Abstract
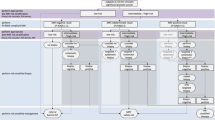
Multiparametric MRI of the prostate is now recommended as the initial diagnostic test for men presenting with suspected prostate cancer, with a negative MRI enabling safe avoidance of biopsy and a positive result enabling MRI-directed sampling of lesions. The diagnostic pathway consists of several steps, from initial patient presentation and preparation to performing and interpreting MRI, communicating the imaging findings, outlining the prostate and intra-prostatic target lesions, performing the biopsy and assessing the cores. Each component of this pathway requires experienced clinicians, optimized equipment, good inter-disciplinary communication between specialists, and standardized workflows in order to achieve the expected outcomes. Assessment of quality and mitigation measures are essential for the success of the MRI-directed prostate cancer diagnostic pathway. Quality assurance processes including Prostate Imaging-Reporting and Data System, template biopsy, and pathology guidelines help to minimize variation and ensure optimization of the diagnostic pathway. Quality control systems including the Prostate Imaging Quality scoring system, patient-level outcomes (such as Prostate Imaging-Reporting and Data System MRI score assignment and cancer detection rates), multidisciplinary meeting review and audits might also be used to provide consistency of outcomes and ensure that all the benefits of the MRI-directed pathway are achieved.
Key points
-
Multiparametric MRI is now recommended as the initial diagnostic test for men presenting with suspected prostate cancer.
-
A negative MRI enables patients to safely avoid biopsy, whereas a positive MRI prompts targeted biopsy and pathologically accurate tissue sampling.
-
The MRI-directed prostate cancer diagnostic pathway involves several steps including acquiring and interpreting MRI, communicating MRI findings, outlining suspicious target lesions, performing biopsy and evaluating the cores.
-
All steps in the pathway are prone to variation; assessment and mitigation of poor quality and variance are essential for a successful delivery of the MRI-directed pathway.
-
Quality assurance systems to minimize variation in performance and prevent poor quality include Prostate Imaging-Reporting and Data System (PI-RADS) imaging guidelines for radiologists, prostate biopsy templates and International Society of Urological Pathology guidelines for histopathologists.
-
Quality control measures include checking MRI compliance with PI-RADS, image quality assessment with the Prostate Imaging Quality scoring system, radiologist certification, multidisciplinary team meeting review and pathology re-review of images, as well as audits of cancer detection rates and biopsy core quality.






Similar content being viewed by others
References
Bjurlin, M. A. et al. Update of the standard operating procedure on the use of multiparametric magnetic resonance imaging for the diagnosis, staging and management of prostate cancer. J. Urol. 203, 706–712 (2020).
Mottet, N. et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer — 2020 update. Part 1: screening, diagnosis, and local treatment with curative intent. Eur. Urol. 79, 243–262 (2021).
Mason, B. R. et al. Current status of MRI and PET in the NCCN guidelines for prostate cancer. J. Natl. Compr. Cancer Netw. 17, 506–513 (2019).
NICE. Prostate cancer: diagnosis and management. NICE https://www.nice.org.uk/guidance/ng131 (2019).
Le Bihan, D. et al. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168, 497–505 (1988).
Bowden, D. & Barrett, T. Angiogenesis imaging in Neoplasia. J. Clin. Imaging Sci. 1, 38 (2011).
Kasivisvanathan, V. et al. MRI-targeted or standard biopsy for prostate-cancer diagnosis. N. Engl. J. Med. 378, 1767–1777 (2018).
van der Leest, M. et al. Head-to-head comparison of transrectal ultrasound-guided prostate biopsy versus multiparametric prostate resonance imaging with subsequent magnetic resonance-guided biopsy in biopsy-naïve men with elevated prostate-specific antigen: a large prospective Mu. Eur. Urol. 75, 570–578 (2019).
Ahmed, H. U. et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 389, 815–822 (2017).
Rouvière, O. et al. Use of prostate systematic and targeted biopsy on the basis of multiparametric MRI in biopsy-naive patients (MRI-FIRST): a prospective, multicentre, paired diagnostic study. Lancet Oncol. 20, 100–109 (2019).
