Abstract
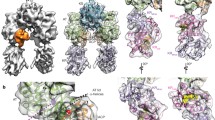
Polyketide synthases (PKSs) are microbial multienzymes for the biosynthesis of biologically potent secondary metabolites. Polyketide production is initiated by the loading of a starter unit onto an integral acyl carrier protein (ACP) and its subsequent transfer to the ketosynthase (KS). Initial substrate loading is achieved either by multidomain loading modules or by the integration of designated loading domains, such as starter unit acyltransferases (SAT), whose structural integration into PKS remains unresolved. A crystal structure of the loading/condensing region of the nonreducing PKS CTB1 demonstrates the ordered insertion of a pseudodimeric SAT into the condensing region, which is aided by the SAT-KS linker. Cryo-electron microscopy of the post-loading state trapped by mechanism-based crosslinking of ACP to KS reveals asymmetry across the CTB1 loading/–condensing region, in accord with preferential 1:2 binding stoichiometry. These results are critical for re-engineering the loading step in polyketide biosynthesis and support functional relevance of asymmetric conformations of PKSs.




Similar content being viewed by others
Change history
10 April 2018
In the version of this article originally published, “Supplementary Text and Figures” in the Supplementary Information section incorrectly linked to the Life Sciences Reporting Summary instead of the file containing Supplementary Tables 1–4 and Supplementary Figures 1–12. The error has been corrected in the HTML version of this article.
References
Hertweck, C. The biosynthetic logic of polyketide diversity. Angew. Chem. Int. Edn. Engl. 48, 4688–4716 (2009).
Herbst, D. A., Jakob, R. P., Zähringer, F. & Maier, T. Mycocerosic acid synthase exemplifies the architecture of reducing polyketide synthases. Nature 531, 533–537 (2016).
Weissman, K. J. The structural biology of biosynthetic megaenzymes. Nat. Chem. Biol. 11, 660–670 (2015).
Tang, Y., Kim, C. Y., Mathews, I. I., Cane, D. E. & Khosla, C. The 2.7-angstrom crystal structure of a 194-kDa homodimeric fragment of the 6-deoxyerythronolide B synthase. Proc. Natl Acad. Sci. USA 103, 11124–11129 (2006).
Maier, T., Leibundgut, M. & Ban, N. The crystal structure of a mammalian fatty acid synthase. Science 321, 1315–1322 (2008).
Crawford, J. M., Vagstad, A. L., Whitworth, K. P., Ehrlich, K. C. & Townsend, C. A. Synthetic strategy of nonreducing iterative polyketide synthases and the origin of the classical “starter-unit effect”. ChemBioChem 9, 1019–1023 (2008).
Vagstad, A. L. et al. Combinatorial domain swaps provide insights into the rules of fungal polyketide synthase programming and the rational synthesis of non-native aromatic products. Angew. Chem. Int. Edn. Engl. 52, 1718–1721 (2013).
Foulke-Abel, J. & Townsend, C. A. Demonstration of starter unit interprotein transfer from a fatty acid synthase to a multidomain, nonreducing polyketide synthase. ChemBioChem 13, 1880–1884 (2012).
Crawford, J. M., Dancy, B. C., Hill, E. A., Udwary, D. W. & Townsend, C. A. Identification of a starter unit acyl-carrier protein transacylase domain in an iterative type I polyketide synthase. Proc. Natl Acad. Sci. USA 103, 16728–16733 (2006).
Newman, A. G., Vagstad, A. L., Storm, P. A. & Townsend, C. A. Systematic domain swaps of iterative, nonreducing polyketide synthases provide a mechanistic understanding and rationale for catalytic reprogramming. J. Am. Chem. Soc. 136, 7348–7362 (2014).
Fujii, I., Watanabe, A., Sankawa, U. & Ebizuka, Y. Identification of Claisen cyclase domain in fungal polyketide synthase WA, a naphthopyrone synthase of Aspergillus nidulans. Chem. Biol. 8, 189–197 (2001).
Dutta, S. et al. Structure of a modular polyketide synthase. Nature 510, 512–517 (2014).
Tang, Y., Chen, A. Y., Kim, C. Y., Cane, D. E. & Khosla, C. Structural and mechanistic analysis of protein interactions in module 3 of the 6-deoxyerythronolide B synthase. Chem. Biol. 14, 931–943 (2007).
Whicher, J. R. et al. Cyanobacterial polyketide synthase docking domains: a tool for engineering natural product biosynthesis. Chem. Biol. 20, 1340–1351 (2013).
Pappenberger, G. et al. Structure of the human fatty acid synthase KS-MAT didomain as a framework for inhibitor design. J. Mol. Biol. 397, 508–519 (2010).
Vagstad, A. L., Bumpus, S. B., Belecki, K., Kelleher, N. L. & Townsend, C. A. Interrogation of global active site occupancy of a fungal iterative polyketide synthase reveals strategies for maintaining biosynthetic fidelity. J. Am. Chem. Soc. 134, 6865–6877 (2012).
