Abstract
In Japan, radiocesium contamination in foods has become of great concern and it is a primary issue to reduce grain radiocesium concentration in rice (Oryza sativa L.). Here, we report a low-cesium rice mutant 1 (lcs1) with the radiocesium concentration in grain about half that in the wild-type cultivar. Genetic analyses revealed that a mutation in OsSOS2, which encodes a serine/threonine-protein kinase required for the salt overly sensitive (SOS) pathway in plants, is responsible for the decreased cesium (Cs) concentrations in lcs1. Physiological analyses showed that Cs+ uptake by lcs1 roots was significantly decreased under low-potassium (K+) conditions in the presence of sodium (Na+) (low K+/Na+). The transcript levels of several K+ and Na+ transporter genes, such as OsHAK1, OsHAK5, OsAKT1, and OsHKT2;1 were significantly down-regulated in lcs1 grown at low K+/Na+. The decreased Cs+ uptake in lcs1 might be closely related to the lower expression of these genes due to the K+/Na+ imbalance in the lcs1 roots caused by the OsSOS2 mutation. Since the lcs1 plant had no significant negative effects on agronomic traits when grown in radiocesium-contaminated paddy fields, this mutant could be used directly in agriculture for reducing radiocesium in rice grains.
Similar content being viewed by others
Introduction
Because of the incident at Tokyo Electric Power Company’s Fukushima Daiichi Nuclear Power Plants (TEPCO-FDNPP), which was triggered by the Great East Japan Earthquake and the subsequent tsunami on March 11, 2011, a large amount of radiocesium was dispersed into the surrounding environment1, 2. Since radiocesium has a relatively long half-life (134Cs, 2.06 years; 137Cs, 30.2 years) and enters easily through the food chain into the human body3, 4, we must pay special attention to the possible contamination of rice (Oryza sativa L.), a staple crop in Japan. To restrict shipments of rice with radiocesium levels over 100 Bq kg−1, which is stipulated by the Food Sanitation Act in 2012, all rice produced in Fukushima Prefecture has been inspected for radiocesium concentration5. The Ministry of Agriculture, Forestry and Fisheries of Japan announced that no rice with a radiocesium level over 100 Bq kg−1 was produced in 20156. From the viewpoint of the potential risk to human health from chronic exposure to radiocesium, however, customers still have great concern about radiocesium concentrations in rice grains. In addition, revitalization of the area where planting is currently restricted is still incomplete.
Depending on the radiocesium concentrations in the soil, several options are recommended for decontamination: topsoil removal, fine-textured topsoil removal using water, and topsoil burying2, 7. On the other hand, many reports revealed that application of potassium (K+) fertilizer is very effective and more practical in reducing radiocesium uptake by paddy rice7, 8. Kato et al. recommended to increase the concentration of exchangeable soil K+ to 200 mg kg−1 soil before planting to reduce the radiocesium concentration in rice grains to the acceptable level8. Although K+ application is widely implemented in the low-contaminated area in the Fukushima Prefecture, K+ must be applied over a long period of time due to the long half-life of 137Cs. Continuous application of K+ is a laborious and costly countermeasure, and therefore rice cultivars that accumulate less radiocesium in their grains have been proposed.
It is well documented that Cs+ is taken up by plants through K+ transport pathways because two ions have similar chemical properties3, 4, 9. The effectiveness of K+ supply can be explained by a competitive effect of K+ on root Cs+ uptake, and K+ transporters and K+ channels are suggested to mediate Cs+ uptake in roots3, 9. In Arabidopsis, high affinity K+ transporter AtHAK5 is a major mediator of Cs+ uptake in roots under K+ starvation because the mutant lacking AtHAK5 accumulates Cs+ in whole seedlings at about half the concentration in wild-type plants10. Under K+-replete conditions, Cs+ is likely to be transported into Arabidopsis roots via cyclic nucleotide–gated channels (AtCNGCs)4. AtCNGC1 is reported to be a candidate gene for determining the natural variation in shoot Cs concentrations11.
There is a substantial genotypic variation in Cs concentrations in rice grains12, 13 and stable 133Cs concentrations are generally lower in grains of japonica cultivars than in those of indica cultivars12. Significant positive correlation between the concentrations of stable 133Cs and radiocesium (134Cs and 137Cs) in grains was found when 85 cultivars were grown in a paddy field located in Fukushima Prefecture, suggesting that the behavior of radiocesium within rice plants is almost identical to that of stable Cs13. The physiological characteristics of Cs+ transport in rice have been well studied using radioactive or stable Cs+ 14,15,16; however, the genetic mechanisms involved in Cs accumulation in rice are still unknown.
A mutant library can be used not only for forward genetics analysis to identify the genes responsible for Cs accumulation in rice but also as breeding material to produce a practically useful low-Cs-accumulating cultivar. We have previously screened a mutant library produced from Koshihikari (the most popular rice cultivar in Japan) by carbon-ion-beam irradiation, and identified mutants with a nearly undetectable level of cadmium (Cd) in grains17. Forward genetic analysis showed that the low-Cd mutants had a mutation in OsNramp5, which encodes a manganese transporter in rice. One of the mutants, line lcd-kmt2 with no agriculturally or economically adverse traits has been registered as a new cultivar, Koshihikari Kan No.1; it is now being introduced into Japanese paddy fields18. This strategy can be applied to produce practically useful rice cultivars that accumulate less radiocesium in their grains.
In this study, we identified a low-cesium rice mutant 1 (lcs1) with a deletion in Os SOS2, which has a significantly lower radiocesium concentration in its grains than the parent cultivar Koshihikari. The serine/threonine protein kinase SOS2 is required for the salt overly sensitive (SOS) regulatory pathway involved in plant salt tolerance19, 20. In Arabidopsis thaliana, the SOS pathway comprises SOS1, SOS2, and SOS3; SOS2 is activated by SOS3, and the SOS2–SOS3 complex phosphorylates and activates SOS1, a plasma membrane Na+/H+ antiporter, under salinity21,22,23. Rice also uses the SOS pathway to avoid salt toxicity24. However, there are no reports of the involvement of SOS2 in Cs accumulation in plants. We also analyzed the physiological mechanism underlying the decrease in Cs concentration by the SOS2 mutation in rice.
Results
lcs1 is a practically useful rice mutant with reduced radiocesium uptake
A mutant library of Koshihikari was produced by ion-beam irradiation17 and the lcs1 mutant was selected from 2710 M2 (second generation after mutagenesis) plants (Supplementary Fig. S1). The grain 133Cs concentration was 0.015 mg kg−1 in lcs1 and 0.05 mg kg−1 in the Koshihikari parent (WT). The lcs1 mutant and WT were cultivated together in three paddy fields about 60 km northwest from TEPCO-FDNPP. Radiocesium concentration in soil was 1057–1705 Bq kg−1 for 134Cs and from 3894–6387 Bq kg−1 for 137Cs; for both isotopes, it was highest at site C (Supplementary Table S1). The exchangeable soil Na+ concentrations were similar among the sites. The exchangeable soil K+ concentration and the K+/Na+ ratios were lowest at site C and highest at site A. The exchangeable soil K+ concentration at all sites was lower than the recommended range (80–250 mg K+ kg−1) for lowland paddy soil8.