Turkbey, B. et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur. Urol. 76, 340–351 (2019).
Venderink, W. et al. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. Part 3: targeted biopsy. Eur. Urol. 77, 481–490 (2020).
Padhani, A. R. et al. PI-RADS steering committee: the PI-RADS multiparametric MRI and MRI-directed biopsy pathway. Radiology 292, 464–474 (2019).
Schoots, I. G. & Padhani, A. R. Risk-adapted biopsy decision based on prostate magnetic resonance imaging and prostate-specific antigen density for enhanced biopsy avoidance in first prostate cancer diagnostic evaluation. BJU Int. 127, 175–178 (2021).
Sathianathen, N. J. et al. Negative predictive value of multiparametric magnetic resonance imaging in the detection of clinically significant prostate cancer in the prostate imaging reporting and data system era: a systematic review and meta-analysis. Eur. Urol. 78, 402–414 (2020).
Park, K. J., Choi, S. H., Kim, M. H., Kim, J. K. & Jeong, I. G. Performance of prostate imaging reporting and data system version 2.1 for diagnosis of prostate cancer: a systematic review and meta-analysis. J. Magn. Reson. Imaging 54, 103–112 (2021).
Westphalen, A. C. et al. Variability of the positive predictive value of PI-RADS for prostate MRI across 26 centers: experience of the society of abdominal radiology prostate cancer disease-focused panel. Radiology 296, 76–84 (2020).
Radtke, J. P. et al. Multiparametric magnetic resonance imaging (MRI) and MRI–transrectal ultrasound fusion biopsy for index tumor detection: correlation with radical prostatectomy specimen. Eur. Urol. 70, 846–853 (2016).
Tan, N. et al. Characteristics of detected and missed prostate cancer foci on 3-T multiparametric MRI using an endorectal coil correlated with whole-mount thin-section histopathology. Am. J. Roentgenol. 205, W87–W92 (2015).
Langer, D. L. et al. Intermixed normal tissue within prostate cancer: effect on MR imaging measurements of apparent diffusion coefficient and T2-sparse versus dense cancers. Radiology 249, 900–908 (2008).
Serrao, E. M. et al. Investigating the ability of multiparametric MRI to exclude significant prostate cancer prior to transperineal biopsy. J. Can. Urol. Assoc. 9, E853–E858 (2015).
Salami, S. S. et al. Biologic significance of magnetic resonance imaging invisibility in localized prostate cancer. JCO Precis. Oncol. https://doi.org/10.1200/po.19.00054 (2019).
Esses, S. J., Taneja, S. S. & Rosenkrantz, A. B. Imaging facilities’ adherence to PI-RADS v2 minimum technical standards for the performance of prostate MRI. Acad. Radiol. 25, 188–195 (2018).
Burn, P. R. et al. A multicentre assessment of prostate MRI quality and compliance with UK and international standards. Clin. Radiol. 74, 894.e19–894.e25 (2019).
Rouvière, O., Souchon, R. & Melodelima, C. Pitfalls in interpreting positive and negative predictive values: application to prostate multiparametric magnetic resonance imaging. Diagn. Interv. Imaging 99, 515–518 (2018).
Barentsz, J. O. et al. ESUR prostate MR guidelines 2012. Eur. Radiol. 22, 746–757 (2012).
Weinreb, J. C. et al. PI-RADS prostate imaging — reporting and data system: 2015, version 2. Eur. Urol. 69, 16–40 (2016).
Sackett, J. et al. Quality of prostate MRI: is the PI-RADS standard sufficient? Acad. Radiol. 28, 199–207 (2021).
van der Leest, M., Israël, B., Engels, R. R. M. & Barentsz, J. O. Reply to Arnaldo Stanzione, Massimo Imbriaco, and Renato Cuocolo’s Letter to the Editor re: Marloes van der Leest, Bas Israël, Eric Bastiaan Cornel, et al. High diagnostic performance of short magnetic resonance imaging protocols for prostate cancer detection in biopsy-naïve men: the next step in magnetic resonance imaging accessibility. Eur. Urol. 2019;76:574-81. Are we meeting our standards? Stringent prostate imaging reporting and data system acquisition requirements might be limiting prostate accessibility. Eur. Urol. 77, e58–e59 (2020).