Crawford, J. M. et al. Deconstruction of iterative multidomain polyketide synthase function. Science 320, 243–246 (2008).
Winter, J. M. et al. Biochemical and structural basis for controlling chemical modularity in fungal polyketide biosynthesis. J. Am. Chem. Soc. 137, 9885–9893 (2015).
Liu, T., Chiang, Y. M., Somoza, A. D., Oakley, B. R. & Wang, C. C. Engineering of an “unnatural” natural product by swapping polyketide synthase domains in Aspergillus nidulans. J. Am. Chem. Soc. 133, 13314–13316 (2011).
Bruegger, J. et al. Probing the selectivity and protein•protein interactions of a nonreducing fungal polyketide synthase using mechanism-based crosslinkers. Chem. Biol. 20, 1135–1146 (2013).
Choquer, M. et al. The CTB1 gene encoding a fungal polyketide synthase is required for cercosporin biosynthesis and fungal virulence of Cercospora nicotianae. Mol. Plant Microbe Interact. 18, 468–476 (2005).
Chung, K. R., Ehrenshaft, M., Wetzel, D. K. & Daub, M. E. Cercosporin-deficient mutants by plasmid tagging in the asexual fungus Cercospora nicotianae. Mol. Genet. Genomics 270, 103–113 (2003).
Daub, M. E. & Ehrenshaft, M. The photoactivated cercospora toxin cercosporin: contributions to plant disease and fundamental biology. Annu. Rev. Phytopathol. 38, 461–490 (2000).
Newman, A. G., Vagstad, A. L., Belecki, K., Scheerer, J. R. & Townsend, C. A. Analysis of the cercosporin polyketide synthase CTB1 reveals a new fungal thioesterase function. Chem. Commun. (Camb.) 48, 11772–11774 (2012).
Nguyen, C. et al. Trapping the dynamic acyl carrier protein in fatty acid biosynthesis. Nature 505, 427–431 (2014).
Whicher, J. R. et al. Structural rearrangements of a polyketide synthase module during its catalytic cycle. Nature 510, 560–564 (2014).
Kapur, S. et al. Reprogramming a module of the 6-deoxyerythronolide B synthase for iterative chain elongation. Proc. Natl Acad. Sci. USA 109, 4110–4115 (2012).
Wang, F. et al. Structural and functional analysis of the loading acyltransferase from avermectin modular polyketide synthase. ACS Chem. Biol. 10, 1017–1025 (2015).
Wattana-amorn, P. et al. Solution structure of an acyl carrier protein domain from a fungal type I polyketide synthase. Biochemistry 49, 2186–2193 (2010).
Leibundgut, M., Jenni, S., Frick, C. & Ban, N. Structural basis for substrate delivery by acyl carrier protein in the yeast fatty acid synthase. Science 316, 288–290 (2007).
Changeux, J. P. & Edelstein, S. Conformational selection or induced fit? 50 years of debate resolved. F1000 Biol. Rep. 3, 19 (2011).
Brignole, E. J., Smith, S. & Asturias, F. J. Conformational flexibility of metazoan fatty acid synthase enables catalysis. Nat. Struct. Mol. Biol. 16, 190–197 (2009).
Maier, T., Jenni, S. & Ban, N. Architecture of mammalian fatty acid synthase at 4.5 A resolution. Science 311, 1258–1262 (2006).
Zhang, L. et al. Crystal structure of FabZ-ACP complex reveals a dynamic seesaw-like catalytic mechanism of dehydratase in fatty acid biosynthesis. Cell Res. 26, 1330–1344 (2016).
Keatinge-Clay, A. T., Maltby, D. A., Medzihradszky, K. F., Khosla, C. & Stroud, R. M. An antibiotic factory caught in action. Nat. Struct. Mol. Biol. 11, 888–893 (2004).
Gibson, D. G. et al. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–345 (2009).
Betancor, L., Fernández, M. J., Weissman, K. J. & Leadlay, P. F. Improved catalytic activity of a purified multienzyme from a modular polyketide synthase after coexpression with Streptomyces chaperonins in Escherichia coli. ChemBioChem 9, 2962–2966 (2008).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, (213–221 (2010).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Cowtan, K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 62, 1002–1011 (2006).
Cowtan, K. Recent developments in classical density modification. Acta Crystallogr. D Biol. Crystallogr. 66, 470–478 (2010).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Edwards, A. L., Matsui, T., Weiss, T. M. & Khosla, C. Architectures of whole-module and bimodular proteins from the 6-deoxyerythronolide B synthase. J. Mol. Biol. 426, 2229–2245 (2014).
Asturias, F. J. et al. Structure and molecular organization of mammalian fatty acid synthase. Nat. Struct. Mol. Biol. 12, 225–232 (2005).
Li, X. et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nat. Methods 10, 584–590 (2013).