The morphology of lcs1 and WT plants was indistinguishable at all three sites (Fig. 1a). Although grain yields varied among the sites, the difference between lcs1 and WT was not significant (Fig. 1b). Straw yield was insignificantly lower in lcs1 than in WT at all sites (Fig. 1c). Total radiocesium (134Cs and 137Cs) concentration in rice grains varied greatly among the sites and between WT and lcs1 (Fig. 1d). It was the highest at site C, where radiocesium concentration in lcs1 was 32% of that in WT. At site B, it exceeded 100 Bq kg−1 in WT, but was almost half the WT level and below the 100 Bq kg−1 limit in lcs1. At site A, it was the lowest and was significantly lower in lcs1 than in WT. Radiocesium concentration in straw was also lower in lcs1 than in WT at all sites, although at site B the difference was not significant (Fig. 1e). These results indicate that lcs1 is a low-Cs mutant line potentially useful for agriculture.
Plant morphology, grain yield, straw yield, and total radiocesium (sum of 134Cs and 137Cs) in wild-type Koshihikari and its low-cesium mutant (lcs1) grown in three paddy fields located in Fukushima Prefecture. (a) Morphology of WT and lcs1 plants grown in three fields (sites A, B, and C). (b) Grain yield. (c) Straw yield. (d) Total radiocesium concentration in grains (unpolished rice). (e) Total radiocesium concentration in straw. Data are means ± SD of three biological replicates. **P < 0.01, *P < 0.05 (t-test).
The concentrations of K+, sodium (Na+), and rubidium (85Rb), which are group I alkali metals, were also compared between the WT and lcs1 at all sites. The differences in K+ concentrations between the WT and lcs1 were small, although lcs1 showed significantly lower K+ concentrations than the WT in grains at site B and in straw at sites A and B (Supplementary Fig. S2a,b). Na+ concentrations in grains and straw were significantly lower in lcs1 than in WT at site C (Supplementary Fig. S2c,d). Stable Rb concentrations in grains and straw were lower in lcs1 than in WT at all sites (Supplementary Fig. S2e,f). Thus, the lcs1 mutation affects the concentrations of not only Cs but also some other alkali elements in plants.
A mutation in OsSOS2 is responsible for reducing the radiocesium level in lcs1
We developed an F2 population (second filial generation) derived from a cross between lcs1 and the indica cultivar Habataki, and performed quantitative trait loci (QTL) analysis. The F2 population segregated widely for 133Cs and 85Rb concentration in grains (Supplementary Fig. S3a,b). QTL analysis revealed that major-effect QTLs for reducing Cs and Rb concentration in lcs1 grains were co-localized on chromosome 6 (Supplementary Fig. S3c,d). Linkage analysis mapped the lcs1 candidate locus to 1.64 Mbp between the markers ad06010939 and RM20377 on chromosome 6 (Supplementary Fig. S3e). To identify the gene responsible for the lcs1 phenotype, we sequenced the whole genomes of WT and lcs1 and compared the candidate regions on chromosome 6; this analysis identified two mutations (Supplementary Table S2). One of them was a 1485 bp deletion in the gene Os06g0606000 (Supplementary Fig. S3f), which is annotated as encoding the SALT OVERLY SENSITIVE 2 protein (OsSOS2) in the Rice Annotation Project Database (http://rapdb.dna.affrc.go.jp/). The other mutation was an A-to-C SNP transition in an intergenic region, suggesting that the deletion in OsSOS2 is responsible for low Cs concentration in lcs1.
As shown in Fig. 2a, OsSOS2 in the lcs1 mutant lacked part of exon 11 and all the downstream exons (12–14). Genomic sequence data revealed that truncated exon 11 was joined to the intergenic region (Fig. 2b). Polymerase chain reaction (PCR) was conducted to amplify the mutated genomic region using a marker primer set (sosP1 and sosP2) designed to recognize the regions flanking the deletion site in lcs1 (Fig. 2a). The sizes of WT and lcs1 PCR fragments were clearly different and consistent with the 1458-bp deletion in lcs1 (Fig. 2c). Reverse transcriptase (RT)-PCR analysis detected a transcript with exons 9 and 10 in lcs1 (Fig. 2d), but no transcripts that would include exons 9–11, although the annealing sites for the primers sosP3 and sosP5 were present in the lcs1 genome. Instead, an abnormal transcript, in which the intron between exons 10 and 11 was not spliced out, was abundant in lcs1 (Fig. 2e). In this transcript, a stop codon appeared immediately downstream of exon 10 (Fig. 2f). These results strongly suggest that OsSOS2 protein molecules in lcs1 are C-terminally truncated.
Identification of the gene responsible for the decreased Cs+ uptake in lcs1. (a) Structure of OsSOS2 (Os06g0606000) and the mutation site in lcs1. (b) Genomic sequence of the mutation site: exon 11 of OsSOS2 is joined to the intergenic region. (c) Fragments of the genomic region containing the mutation site amplified using the marker primer set sosP1and sosP2 shown in (a). Het, an F1 plant derived from a cross between WT and lcs1. (d) RT-PCR analyses of OsSOS2 transcripts using the primers sosP3, sosP4, and sosP5 designed to recognize exons 9–11. RNA was extracted from the whole roots of 8-day-old seedlings. (e) RT-PCR analysis of transcripts using the primers sosP3, sosP4, and sosP6. (f) Codons and the amino acid sequence around the junction between exon 10 and the unspliced intron of OsSOS2 in lcs1. The FISL motif is a site for SOS3 binding.
Low Cs+ uptake in lcs1 requires the presence of Na+ under low-K+ conditions
Since the sos2 mutant of A. thaliana is hypersensitive to NaCl20, we examined whether lcs1 is less tolerant to high-Na+ stress than is WT. No difference in growth was found between WT and lcs1 seedlings grown on a nutrient solution without Na+ (Supplementary Fig. S4). When the plants were treated with 100 mM NaCl for 5 days, the lcs1 leaves rolled up and yellowed (Supplementary Fig. S4a), and the dry weight of shoots and roots became significantly lower in lcs1 than in WT (Supplementary Fig. S4b,c). This result suggests that the OsSOS2 mutation in rice increased sensitivity to NaCl.
To analyze the physiological mechanism underlying the decrease in Cs accumulation in lcs1, we mimicked the difference between WT and lcs1 in long-term radiocesium accumulation in the field by performing short-term hydroponic experiments. Seedlings were grown in half-strength Kimura B solution, which is widely used for rice25. K+, Na+, and stable 133Cs+ levels in the nutrient solution were selected in our preliminary experiments with reference to published data26,27,28. The 133Cs+ level (0.1 μM) was set as low as possible. First, we examined 133Cs+ uptake in WT and lcs1 at different Na+ levels for 24 h (Fig. 3). 133Cs concentrations gradually decreased with increasing external Na+ levels; they were significantly lower in lcs1 than in WT at ≥5 mM Na+ in roots (Fig. 3b), but only at 10 mM Na+ in shoots (Fig. 3a). Na+ did not affect shoot K+ concentrations (Fig. 3c), but those in roots were significantly lower in lcs1 than in WT at 10 and 50 mM Na+ (Fig. 3d). Na+ concentrations gradually increased with increasing external Na+ levels; they were significantly higher in lcs1 than in WT at ≥5 mM Na+ in roots (Fig. 3f) but tended to be lower in lcs1 than in WT in shoots, with a significant difference at 50 mM Na+ (Fig. 3e). These data indicate that the decreased Cs+ uptake by lcs1 roots is linked to an increase in root Na+ concentrations.
Effects of varying levels of Na+ on the concentrations of stable 133Cs, K+, and Na+ in the shoots and roots of WT and lcs1. (a,b) 133Cs concentrations in (a) shoots and (b) roots. (c,d) K+ concentrations in (c) shoots and (d) roots. (e,f) Na+ concentrations in (e) shoots and (f) roots. Plants pre-cultured in 0.2 mM K+ for 7 days were treated with nutrient solution containing 0.1 µM Cs+ and low K+ (0.05 mM) at varying levels of Na+ for 24 h. Data are means ± SD of three biological replicates. **P < 0.01, *P < 0.05 (t-test).