Stabile, A. et al. Factors influencing variability in the performance of multiparametric magnetic resonance imaging in detecting clinically significant prostate cancer: a systematic literature review. Eur. Urol. Oncol. 3, 145–167 (2020).
Akin, O. et al. Interactive dedicated training curriculum improves accuracy in the interpretation of MR imaging of prostate cancer. Eur. Radiol. 20, 995–1002 (2010).
Gaziev, G. et al. Defining the learning curve for multiparametric magnetic resonance imaging (MRI) of the prostate using MRI-transrectal ultrasonography (TRUS) fusion-guided transperineal prostate biopsies as a validation tool. BJU Int. 117, 80–86 (2016).
Stolk, T. T. et al. False positives in PIRADS (V2) 3, 4, and 5 lesions: relationship with reader experience and zonal location. Abdom. Radiol. 44, 1044–1051 (2019).
Hansen, N. L. et al. Comparison of initial and tertiary centre second opinion reads of multiparametric magnetic resonance imaging of the prostate prior to repeat biopsy. Eur. Radiol. 27, 2259–2266 (2017).
Wibmer, A. et al. Diagnosis of extracapsular extension of prostate cancer on prostate MRI: Impact of second-opinion readings by subspecialized genitourinary oncologic radiologists. Am. J. Roentgenol. 205, W73–W78 (2015).
Ecke, T. H. et al. Comparison of initial and second opinion reads of multiparametric magnetic resonance imaging of the prostate for transperineal template-guided biopsies with MRI-Ultrasound fusion. Urol. Oncol. Semin. Orig. Investig. 39, 781.e1–781.e7 (2021).
de Rooij, M. et al. ESUR/ESUI consensus statements on multi-parametric MRI for the detection of clinically significant prostate cancer: quality requirements for image acquisition, interpretation and radiologists’ training. Eur. Radiol. 30, 5404–5416 (2020).
Barrett, T. et al. Certification in reporting multiparametric magnetic resonance imaging of the prostate: recommendations of a UK consensus meeting. BJU Int. 127, 304–306 (2021).
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
NHS. Cancer referral to treatment period start date. NHS https://www.datadictionary.nhs.uk/data_elements/cancer_referral_to_treatment_period_start_date.html (2022).
Redaniel, M. T., Martin, R. M., Gillatt, D., Wade, J. & Jeffreys, M. Time from diagnosis to surgery and prostate cancer survival: a retrospective cohort study. BMC Cancer 13, 559 (2013).
Panebianco, V. et al. Clinical utility of multiparametric magnetic resonance imaging as the first-line tool for men with high clinical suspicion of prostate cancer. Eur. Urol. Oncol. 1, 208–214 (2018).
van der Leest, M. et al. High diagnostic performance of short magnetic resonance imaging protocols for prostate cancer detection in biopsy-naïve men: the next step in magnetic resonance imaging accessibility. Eur. Urol. 76, 574–581 (2019).
Kuhl, C. K. et al. Abbreviated biparametric prostate MR imaging in men with elevated prostate-specific antigen. Radiology 285, 493–505 (2017).
Sushentsev, N. et al. The effect of capped biparametric magnetic resonance imaging slots on weekly prostate cancer imaging workload. Br. J. Radiol. 93, 20190929 (2020).
Zawaideh, J. P. et al. Diagnostic accuracy of biparametric versus multiparametric prostate MRI: assessment of contrast benefit in clinical practice. Eur. Radiol. 30, 4039–4049 (2020).
Bass, E. J. et al. Prostate cancer diagnostic pathway: Is a one-stop cognitive MRI targeted biopsy service a realistic goal in everyday practice? A pilot cohort in a tertiary referral centre in the UK. BMJ Open 8, 24941 (2018).
Purysko, A. S. & Rosenkrantz, A. B. Technique of multiparametric MR imaging of the prostate. Urol. Clin. North. Am. 45, 427–438 (2018).
Franiel, T. et al. MpMRI of the prostate (MR-prostatography): updated recommendations of the DRG and BDR on patient preparation and scanning protocol. Rofo 193, 763–776 (2021).
