisms of penile erection and basis for pharmacological treatment of erectile dysfunction. Pharmacol. Rev. 63, 811–59 (2011).
Sanchez, A. et al. Mechanisms of the relaxant effect of vardenafil in rat penile arteries. Eur. J. Pharmacol. 586, 283–287 (2008).
Angulo, J. et al. Calcium dobesilate potentiates endothelium-derived hyperpolarizing factor-mediated relaxation of human penile resistance arteries. Br. J. Pharmacol. 139, 854–62 (2003).
Angulo, J. et al. Diabetes impairs endothelium-dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem. Biophys. Res. Commun. 312, 1202–1208 (2003).
Werner, M. E., Zvara, P., Meredith, A. L., Aldrich, R. W. & Nelson, M. T. Erectile dysfunction in mice lacking the large-conductance calcium-activated potassium (BK) channel. J. Physiol. 567, 545–556 (2005).
Kun, A. et al. NS11021, a novel opener of large-conductance Ca 2+ -activated K+ channels, enhances erectile responses in rats. Br. J. Pharmacol 158, 1465–1476 (2009).
Sung, H. H. et al. Effect of the novel BKCa channel opener LDD175 on the modulation of corporal smooth muscle tone. J. Sex. Med. 12, 29–38 (2015).
Wandall-Frostholm, C. et al. Pulmonary hypertension in wild type mice and animals with genetic deficit in KCa2.3 and KCa3.1 channels. PLoS One 9, 1–11 (2014).
Nilsson, H., Ljung, B., Sjoblom, N. & Wallin, B. The influence of the sympathetic impulse pattern on contractile responses of rat mesenteric arteries and viens. Acta.Physiol.Scand. 123, 303–309 (1983).
Hallin, R. G. & Torebjörk, H. E. Single unit sympathetic activity in human skin nerve during rest and various manoeuvres. Acta Physiol. Scand. 92, 303–317 (1974).
Christ, G. J. Gap junctions and ion channels: relevance to erectile dysfunction. Int J Impot Res 12(Suppl 4), S15–25 (2000).
Torres, Y. P., Morera, F. J., Carvacho, I. & Latorre, R. A marriage of convenience: beta-subunits and voltage-dependent K+ channels. J. Biol. Chem. 282, 24485–24489 (2007).
Valverde, M. A. et al. Acute activation of maxi-K channels (hSlo) by estradiol binding to the beta subunit. Science (80-.) 285, 1929–1931 (1999).
Goldin Diness, J. et al. Effects on atrial fibrillation in aged hypertensive rats by Ca2+ -activated K+ channel inhibition. Hypertension 57, 1129–1135 (2011).
Qi, X. Y. et al. Role of small-conductance calcium-activated potassium channels in atrial electrophysiology and fibrillation in the dog. Circulation 129, 430–440 (2014).
Coleman, N. et al. New Positive Ca2+ -Activated K+ Channel Gating Modulators with Selectivity for KCa3.1. Mol. Pharmacol. 86, 342–357 (2014).
Oliván-Viguera, A. et al. A novel pan-negative-gating modulator of KCa2/3 channels, fluoro-di-benzoate, RA-2, inhibits endothelium-derived hyperpolarization-type relaxation in coronary artery and produces bradycardia in vivo. Mol. Pharmacol. 87, 338–348 (2015).
McGrath, J. C., Drummond, G. B., McLachlan, E. M., Kilkenny, C. & Wainwright, C. L. Editorial: Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 160, 1573–1576 (2010).
Christensen, B. M., Kim, Y.-H., Kwon, T.-H. & Nielsen, S. Lithium treatment induces a marked proliferation of primarily principal cells in rat kidney inner medullary collecting duct. Am. J. Physiol. Renal Physiol. 291, F39–48 (2006).
Mizusawa, H., Hedlund, P., Håkansson, A., Alm, P. & Andersson, K. E. Morphological and functional in vitro and in vivo characterization of the mouse corpus cavernosum. Br. J. Pharmacol. 132, 1333–1341 (2001).
Acknowledgements
The authors thank Henriette Johanson and Heidi Knudsen for their technical assistance during the in vitro research. Thanks to Inger M. Paulsen, Else M. Løcke and Christina Schmidt for their help on microscopy processing. Thanks to Søren Brandt Poulsen for help with taking the immunofluorescence images. This work was supported by Aarhus University, Denmark; and from the “Consejo de Desarrollo Cientifico y Humanistico, Universidad Central de Venezuela” (CDCH-UCV), Venezuela for S. Comerma-Steffensen. Additional support came from the Danish Heart Foundation, The Danish Research Council, and the Novo Nordisk Foundation for U. Simonsen and R. Köhler.
Author information
Authors and Affiliations
Contributions
A.K. produced part of the in vivo studies, provided discussion of the manuscript; B.M. contributed with support-discussion on histo-immunological studies and manuscript writing; C.A. contributed with ideas, discussion and writing of the manuscript; E.H. contributed with western blot ideas, writing of the manuscript; S.C.S. participated in the design of the study and performed in vitro and in vivo experiments, western blot data, primary sampling methodology and evaluation for immunohistochemistry-immune fluorescence-electronic microscopy, as well as wrote the first manuscript draft, corrected and processed the submission; S.M. contributed with PCR and writing of the manuscript; U.S. and R.K. participated in the design of the study, discussion, and writing of the manuscript. All authors reviewed the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Comerma-Steffensen, S., Kun, A., Hedegaard, E.R. et al. Down-regulation of KCa2.3 channels causes erectile dysfunction in mice. Sci Rep 7, 3839 (2017). https://doi.org/10.1038/s41598-017-04188-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-04188-5
- Springer Nature Limited
This article is cited by
-
MYPT1 reduction is a pathogenic factor of erectile dysfunction
Communications Biology (2022)