Next, we examined the time course of changes in 133Cs concentration in shoots and roots of WT and lcs1 grown in the presence or absence of 10 mM Na+ under low-K+ (0.05 mM) conditions (Fig. 4). Our preliminary data showed that the weight of lcs1 plants did not significantly differ from that of WT plants after a 1-week treatment with 10 mM Na+, but brown spots and the yellowing of lower leaves were more noticeable in lcs1 than in WT. We did not find any differences in Cs concentrations in roots or shoots between the WT and lcs1 grown in the absence of Na+ during the 6-day treatment. Na+ supply to low-K+ medium markedly decreased Cs concentrations in shoots and roots; Cs concentrations were clearly lower in lcs1 than in WT. The difference in shoot Cs concentration was observed from day 2 of treatment; shoot Cs concentration in lcs1 was lower than that in WT by 35% on day 4 and by 40% on day 6 (Fig. 4a). Root Cs concentration was lower in lcs1 than in WT from day 1 of treatment, and the difference increased thereafter. At day 6, root Cs concentration in lcs1 was approximately half that of WT (Fig. 4b). Therefore, Na+ is essential for suppressing Cs accumulation in lcs1.
We tested the effect of varying levels of K+ on Cs+ uptake of plants grown in the presence of 10 mM Na+ for 6 days (Fig. 5). In both WT and lcs1, 133Cs concentrations in roots and shoots decreased remarkably with increasing K+ supply, in agreement with the reported inhibitory effect of K+ on Cs+ uptake by the plants29,30,31. At low K+ (0.02 or 0.05 mM) in the presence of Na+, root and shoot Cs concentrations were significantly lower in lcs1 than in WT. At medium K+ (0.2 mM) and high K+ (1 mM) in the presence of Na+, root Cs concentrations were still lower in lcs1 than in WT, although not significantly so. These results suggest that low external K+ and the presence of Na+ are necessary for lower Cs+ uptake in lcs1 than in WT.
Effects of varying K+ levels on 133Cs concentrations in (a) shoots and (b) roots of WT and lcs1. Plants were treated with nutrient solution containing 0.1 µM Cs+ in the presence of 10 mM Na+ and varying levels of K+ for 6 days. Data are means ± SD of three biological replicates. **P < 0.01 (t-test).
Similar to the situation with Cs+ uptake, 85Rb concentrations in shoots and roots were also significantly lower in lcs1 than in WT when plants were grown for 6 days in nutrient solution containing 10 mM Na+ and low K+ (0.05 mM) (Supplementary Fig. S5a,b), suggesting that Cs+ and Rb+ might be transported into rice roots via similar pathways. Taken together, the physiological analyses show that the low-Cs phenotype of lcs1 appears in the presence of Na+ under low-K+ conditions.
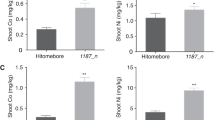
Next, we verified that a mutation in the A. thaliana SOS2 gene inhibits Cs+ uptake. The shoot and root 133Cs concentrations were significantly lower in sos2-1 21 than in WT (Columbia) grown in low K+ (0.05 mM) and the presence of 10 mM Na+ (Fig. 6a,b), especially in sos2-1 roots, where the decrease in Cs was accompanied by an increase in root Na+ concentration (Fig. 6d). No difference in shoot Na+ concentration between WT and sos2-1 was found (Fig. 6c). At Na+ supply, K+ concentrations in roots and shoots in sos2-1 were significantly lower than those in WT, indicating that 10 mM Na+ severely inhibits K+ uptake in the sos2-1 mutant (Fig. 6e,f). Na+ supply had the strongest effect on 85Rb concentrations in roots and shoots in sos2-1 (Supplementary Fig. S5c,d). These results verified the inhibitory effect of SOS2 mutations on Cs+ uptake.
Effect of Na+ on the concentrations of 133Cs, K+, and Na+ in the shoots and roots of Arabidopsis thaliana Columbia (WT) and sos2-1 mutant. (a,b) 133Cs concentrations in (a) shoots and (b) roots. (c,d) Na+ concentrations in (c) shoots and (d) roots. (e,f) K+ concentrations in (e) shoots and (f) roots. Three-week-old plants grown in MS medium were treated with nutrient solution containing 1 µM Cs+, 1 µM Rb+ and 10 mM Na+ under low-K+ (0.05 mM) conditions for 6 days. Data are means ± SD of three biological replicates. **P < 0.01 (t-test).
Next, we tested whether the lcs1 allele of OsSOS2 confers a low-Cs phenotype in a rice cultivar carrying the wild-type OsSOS2 allele. We produced an F2 population from a cross between lcs1 and lcd-kmt2, a mutant with low-Cd phenotype in the Koshihikari background17. In lcs1 homozygotes, 133Cs concentrations in shoots and roots were lower than in WT homozygotes and heterozygotes (Supplementary Fig. S6). This demonstrates that the lcs1 allele decreases Cs in rice plants. Among 91 F2 plants, 5 carried both lcs1 and lcd-kmt2 alleles and had decreased concentrations of Cs (Supplementary Fig. S6) and Cd in shoots and roots, indicating that it is possible to produce new cultivars with both traits (low Cs and low Cd).
Sodium inhibits the expression of several genes encoding monovalent cation transporters in lcs1
To investigate the molecular mechanism of decreased Cs+ uptake in lcs1, we used microarray analysis to compare the root transcriptomes of WT and lcs1 grown in the presence or absence of 10 mM Na+ at 0.05 or 0.2 mM K+ for 3 days. Because K+ transporters are suggested to mediate Cs+ uptake in plants3, 9, we focused on the expression of four gene families (OsHAK, OsAKT, OsHKT, and OsCNGC) involved in K+ transport (Table S3). After excluding the genes with signal values less than 50, we selected four genes (OsHAK1, OsHAK5, OsAKT1, and OsHKT2;1) whose down-regulation was stronger in lcs1 than in WT at low K+ in the presence of Na+.
Quantitative real-time PCR (qRT-PCR) was conducted to compare the time courses of the expression of OsHAK1 (Os04g0401700; Fig. 7a), OsHAK5 (Os01g0930400; Fig. 7b), OsAKT1 (Os01g0648000; Fig. 7c), and OsHKT2;1 (Os06g0701700; Fig. 7d) between WT and lcs1. In plants pre-grown at low K+ (0.02 mM) for 7 days, the expression levels of the four genes were higher than in plants grown at high K+ (1 mM). The expression of OsHKT2;1 was particularly strongly upregulated (>60 times) in K+-starved plants. OsHAK5 was the only gene whose expression was usually lower in lcs1 than in the WT, irrespective of K+ or Na+ level. In both WT and lcs1, the low K+–enhanced expression of the four genes was drastically decreased at 2 h and 6 h after the addition of Na+. The expression of all genes was increased at 12 h and 24 h after Na+ addition in WT but not in lcs1, resulting in significant differences between WT and lcs1.
Relative mRNA levels of OsHAK1 (a), OsHAK5 (b), OsAKT1 (c), and OsHKT2;1 (d) in the roots of WT and lcs1. Plants were treated with nutrient solution containing 1 mM K (high K+) or 0.02 mM (low K+) for 7 days. Then the plants grown at low K+ were transferred into a solution with 10 mM Na+ under low-K+ conditions (Na+ + low K+) and treated for 2, 6, 12, or 24 h. Total RNA was extracted from the roots and the expression of each gene was determined by qRT-PCR. Actin 1 was used as an internal control. The expression of each gene in wild-type treated with high K+ was defined as 1. Data are means ± SD of five biological replicates. **P < 0.01, *P < 0.05 (t-test).