Schoots, I. G. et al. PI-RADS committee position on MRI without contrast medium in biopsy-naive men with suspected prostate cancer: narrative review. Am. J. Roentgenol. 216, 3–19 (2021).
Ippoliti, S. et al. Optimal biopsy approach for detection of clinically significant prostate cancer. Br. J. Radiol. 95, 20210413 (2021).
Hansen, N. et al. Magnetic resonance and ultrasound image fusion supported transperineal prostate biopsy using the Ginsburg protocol: technique, learning points, and biopsy results. Eur. Urol. 70, 332–340 (2016).
Immerzeel, J. et al. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. Part 4: transperineal magnetic resonance–ultrasound fusion guided biopsy using local anesthesia. Eur. Urol. 81, 110–117 (2022).
Brisbane, W. G. et al. Targeted prostate biopsy: umbra, penumbra, and value of perilesional sampling. Eur. Urol. https://doi.org/10.1016/j.eururo.2022.01.008 (2022).
Hansen, N. L. et al. Optimising the number of cores for magnetic resonance imaging-guided targeted and systematic transperineal prostate biopsy. BJU Int. 125, 260–269 (2020).
Epstein, J. I. et al. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 40, 244–252 (2016).
Drost, F.-J. H. et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst. Rev. https://doi.org/10.1002/14651858.cd012663.pub2 (2019).
Caglic, I. & Barrett, T. Optimising prostate mpMRI: prepare for success. Clin. Radiol. 74, 831–840 (2019).
Coskun, M. et al. Impact of bowel preparation with Fleet’sTM enema on prostate MRI quality. Abdom. Radiol. 45, 4252–4259 (2020).
Czarniecki, M. et al. Role of PROPELLER-DWI of the prostate in reducing distortion and artefact from total hip replacement metalwork. Eur. J. Radiol. 102, 213–219 (2018).
Caglic, I., Hansen, N. L., Slough, R. A., Patterson, A. J. & Barrett, T. Evaluating the effect of rectal distension on prostate multiparametric MRI image quality. Eur. J. Radiol. 90, 174–180 (2017).
Engels, R. R. M., Israël, B., Padhani, A. R. & Barentsz, J. O. Multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer: what urologists need to know. part 1: acquisition. Eur. Urol. 77, 457–468 (2020).
Slough, R. A., Caglic, I., Hansen, N. L., Patterson, A. J. & Barrett, T. Effect of hyoscine butylbromide on prostate multiparametric MRI anatomical and functional image quality. Clin. Radiol. 73, 216.e9–216.e14 (2018).
Ullrich, T. et al. Hyoscine butylbromide significantly decreases motion artefacts and allows better delineation of anatomic structures in mp-MRI of the prostate. Eur. Radiol. 28, 17–23 (2018).
Purysko, A. S. et al. Influence of enema and dietary restrictions on prostate MR image quality: a multireader study. Acad. Radiol. 29, 4–14 (2022).
Reischauer, C., Cancelli, T., Malekzadeh, S., Froehlich, J. M. & Thoeny, H. C. How to improve image quality of DWI of the prostate — enema or catheter preparation? Eur. Radiol. 31, 6708–6716 (2021).
Lim, C. et al. Does a cleansing enema improve image quality of 3T surface coil multiparametric prostate MRI? J. Magn. Reson. Imaging 42, 689–697 (2015).
Czyzewska, D., Sushentsev, N., Latoch, E., Slough, R. A. & Barrett, T. T2-PROPELLER compared to T2-FRFSE for image quality and lesion detection at prostate MRI. Can. Assoc. Radiol. J. https://doi.org/10.1177/08465371211030206 (2021).
Meier-Schroers, M. et al. Revised PROPELLER for T2-weighted imaging of the prostate at 3 Tesla: impact on lesion detection and PI-RADS classification. Eur. Radiol. 28, 24–30 (2018).
Koch, K. M. et al. Analysis and evaluation of a deep learning reconstruction approach with denoising for orthopedic MRI. Radiol. Artif. Intell. 3, e200278 (2021).
Gassenmaier, S. et al. Deep learning — accelerated T2-weighted imaging of the prostate: reduction of acquisition time and improvement of image quality. Eur. J. Radiol. 137, 109600 (2021).