McLeod, R. A., Kowal, J., Ringler, P. & Stahlberg, H. Robust image alignment for cryogenic transmission electron microscopy. J. Struct. Biol. 197, 279–293 (2017).
Rohou, A. & Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 192, 216–221 (2015).
Tang, G. et al. EMAN2: an extensible image processing suite for electron microscopy. J. Struct. Biol. 157, 38–46 (2007).
Kimanius, D., Forsberg, B. O., Scheres, S. H. & Lindahl, E. Accelerated cryo-EM structure determination with parallelisation using GPUs in RELION-2. eLife 5, e18722 (2016).
Zhang, K. Gctf: Real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Rosenthal, P. B. & Henderson, R. Optimal determination of particle orientation, absolute hand, and contrast loss in single-particle electron cryomicroscopy. J. Mol. Biol. 333, 721–745 (2003).
Chen, S. et al. High-resolution noise substitution to measure overfitting and validate resolution in 3D structure determination by single particle electron cryomicroscopy. Ultramicroscopy 135, 24–35 (2013).
Urnavicius, L. et al. The structure of the dynactin complex and its interaction with dynein. Science 347, 1441–1446 (2015).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Bai, X. C., Rajendra, E., Yang, G., Shi, Y. & Scheres, S. H. Sampling the conformational space of the catalytic subunit of human γ-secretase. eLife 4, e11182 (2015).
Collaborative Computational Project, Number 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Pettersen, E. F. et al. UCSF Chimera–a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
McLeod, R. A., Diogo Righetto, R., Stewart, A. & Stahlberg, H. MRCZ - A file format for cryo-TEM data with fast compression. J. Struct. Biol. 201, 252–257 (2017).
Schwede, T., Kopp, J., Guex, N. & Peitsch, M. C. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31, 3381–3385 (2003).
Krissinel, E. & Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 60, 2256–2268 (2004).
Kleywegt, G. J. Validation of protein models from Cα coordinates alone. J. Mol. Biol. 273, 371–376 (1997).
Kleywegt, G. J. Use of non-crystallographic symmetry in protein structure refinement. Acta Crystallogr. D Biol. Crystallogr. 52, 842–857 (1996).
Kahraman, A., Malmström, L. & Aebersold, R. Xwalk: computing and visualizing distances in cross-linking experiments. Bioinformatics 27, 2163–2164 (2011).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Acknowledgements
We thank P. Leadlay and L. Betancor for providing plasmid pETcoco-2A-L1SL2. X-ray diffraction data were collected at beamline PXI of the Paul Scherrer Institute, Villigen, Switzerland, and cryo-EM data were collected at the BioEM facility of the University of Basel; we acknowledge excellent support by teams of both facilities. This work was supported by the Swiss National Science Foundation (SNF) project grants 138262, 159696, SNF R’equip grants 145023 and 164074, and the National Institutes of Health (ES001670). D.A.H. acknowledges a fellowship from the Werner-Siemens Foundation.
Author information
Authors and Affiliations
Contributions
R.P.J. expressed, purified and crystallized CTB1 SAT-KS-MAT. D.A.H., R.P.J., and T.M. solved the crystal structure. D.A.H. performed cryo-EM, data processing, modeling, refinement and analysis of all structural data. C.R.H.-R. optimized and prepared crosslinked CTB1 SAT°-KS-MAT°=ACP2 for structural analysis and performed mutational experiments for structural validation. J.M.K. synthesized the α-bromopropionyl crosslinker. P.A.S. and J.R.A. performed initial exploratory experiments, and J.R.A. prepared the CTB1 SAT°-KS-MAT° construct for crosslinking. C.A.T. and T.M. designed research. The manuscript was written by D.A.H., T.M., C.R.H.-R., and C.A.T.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Text and Figures
Supplementary Tables 1–4 and Supplementary Figures 1–12
Supplementary Note 1:
Synthetic Procedures
Supplementary Video 1
ACP2 binding to the CTB1 loading/condensing region
Rights and permissions
About this article
Cite this article
Herbst, D.A., Huitt-Roehl, C.R., Jakob, R.P. et al. The structural organization of substrate loading in iterative polyketide synthases. Nat Chem Biol 14, 474–479 (2018). https://doi.org/10.1038/s41589-018-0026-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-018-0026-3
- Springer Nature America, Inc.
This article is cited by
-
Structure and dynamics of the essential endogenous mycobacterial polyketide synthase Pks13
Nature Structural & Molecular Biology (2023)
-
Enzymology of assembly line synthesis by modular polyketide synthases
Nature Chemical Biology (2023)
-
Insights into azalomycin F assembly-line contribute to evolution-guided polyketide synthase engineering and identification of intermodular recognition
Nature Communications (2023)
-
Solution structure of the type I polyketide synthase Pks13 from Mycobacterium tuberculosis
BMC Biology (2022)
-
Engineering of PKS Megaenzymes—A Promising Way to Biosynthesize High-Value Active Molecules
Topics in Catalysis (2022)