Discussion
A goal of this study was to provide a practically useful rice line that would accumulate less radiocesium in its grains to improve food safety in Fukushima Prefecture. The lcs1 mutant was selected from a mutant library of the most popular Japanese rice cultivar Koshihikari (Supplementary Fig. S1). A lower radiocesium concentration in lcs1 than in WT grains was confirmed in field trials (Fig. 1d). Genetic analysis revealed that lcs1 had a 1485-bp deletion, which included the sequence encoding the C-terminal region of OsSOS2 (Fig. 2a, Supplementary Fig. S3f). SOS2 consists of the N-terminal catalytic domain, which was preserved in lcs1, and the C-terminal regulatory domain, which was truncated in lcs1. The regulatory domain contains a FISL motif, which binds to the calcium-binding protein SOS3, and an adjacent protein phosphatase interaction (PPI) motif32. In lcs1, the FISL but not the PPI motif was present (Fig. 2f). The PPI motif binds to the type 2 C protein phosphatase ABI232, which may deactivate SOS2 or dephosphorylate its target ion transporters such as SOS133. In the Arabidopsis abi2-1 mutant, the interaction between ABI2 and SOS2 is disrupted, and this mutant is more tolerant to salt shock than is WT33. Mutations in the PPI motif also weaken the SOS2–SOS3 interaction, possibly because of a steric hindrance effect on the neighboring FISL motif33. In the lcs1 mutant, the absence of the PPI motif in OsSOS2 would prevent its interaction with ABI2 and weaken the OsSOS2–OsSOS3 interaction, increasing Na+ sensitivity of the mutant (Supplementary Fig. S4) and decreasing Na+ extrusion from the roots by OsSOS1. The lcs1 mutant accumulated more Na+ in the roots than did WT (Fig. 3f), which strongly supports this speculation, although shoot Na+ concentration was lower in lcs1 than in WT (Fig. 3e). In Arabidopsis shoots, the complex of SOS2 with the calcium sensor CBL10 plays a role in Na+ sequestration into the vacuole by regulating tonoplast Na+/H+ antiporter in shoots and leaves34, 35; the cbl10 mutant contains less Na+ in shoots than does WT, and is more sensitive to high Na+ 34. The lcs1 mutant might also be defective in vacuolar Na+ sequestration in shoots.
SOS2 is well studied as a multifunctional protein kinase that regulates salt tolerance in plants19,20,21,22,23; however, to the best of our knowledge, its involvement in plant Cs+ uptake has not been documented. SOS2 has no potential transporter domains to mediate Cs+ uptake. SOS1 is also unlikely to be related to Cs+ influx into the roots because SOS1 is an Na+ efflux transporter23. The CBL10–SOS2 complex is suggested to regulate a tonoplast Na+/H+ antiporter in shoots and leaves34, 35. These data suggest the involvement of other genes related to Cs+ transport together with an alteration in the SOS pathway. Increasing K+ supply decreased root Cs+ uptake in both WT and lcs1 (Fig. 5); this indicates that rice Cs+ uptake is mediated by a K+ transport system14. In Arabidopsis roots, AtHAK5 mediates K+ uptake at external K+ concentrations <0.01 mM, whereas AtAKT1 is more important for K+ uptake in a range of 0.05–1 mM36. In rice, two HAK transporters (OsHAK126 and OsHAK537) and OsAKT138 are crucial for K+ uptake at ≤1 mM K+. OsHAK5 mediates K+ uptake at low K+ (0.1 or 0.3 mM), but not at high K+ (1 mM)37, whereas OsHAK1 and OsAKT1 function in the wide range of 0.05–1 mM K+ 26, 38. Because significant differences in Cs+ uptake between WT and lcs1 were found at ≤0.2 mM K+, OsHAK1, OsHAK5, and OsAKT1 might contribute to the decreased Cs+ uptake in lcs1. Under K+-depleted conditions, high Na+ directly represses the expression of OsHAK1 26 in rice and AtHAK5 28 in Arabidopsis. Na+ may affect not only K+ uptake but also Cs+ uptake through K+ transporters. As the first step to clarify the molecular mechanisms of Cs accumulation in WT and lcs1, we analyzed the differences in the transcription levels of genes involved in K+ transport by microarray and qRT-PCR analyses. The transcript levels of OsHAK1, OsHAK5, and OsAKT1 were increased in the roots of lcs1 and WT grown at low K+ (0.02 or 0.05 mM) in the absence of Na+ (Fig. 7 and Supplementary Table S3). The expression of OsHKT2;1, which encodes the primary Na+ transporter in rice under K+-starvation conditions27, was also remarkably upregulated at low K+. However, the time-dependent differences in Cs+ uptake (Fig. 4) and the differences in transcript levels (except for OsHAK5) were not observed between WT and lcs1 at low K+ alone. In the lcs1 mutant, the suppression of Cs+ uptake into roots and its subsequent translocation to shoots required low K+ in the presence of Na+ (Figs 3–5). This phenomenon was also observed in the sos2-1 mutant of Arabidopsis (Fig. 6). The transcript levels of OsHAK1, OsHAK5, OsAKT1, and OsHKT2;1 were significantly lower in lcs1 roots than in WT roots after adding Na+ to low-K+ medium (Fig. 7). These results suggest that the K+/Na+ ratio in medium is a key factor that determines the difference in Cs accumulation between WT and lcs1; the decreased Cs+ uptake in lcs1 roots might be caused by suppressed expression of any of these genes.
A rice–barley chimeric HAK1 protein can mediate K+, Rb+, and Cs+ influx in a yeast and has a low capacity to discriminate these ions39. OsHAK5 is also likely to mediate Cs+ influx in rice roots, especially at low-K+ conditions, but the OsHAK5 transcript level in microarray analysis was substantially lower than that of OsHAK1 (Supplementary Table S3), and the OsHAK5 transcript level measured by qRT-PCR was lower in lcs1 roots than in WT roots irrespective of the supply levels of K+ and Na+ (Fig. 7b). Therefore, the contribution of OsHAK5 might be smaller than that of OsHAK1, and the decreased Cs+ uptake in lcs1 might be caused by the lower transcript levels of OsHAK1 rather than OsHAK5. In yeast expressing AtHAK5, the selectivity for K+ over Na+ and Cs+ was increased by a single point mutation, F130S, which is conserved in HAK1-type and HAK5-type transporters of several species, including OsHAK140. Modification of ion selectivity of a specific transporter (e.g., OsHAK1) might be useful for the production of low-Cs rice without decreasing K+ level in the plant.
AtAKT1 and AtHAK5 act as the main system of Rb+ absorption in K+-starved Arabidopsis 41, although root Cs+ influx is similar in the akt1 knockout mutant and WT42. 85Rb concentrations as well as 133Cs concentrations in lcs1 and sos2-1 mutants were significantly decreased by low K+ and the presence of Na+ (Supplementary Fig. S5). Because of the similarity of the hydrated ionic radii of Rb+ and Cs+ (both are 0.329 nm)43, some channels and transporters discriminate poorly between them. Kobayashi et al. suggested that up to 50% of Cs+ uptake in rice roots grown at 0.02 mM K+ in the presence of 0.1 μM Cs+ is mediated by tetraethyl ammonium–sensitive inward-rectifying K+ channels such as OsAKT114. OsAKT1 might mediate Cs+ uptake in rice roots, and down-regulation of OsAKT1 may contribute to the decreased Cs+ uptake in lcs1 grown at a low K+/Na+ ratio.