Ueda, T. et al. Deep learning reconstruction of diffusion-weighted MRI improves image quality for prostatic imaging. Radiology https://doi.org/10.1148/radiol.204097 (2022).
Moldovan, P. C. et al. What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy? A systematic review and meta-analysis from the European Association of Urology Prostate Cancer Guidelines Panel. Eur. Urol. 72, 250–266 (2017).
Leeflang, M. M. G., Rutjes, A. W. S., Reitsma, J. B., Hooft, L. & Bossuyt, P. M. M. Variation of a test’s sensitivity and specificity with disease prevalence. CMAJ 185, E537–E544 (2013).
Tan, N., Lakshmi, M., Hernandez, D. & Scuderi, A. Upcoming American College of Radiology prostate MRI designation launching: what to expect. Abdom. Radiol. 45, 4109–4111 (2020).
Belue, M. J., Yilmaz, E. C., Daryanani, A. & Turkbey, B. Current status of biparametric MRI in prostate cancer diagnosis: literature analysis. Life 12, 804 (2022).
Barrett, T., Rajesh, A., Rosenkrantz, A. B., Choyke, P. L. & Turkbey, B. PI-RADS version 2.1: one small step for prostate MRI. Clin. Radiol. 74, 841–852 (2019).
Barrett, T., Turkbey, B. & Choyke, P. L. PI-RADS version 2: what you need to know. Clin. Radiol. 70, 1165–1176 (2015).
Papoutsaki, M. V. et al. Standardisation of prostate multiparametric MRI across a hospital network: a London experience. Insights Imaging 12, 52 (2021).
Giganti, F., Allen, C., Emberton, M., Moore, C. M. & Kasivisvanathan, V. Prostate imaging quality (PI-QUAL): a new quality control scoring system for multiparametric magnetic resonance imaging of the prostate from the PRECISION trial. Eur. Urol. Oncol. 3, 615–619 (2020).
Giganti, F. et al. Understanding PI-QUAL for prostate MRI quality: a practical primer for radiologists. Insights Imaging 12, 59 (2021).
Giganti, F. et al. Prostate MRI quality: a critical review of the last 5 years and the role of the PI-QUAL score. Br. J. Radiol. 95, 20210415 (2021).
Giganti, F. et al. Inter-reader agreement of the PI-QUAL score for prostate MRI quality in the NeuroSAFE PROOF trial. Eur. Radiol. https://doi.org/10.1007/s00330-021-08169-1 (2021).
Boschheidgen, M. et al. Comparison and prediction of artefact severity due to total hip replacement in 1.5 T versus 3 T MRI of the prostate. Eur. J. Radiol. 144, 109949 (2021).
Karanasios, E., Caglic, I., Zawaideh, J. P. & Barrett, T. Prostate MRI quality: clinical impact of the PI-QUAL score in prostate cancer diagnostic work-up. Br. J. Radiol. https://doi.org/10.1259/bjr.20211372 (2022).
Arnoldner, M. A. et al. Rectal preparation significantly improves prostate imaging quality: assessment of the PI-QUAL score with visual grading characteristics. Eur. J. Radiol. 147, 110145 (2022).
Turkbey, B. Better image quality for diffusion-weighted MRI of the prostate using deep learning. Radiology https://doi.org/10.1148/radiol.212078 (2022).
de Rooij, M. & Barentsz, J. O. PI-QUAL v.1: the first step towards good-quality prostate MRI. Eur. Radiol. 32, 876–878 (2022).
Cipollari, S. et al. Convolutional neural networks for automated classification of prostate multiparametric magnetic resonance imaging based on image quality. J. Magn. Reson. Imaging 55, 480–490 (2022).
Brizmohun Appayya, M. et al. National implementation of multi-parametric magnetic resonance imaging for prostate cancer detection–recommendations from a UK consensus meeting. BJU Int. 122, 13–25 (2018).
Puech, P. et al. How are we going to train a generation of radiologists (and urologists) to read prostate MRI? Curr. Opin. Urol. 25, 522–535 (2015).