Under low K+/Na+ conditions, Na+ substitutes K+ to improve rice growth44, and OsHKT2;1 is crucial for nutritional Na+ uptake into K+-starved rice roots45. The expression of OsHKT2;1 was most sensitive to Na+ supply at low K+ and was significantly lower in the roots of lcs1 than of WT (Fig. 7d). However, OsHKT2;1 is unlikely to mediate Cs+ uptake in rice because of a small permeability to K+ and Rb+ via OsHKT2;127. The OsCNGC family is also unlikely to be involved in the difference in Cs+ accumulation between lcs1 and WT under low K+/Na+ conditions because CNGCs mediate Cs+ uptake by the roots of K+-replete plants4. In this study, we could not conclusively identify the K+ transporters that are most likely to mediate the suppression of Cs+ uptake in lcs1 grown at low K+/Na+, and further studies such as post-transcriptional analysis will be necessary to identify these transporters.
Based on the results of hydroponic experiments with seedlings, we analyzed why radiocesium concentrations in lcs1 grains were lower than in those of WT under the field conditions. Four genes (OsHAK1, OsHAK5, OsAKT1, and OsHKT2;1) were expected to be up-regulated in both WT and lcs1 roots under the field conditions, because exchangeable soil K+ levels were lower than the recommended levels (80–250 mg K+ kg−1)8. Although the exchangeable soil Na+ levels do not affect rice growth, Na+ uptake via OsHKT2;1 must be promoted to substitute for K+. Because lcs1 is unable to extrude Na+ from roots, Na+ concentration in the roots would gradually increase, and a low K+/Na+ ratio in roots would down-regulate the levels of OsHAK1, OsHAK5, and OsAKT1 transcripts and radiocesium concentrations in lcs1 grains due to the suppression of radiocesium uptake into the roots. Suppression of the transcript levels of K+ transport genes in lcs1 is a disadvantage for K+ uptake, but a large decrease (such as that observed for Cs concentrations) was not found in lcs1, probably indicating that K+ is rapidly exported from roots to shoots (unlike Cs+) to maintain K+ homeostasis in the shoots14. The exchangeable K+/Na+ ratio in soil is a key factor that determines the difference in radiocesium concentration in grains between WT and lcs1. Significant correlation was observed between the difference in radiocesium concentration (y) and the exchangeable K+/Na+ ratio (x) if the former was logarithmically transformed (y = 348.51x −2.402, R 2 = 0.913). Lower Na+ concentration in grains and straw in lcs1 at site C, which had the lowest K+/Na+ ratio among the three sites, can be explained by an alteration in Na+ transport systems via OsSOS1 or tonoplast Na+/H+ antiporter. Although lcs1 was more sensitive to high-salt stress than was WT in hydroponic experiments (Supplementary Fig. S4), this mutation had no considerable negative effects on plant morphology (Fig. 1a) or grain yield (Fig. 1b) at any of the three field sites. Therefore, the lcs1 mutant can be directly introduced into paddy fields for reducing radiocesium in rice grains. In fields like site C (high radiocesium concentration and low K+/Na+ ratio in soil), a combination of K+ supply with the use of lcs1 will be a better method to reduce radiocesium without decreasing grain and straw yield. Moreover, we demonstrated that it is possible to produce rice cultivars with both low-Cs and low-Cd traits (Supplementary Fig. S6). In Japan, there is still a great concern about Cd contamination in rice17. We believe that rice cultivars carrying the lcs1 allele would be an innovative countermeasure to reduce long-term exposure to radiocesium via the food chain.
Materials and Methods
Screening for low-Cs mutants
We irradiated seeds of the most popular Japanese cultivar, Koshihikari, with accelerated carbon ions following the method of Ishikawa et al.17. After irradiation, bulked M2 seeds were sowed and about 3000 M2 seedlings and 300 WT seedlings were transplanted into plastic pots filled with paddy soil containing 3.0 mg kg−1 of 133Cs under flooded conditions. The plants were grown in a greenhouse from mid-May to mid-September 2014 at ambient temperature (20–35 °C) under sunlight until grain filling. Grains were harvested from 2710 M2 plants and their 133Cs concentrations were determined as described below.
Field experiments
Field experiments were conducted in three paddy fields located 60 km northwest from TEPCO-FDNPP. Bulked soil samples were collected from each site to a depth of 15 cm before planting and used for chemical analysis including radiocesium. M5 or M6 lcs1 plants and WT Koshihikari were grown at sites B and C in 2014 and at site A in 2015. Commercial fertilizer was applied before transplanting (60 kg N and 60 kg P2O5 per ha). Potassium was not applied to avoid competition with radiocesium for uptake by rice roots. At maturing stage, rice was harvested by cutting the stems 10–15 cm above the soil level to prevent contamination with radiocesium from the soil, and separated into grains and straw. Husked grains (brown rice) were sieved through a 1.8-mm mesh to remove immature grains and weighed to evaluate grain yield. After weighing, grain and straw samples were used to analyze the concentration of radiocesium and other metals as described below.
Radiocesium and metal analysis
For the determination of total 137Cs and 134Cs, soil, rice grain, and straw samples were placed in a U8 or 2 L Marinelli container and analyzed using a germanium semiconductor detector (GX5520, Canberra Co.)46. The concentrations of 134Cs and 137Cs were corrected to the date of harvest based on radioactive decay. Plant samples were digested with concentrated HNO3–H2O2 18 and the stable isotopes 133Cs and 85Rb were analyzed by inductively coupled plasma mass spectroscopy (ICP-MS; ELAN DRC-e, Perkin-Elmer Sciex). The concentrations of K+ and Na+ in plant samples were determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES; 700 Series, Agilent Technologies) after digestion with HNO3–H2O2. Exchangeable K+ and Na+ were extracted from soil with 1 M CH3COONH4 (=1:10, pH 7.0) and analyzed by ICP-OES.
Gene identification by using a combination of SNP linkage mapping and whole-genome resequencing
An F2 population was derived from a cross between lcs1 and the indica rice cultivar Habataki. The F2 progeny consisting of 89 individuals and the parents were grown until grain filling under the same conditions as for the mutant screening. The concentrations of 133Cs and 85Rb in individual plants were analyzed by ICP-MS as described above. At the seedling stage, a piece of leaf blade was collected for genomic DNA extraction. To genotype the F2 population, 355 SNPs were selected from a world core SNP set, which includes 768 SNPs that are relatively evenly spaced on the 12 chromosomes47. SNPs were detected using the GoldenGate BeadArray technology platform and BeadStation 500 G system (Illumina) according to the manufacturer’s instructions. QTL analysis was conducted to map the candidate gene loci for lcs1 according to Ishikawa et al.17. Five simple sequence repeat (SSR) markers were added to delimit the interval containing the candidate gene.
Whole-genome resequencing of WT and lcs1 was performed according to Takagi et al.48. The libraries for DNA sequencing were constructed with a TruSeq DNA PCR-Free LT Sample Preparation Kit (Illumina) and sequenced on an Illumina NextSeq 500 platform (125 bp pared end sequence). Short reads were trimmed in 115 bp, and those in which >20% of the sequence had a Phred quality score of <20 were excluded from further analysis. Large deletions in lcs1 were detected by comparing the sequence depth between WT and lcs1. To avoid library bias, three sequence libraries (n = 3) were constructed for WT and lcs1 (Supplementary Table S4). Sequence reads obtained from each library were independently aligned to the Nipponbare genome (http://rapdb.dna.affrc.go.jp/download/irgsp1.html) by using BWA49, and the aligned data were converted to SAM/BAM files using SAMtools50. The average depth was calculated for the three WT libraries in regions longer than 15 bp where all WT libraries, but not lcs1 libraries had at least an aligned sequence reads. The regions with an average depth of >5 in WT were defined as large deletions in lcs1. SNPs between WT and lcs1 were detected by using MutMap pipeline version 1.4.4 (http://genome-e.ibrc.or.jp/home/bioinformatics-team/mutmap) using combined sequence reads the three WT and lcs1 libraries, respectively. SNPs with the depth > 7 and SNP-index = 1 were selected.
To confirm the results of the whole-genome resequencing, we designed PCR primers (sosP1 and sosP2) corresponding to the region flanking the deletion site in the candidate gene (OsSOS2; Os06g0606000) in lcs1 (Supplementary Table S5). PCR products obtained by amplification of genomic DNA were directly sequenced using the BigDye Terminator Cycle Sequencing Kit 3.1 (Invitrogen) in an ABI3130xl genetic analyzer (Applied Biosystems). The same primers were used to distinguish the WT and lcs1 alleles.
Analysis of OsSOS2 transcript
Total RNA was extracted from whole roots of WT and lcs1 plants using an RNeasy mini Kit (Qiagen) and quantified using a Nanodrop 8000 spectrophotometer (Thermo Scientific). First-strand cDNA was synthesized from 0.5 μg RNA with ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo) according to the manufacturers’ instructions. The transcript of each exon in OsSOS2 was analyzed by RT-PCR using the primers (sosP3, sosP4, sosP5, and sosP6) listed in Supplementary Table S5.
Physiological analysis of the lcs1 mutant in hydroponics
Seeds of WT and lcs1 were surface-sterilized and one-week-old seedlings were hydroponically cultured in a 1.3-L plastic pot filled with K+- and Na+-free modified 1/2 strength Kimura B nutrient solution25. Then, the seedlings were pre-cultured in the nutrient solution supplemented with 0.2 mM KCl for 5 days. In treatments, K+ was added to the nutrient solution as KCl, Na+ as NaCl, 133Cs+ as CsCl, and 85Rb+ as RbCl. To examine the Na+ sensitivity, pre-cultured WT and lcs1 plants were treated with 100 mM Na+ in the presence of 0.2 mM K+ for 5 days. To examine the effect of different Na+ levels on Cs+ uptake, pre-cultured plants were treated for 24 h with varying levels of Na+ (0, 1, 5, 10, or 50 mM) in the presence of 0.1 μM Cs+ under low-K+ (0.05 mM) conditions. For the time-course Cs+ uptake experiment, pre-cultured plants were treated with 0.1 μM Cs+ in the presence or absence of 10 mM Na+ under low-K+ (0.05 mM) conditions for 1, 2, 4, or 6 days. To examine the effect of different K+ levels on Cs+ uptake, pre-cultured plants were treated with varying levels of K+ (0.02, 0.05, 0.2, or 1 mM) in the presence of 0.1 μM Cs+ plus 10 mM Na+ for 6 days. In the Rb+ uptake experiment, pre-cultured plants were treated with 0.1 μM Rb+ in the presence or absence of 10 mM Na+ under low-K+ (0.05 mM) conditions for 6 days. The pH of all solutions was adjusted to 5.0 with H2SO4 and NH4OH, and the solution was renewed every day. All plants were grown in a Biotron (NC350, NK System) with a 16-h-light (28 °C) /8-h-dark (25 °C) photoperiod, relative humidity of 60%, and light intensity of 400 μmol m−2 s−1. All plants were divided into shoots and roots, dried at 70 °C for 3 days, and weighed. The concentrations of K+, Na+, 133Cs, and 85Rb in shoots and roots were analyzed by ICP-OES or ICP-MS after digestion with HNO3–H2O2, as described above.
Functional complementation test with the Arabidopsis sos2-1 mutant and an F2 population from a cross between lcs1 and lcd-kmt2
Seeds of Arabidopsis ecotype Columbia and the sos2-1 mutant21 were surface-sterilized and sown on MS plates containing 0.8% (w/v) agar and 2% sucrose. Plates were placed in a growth chamber (EYELATRON FLI-301N, EYELA) with a 16-h-light (22 °C)/8-h-dark (22 °C) photoperiod and light intensity of 150 μmol m−2 s−1. Three-week-old plants were transferred into the nutrient solution described above. The solution contained 0.05 mM K+, 1 μM Cs+, and 1 μM Rb+ in the presence or absence of 10 mM NaCl (pH 5.5). The solution was renewed every day. The plants were harvested after 6 days, and the concentrations of 133Cs, 85Rb, K+, and Na+ in shoots and roots were analyzed by ICP-MS or ICP-OES after digestion with HNO3–H2O2, as described above.
To demonstrate that the lcs1 allele confers a low-Cs phenotype on a cultivar carrying wild-type OsSOS2 and to produce double mutants with low concentrations of both Cs+ and Cd, lcs1 and lcd-kmt2 (a low-Cd mutant)17 were crossed and the F2 seedlings (91 individuals) were pre-cultured in the nutrient solution as described above. A small leaf piece was collected from each seedling for genomic DNA extraction. Pre-cultured plants were treated with solution containing 1 µM Cs+ and 0.18 µM Cd2+ in the presence of 10 mM Na+ under low-K+ (0.05 mM) conditions for 3 days. The F2 population was genotyped by using genetic markers that can detect the lcs1 (sosP1 and sosP2) and lcd-kmt2 alleles17. The concentrations of 133Cs and 114Cd in shoots and roots were analyzed by ICP-MS after digestion with HNO3–H2O2 as described above.
Microarray and qRT-PCR
For microarray analysis, pre-cultured WT and lcs1 plants were transferred to the nutrient solution containing K+ (0.05 mM or 0.2 mM) in the presence or absence of 10 mM Na+ and grown for 3 days. Total RNA was isolated from whole roots using an RNeasy mini kit, quantified using Nanodrop, and its quality was checked using an Agilent 2100 bioanalyzer (Agilent Technologies). Microarray analyses were performed according to the manufacturer’s protocol and Yamatani et al.51. Briefly, RNA samples were labeled with Cy-3 and Cy-5 using a Quick Amp Labeling Kit, Two-Color (Agilent Technologies). A mixture of Cy3-labeled and Cy5-labeled cRNA (825 ng each) was fragmented and hybridized with the rice 4 × 44 K microarray RAP-DB (Agilent Technologies; G2519F#15241) at 65 °C for 17 h. Slides were washed with washing buffer, and scanned on an Agilent G2505B DNA microarray scanner. Background correction of the raw signals was performed using Agilent Feature Extraction software (version 9.5.3.1).
To confirm the differences in gene expression between WT and lcs1 plants, qRT-PCR was performed. One-week-old seedlings were treated with 0.02 mM or 1 mM K+ for 7 days. Plants exposed to 0.02 mM K+ were then treated with 10 mM Na+ for 2, 6, 12, or 24 h. All samples were frozen immediately in liquid nitrogen and stored at −80 °C until RNA extraction. Total RNA was extracted and quantified as above. Its integrity was checked using agarose gel electrophoresis, and cDNA was synthesized from 0.5 μg RNA with ReverTra Ace qPCR RT Master Mix with gDNA Remover as described above. cDNA was diluted 1:8 in 1/10 TE buffer (1 mM Tris-HCl, 0.1 mM EDTA, pH 8.0). The reaction mixture contained 4 µL of diluted cDNA, 10 µL of 2 × SYBR Green PCR Master Mix (Toyobo), 1 µL of forward and reverse primers (final concentration, 0.3 µM), and 5 µL of PCR-grade H2O. Four reference genes, Actin 1 (Os03g0718100), Ubiquitin 5 (Os01g0328400), β-tubulin 4 (Os01g0805900), and Histone H3 (Os06g0130900) were tested to choose the most reliable reference gene for data normalization. The primers for OsHAK1, OsHAK5, OsAKT1;1, OsHKT2;1, and four reference genes are listed in Supplementary Table S5. Real-time PCR was performed on a LightCycler 96 (Roche) as follows: 1 min at 95 °C, then 40 cycles of 15 s at 95 °C and 1 min at 60 °C. The LightCycler 96 system software version 1.1.0.1320 was used to visualize and analyze the data including mean quantification cycle (Cq) values, amplification efficiencies, and melting curve. PCR efficiency was evaluated using a standard curve and was found to be 1.94 or 2.03 depending on the primer pairs. The expression stability and pairwise variation of the reference genes was analyzed using the geNorm algorithm in the qbase+ software (https://www.qbaseplus.com/), and Actin 1 and β-tubulin 4 were chosen as the best reference genes. Because Actin 1 was most stably expressed during the treatments, we used it as an internal standard to normalize the expression data of the target genes. Five biological replicates per sample were used for qRT-PCR and two technical replicates were analyzed per biological replicate. The relative expression levels of target genes were evaluated using the 2−ΔΔCq method according to Livak and Schmittgen52.
Statistics
All values are presented as means ± standard deviation of the means (SD). Significance of the differences between WT and lcs1 was analyzed by Student’s t-test using SPSS Statistics version 20.0 (IBM Japan). Significance levels (*P < 0.05 or **P < 0.01) and the number of replicates are given in each figure legend.
References
Yasunari, T. J. et al. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident. Proc. Natl. Acad. Sci. USA 108, 19530–19534, doi:10.1073/pnas.1112058108 (2011).
Takata, Y. et al. Spatial prediction of radioactive Cs concentration in agricultural soil in eastern Japan. Soil Sci. Plant Nutr. 60, 393–403, doi:10.1080/00380768.2013.879528 (2014).
White, P. J. & Broadley, M. R. Mechanisms of caesium uptake by plants. New Phytol. 147, 241–256, doi:10.1046/j.1469-8137.2000.00704.x (2000).
Hampton, C. R., Broadley, M. R. & White, P. J. Short review: The mechanisms of radiocaesium uptake by arabidopsis roots. Nukleonika 50, S3–S8, doi:10.1104/pp.104.046672 (2005).
Nihei, N., Tanoi, K. & Nakanishi, T. M. Inspections of radiocesium concentration levels in rice from Fukushima Prefecture after the Fukushima Dai-ichi Nuclear Power Plant accident. Sci. Rep. 5, 8653, doi:10.1038/srep08653 (2015).
Ministry of Agriculture, Forestry and Fisheries of Japan: Results of radiocesium concentration in agriculutiral products in 2015. http://www.maff.go.jp/j/kanbo/joho/saigai/s_chosa/h27/gaiyou_150400.html (Date of access:07/10/2016) (2016).
Yamaguchi, N., Taniyama, I., Kimura, T., Yoshioka, K. & Saito, M. Contamination of agricultural products and soils with radiocesium derived from the accident at TEPCO Fukushima Daiichi Nuclear Power Station: monitoring, case studies and countermeasures. Soil Sci. Plant Nutr. 62, 303–314, doi:10.1080/00380768.2016.1196119 (2016).
Kato, N. et al. Potassium fertilizer and other materials as countermeasures to reduce radiocesium levels in rice: Results of urgent experiments in 2011 responding to the Fukushima Daiichi Nuclear Power Plant accident. Soil Sci. Plant Nutr. 61, 179–190, doi:10.1080/00380768.2014.995584 (2015).
Zhu, Y. G. & Smolders, E. Plant uptake of radiocaesium: a review of mechanisms, regulation and application. J. Exp. Bot. 51, 1635–1645, doi:1093/jexbot/51.351.1635 (2000).
Qi, Z. et al. The high affinity K+ transporter AtHAK5 plays a physiological role in planta at very low K+ concentrations and provides a caesium uptake pathway in. Arabidopsis. J. Exp. Bot. 59, 595–607, doi:10.1093/jxb/erm330 (2008).
Kanter, U., Hauser, A., Michalke, B., Draxl, S. & Schaffner, A. R. Caesium and strontium accumulation in shoots of Arabidopsis thaliana: genetic and physiological aspects. J. Exp. Bot. 61, 3995–4009, doi:10.1093/jxb/erq213 (2010).
Yamaguchi, N. et al. Behavior of radiocesium in soil-plant system and its controlling factor: a review. Reprinted the Bulletin of the National Institute for Agro-Environmental Sciences 31, 75–129 (Japanese with English sumary) (2012).
Ohmori, Y. et al. Difference in cesium accumulation among rice cultivars grown in the paddy field in Fukushima Prefecture in 2011 and 2012. J. Plant Res. 127, 57–66, doi:10.1007/s10265-013-0616-9 (2014).
Kobayashi, N. I., Sugita, R., Nobori, T., Tanoi, K. & Nakanishi, T. M. Tracer experiment using 42K+ and 137Cs+ revealed the different transport rates of potassium and caesium within rice roots. Funct. Plant Biol. 43, 151–160, doi:10.1071/FP15245 (2016).
Tsukada, H., Hasegawa, H., Hisamatsu, S. & Yamasaki, S. Rice uptake and distributions of radioactive 137Cs, stable 133Cs and K from soil. Environ. Pollut. 117, 403–409, doi:10.1016/S0269-7491(01)00199-3 (2002).
Tsukada, H., Hasegawa, H., Hisamatsu, S. & Yamasaki, S. Transfer of 137Cs and stable Cs from paddy soil to polished rice in Aomori, Japan. J. Environ. Radioactiv. 59, 351–363, doi:10.1016/S0265-931X(01)00083-2 (2002).
Ishikawa, S. et al. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 109, 19166–19171, doi:10.1073/pnas.1211132109 (2012).
Ishikawa, S. et al. Low-cadmium rice (Oryza sativa L.) cultivar can simultaneously reduce arsenic and cadmium concentrations in rice grains. Soil Sci. Plant Nutr. 62, 327–339, doi:10.1080/00380768.2016.1144452 (2016).
Zhu, J. K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 53, 247–273, doi:10.1146/annurev.arplant.53.091401.143329 (2002).
Zhu, J. K., Liu, J. & Xiong, L. Genetic analysis of salt tolerance in arabidopsis: Evidence for a critical role of potassium nutrition. Plant Cell 10, 1181–1191, doi:10.1105/tpc.10.7.1181 (1998).
Liu, J. P., Ishitani, M., Halfter, U., Kim, C. S. & Zhu, J. K. The Arabidopsis thaliana SOS2 gene encodes a protein kinase that is required for salt tolerance. Proc. Natl. Acad. Sci. USA 97, 3730–3734, doi:10.1073/pnas.97.7.3730 (2000).
Qiu, Q. S., Guo, Y., Dietrich, M. A., Schumaker, K. S. & Zhu, J. K. Regulation of SOS1, a plasma membrane Na+/H+ exchanger in Arabidopsis thaliana, by SOS2 and SOS3. Proc. Natl. Acad. Sci. USA 99, 8436–8441, doi:10.1073/pnas.122224699 (2002).
Shi, H. Z., Ishitani, M., Kim, C. S. & Zhu, J. K. The Arabidopsis thaliana salt tolerance gene SOS1 encodes a putative Na+/H+ antiporter. Proc. Natl. Acad. Sci. USA 97, 6896–6901, doi:10.1073/pnas.120170197 (2000).
Martinez-Atienza, J. et al. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 143, 1001–1012, doi:10.1104/pp.106.092635 (2007).
Ishikawa, S. et al. Real-time imaging and analysis of differences in cadmium dynamics in rice cultivars (Oryza sativa) using positron-emitting 107Cd tracer. BMC Plant Biol 11, 172, doi:10.1186/1471-2229-11-172 (2011).
Chen, G. et al. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 38, 2747–2765, doi:10.1111/pce.12585 (2015).
Horie, T. et al. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 26, 3003–3014, doi:10.1038/sj.emboj.7601732 (2007).
Nieves-Cordones, M., Aleman, F., Martinez, V. & Rubio, F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from Low K+ solutions under saline conditions. Molecular Plant 3, 326–333, doi:10.1093/mp/ssp102 (2010).
Shaw, G. & Bell, J. N. B. Competitive effects of potassium and ammonium on cesium uptake kinetics in wheat. J. Environmen. Radioactiv. 13, 283–296, doi:10.1016/0265-931X(91)90002-W (1991).
Smolders, E., Kiebooms, L., Buysse, J. & Merckx, R. 137Cs uptake in spring wheat (Triticum aestivum L. cv Tonic) at varying K supply. 1. The effect in solution culture. Plant Soil 181, 205–209, doi:10.1007/BF00012054 (1996).
Smolders, E., Kiebooms, L., Buysse, J. & Merckx, R. 137Cs uptake in spring wheat (Triticum aestivum L. cv Tonic) at varying K supply. 2. A potted soil experiment. Plant Soil 181, 211–220, doi:10.1007/BF00012055 (1996).
Bertorello, A. M. & Zhu, J. K. SIK1/SOS2 networks: decoding sodium signals via calcium-responsive protein kinase pathways. Pflug. Arch. Eur. J. Phy. 458, 613–619, doi:10.1007/s00424-009-0646-2 (2009).
Ohta, M., Guo, Y., Halfter, U. & Zhu, J. K. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C AB12. Proc. Natl. Acad. Sci. USA 100, 11771–11776, doi:10.1073/pnas.2034853100 (2003).
Kim, B. G. et al. The calcium sensor CBL10 mediates salt tolerance by regulating ion homeostasis in Arabidopsis. Plant J 52, 473–484, doi:10.1111/j.1365-313X.2007.03249.x (2007).
Li, R. F. et al. Functions and mechanisms of the CBL-CIPK signaling system in plant response to abiotic stress. Prog. Nat. Sci. 19, 667–676, doi:10.1016/j.pnsc.2008.06.030 (2009).
Aleman, F., Nieves-Cordones, M., Martinez, V. & Rubio, F. Root K+ acquisition in plants: the Arabidopsis thaliana model. Plant Cell Physiol 52, 1603–1612, doi:10.1093/pcp/pcr096 (2011).
Yang, T. Y. et al. The role of a potassium transporter OsHAK5 in potassium acquisition and transport from roots to shoots in rice at low potassium supply levels. Plant Physiol. 166, 945–959, doi:10.1104/pp.114.246520 (2014).
Li, J. et al. The Os-AKT1 channel Is critical for K+ uptake in rice roots and is modulated by the rice CBL1-CIPK23 complex. Plant Cell 26, 3387–3402, doi:10.1105/tpc.114.123455 (2014).
Banuelos, M. A., Garciadeblas, B., Cubero, B. & Rodriguez-Navarro, A. Inventory and functional characterization of the HAK potassium transporters of rice. Plant Physiol 130, 784–795, doi:10.1104/pp.007781 (2002).
Aleman, F. et al. The F130S point mutation in the Arabidopsis high-affinity K+ transporter AtHAK5 increases K+ over Na+ and Cs+ selectivity and confers Na+ and Cs+ tolerance to yeast under heterologous expression. Front Plant Sci 5, 430, doi:10.3389/fpls.2014.00430 (2014).
Rubio, F., Nieves-Cordones, M., Aleman, F. & Martinez, V. Relative contribution of AtHAK5 and AtAKT1 to K+ uptake in the high-affinity range of concentrations. Physiol. Planta 134, 598–608, doi:10.1111/ppl.2008.134.issue-4 (2008).
Broadley, M. R., Escobar-Gutierrez, A. J., Bowen, H. C., Willey, N. J. & White, P. J. Influx and accumulation of Cs+ by the akt1 mutant of Arabidopsis thaliana (L.) Heynh. lacking a dominant K+ transport system. J. Exp. Bot 52, 839–844, doi:10.1093/jexbot/52.357.839 (2001).
Howitt, S. M. & Udvardi, M. K. Structure, function and regulation of ammonium transporters in plants. BBA-Biomembranes 1465, 152–170, doi:10.1016/S0005-2736(00)00136-X (2000).
Miyamoto, T., Ochiai, K., Takeshita, S. & Matoh, T. Identification of quantitative trait loci associated with shoot sodium accumulation under low potassium conditions in rice plants. Soil Sci. Plant Nutr. 58, 728–736, doi:10.1080/00380768.2012.745797 (2012).
Miyamoto, T. et al. Expression level of the sodium transporter gene OsHKT2;1 determines sodium accumulation of rice cultivars under potassium-deficient conditions. Soil Sci. Plant Nutr. 61, 481–492, doi:10.1080/00380768.2015.1005539 (2015).
Kondo, M. et al. Exchangeable Cs/K ratio in soil is an index to estimate accumulation of radioactive and stable Cs in rice plant. Soil Sci. Plant Nutr. 61, 133–143, doi:10.1080/00380768.2014.973347 (2015).
Yonemaru, J., Ebana, K. & Yano, M. HapRice, an SNP Haplotype Database and a Web Tool for Rice. Plant Cell Physiol. 55(e9), 1–12, doi:10.1093/pcp/pct188 (2014).
Takagi, H. et al. MutMap accelerates breeding of a salt-tolerant rice cultivar. Nat. Biotechnol. 33, 445–449, doi:10.1038/nbt.3188 (2015).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760, doi:10.1093/bioinformatics/btp324 (2009).
Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 27, 2987–2993, doi:10.1093/bioinformatics/btr509 (2011).
Yamatani, H. et al. NYC4, the rice ortholog of Arabidopsis THF1, is involved in the degradation of chlorophyll protein complexes during leaf senescence. Plant J. 74, 652–662, doi:10.1111/tpj.2013.74.issue-4 (2013).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 25, 402–408, doi:10.1006/meth.2001.1262 (2001).
Acknowledgements
This work was partly supported by the Ministry of Agriculture, Forestry and Fisheries of Japan (Development of Decontamination Technologies for Radioactive Substances in Agricultural Land, 24303-2). SNP array was supported by a genome-support grant from the National Institute of Agrobiological Sciences. We thank Dr. Y. Hase (National Institutes for Quantum and Radiological Science and Technology) for irradiation of the Koshihikari seeds, Dr. T. Taji (Tokyo University of Agriculture) for providing the seeds of the Arabidopsis sos2-1 mutant, Dr. Y. Nagamura and Ms. R. Motoyama (National Agriculture and Food Research Organization, NARO) for their technical assistance with the microarray analysis, and Ms. A. Arai, Ms. Y. Yamanaka, Ms. M. Chiba, Mr. H. Yamaguchi, Mr. H. Iino, and Mr. T. Kamata (NARO) for their field and laboratory assistance.
Author information
Authors and Affiliations
Contributions
S.I. designed the research and wrote the paper. S.I., S.H., T.A., M.K., and M.I. contributed to the genetic and physiological analyses. M.I. performed the radiocesium analysis. S.I., M.K., and H.T. performed the metal analysis. S.I., T.A., T.S., Y.O., T.I., S.F., and A.G. performed the field tests. H.T. performed the whole-genome resequencing. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ishikawa, S., Hayashi, S., Abe, T. et al. Low-cesium rice: mutation in OsSOS2 reduces radiocesium in rice grains. Sci Rep 7, 2432 (2017). https://doi.org/10.1038/s41598-017-02243-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-017-02243-9
- Springer Nature Limited