Rosenkrantz, A. B. et al. The learning curve in prostate MRI interpretation: self-directed learning versus continual reader feedback. Am. J. Roentgenol. 208, W92–W100 (2017).
Greer, M. D. et al. Interreader variability of prostate imaging reporting and data system version 2 in detecting and assessing prostate cancer lesions at prostate MRI. Am. J. Roentgenol. 212, 1197–1205 (2019).
Bhayana, R. et al. PI-RADS versions 2 and 2.1: interobserver agreement and diagnostic performance in peripheral and transition zone lesions among six radiologists. Am. J. Roentgenol. 217, 141–151 (2021).
Smith, C. P. et al. Intra- and interreader reproducibility of PI-RADSv2: a multireader study. J. Magn. Reson. Imaging 49, 1694–1703 (2019).
Park, K. J. et al. Risk stratification of prostate cancer according to PI-RADS® version 2 categories: meta-analysis for prospective studies. J. Urol. 204, 1141–1149 (2020).
de Rooij, M. et al. Focus on the quality of prostate multiparametric magnetic resonance imaging: synopsis of the ESUR/ESUI recommendations on quality assessment and interpretation of images and radiologists’ training. Eur. Urol. 78, 483–485 (2020).
Barrett, T. et al. Prostate MRI qualification: AJR expert panel narrative review. Am. J. Roentgenol. https://doi.org/10.2214/ajr.22.27615 (2022).
Butler, P. F. MQSA (Mammography Quality Standards Act) update-focusing on quality assurance. Radiol. Manag. 20, 40–50 (1998).
Reis, C., Pascoal, A., Sakellaris, T. & Koutalonis, M. Quality assurance and quality control in mammography: a review of available guidance worldwide. Insights Imaging 4, 539–553 (2013).
Pontone, G. et al. Training in cardiac computed tomography: EACVI certification process. Eur. Heart J. Cardiovasc. Imaging 19, 123–126 (2018).
Caglic, I. et al. Integration of prostate biopsy results with pre-biopsy multiparametric magnetic resonance imaging findings improves local staging of prostate cancer. Can. Assoc. Radiol. J. https://doi.org/10.1177/08465371211073158 (2022).
Wassberg, C. et al. The incremental value of contrast-enhanced MRI in the detection of biopsy-proven local recurrence of prostate cancer after radical prostatectomy: effect of reader experience. Am. J. Roentgenol. 199, 360–366 (2012).
Gatti, M. et al. Prostate cancer detection with biparametric magnetic resonance imaging (bpMRI) by readers with different experience: performance and comparison with multiparametric (mpMRI). Abdom. Radiol. 44, 1883–1893 (2019).
Greer, M. D. et al. Validation of the dominant sequence paradigm and role of dynamic contrast-enhanced imaging in Pi-RADS version 2. Radiology 285, 859–869 (2017).
Rothschild, J., Lourenco, A. P. & Mainiero, M. B. Screening mammography recall rate: does practice site matter? Radiology 269, 348–353 (2013).
Greer, M. D. et al. All over the map: an interobserver agreement study of tumor location based on the PI-RADSv2 sector map. J. Magn. Reson. Imaging 48, 482–490 (2018).
Shaish, H. et al. Impact of a structured reporting template on adherence to prostate imaging reporting and data system version 2 and on the diagnostic performance of prostate MRI for clinically significant prostate cancer. J. Am. Coll. Radiol. 15, 749–754 (2018).
Rudolph, M. M. et al. Validation of the PI-RADS language: predictive values of PI-RADS lexicon descriptors for detection of prostate cancer. Eur. Radiol. 30, 4262–4271 (2020).
Purysko, A. S. et al. PI-RADS version 2.1: a critical review, from the AJR special series on radiology reporting and data systems. Am. J. Roentgenol. 216, 20–32 (2021).
Snoj, Ž., Rundo, L., Gill, A. B. & Barrett, T. Quantifying the effect of biopsy lateral decubitus patient positioning compared to supine prostate MRI scanning on prostate translocation and distortion. Can. Urol. Assoc. J. 14, E445–E452 (2020).
Zawaideh, J. P. et al. Comparison of Likert and PI-RA DS version 2 MRI scoring systems for the detection of clinically significant prostate cancer. Br. J. Radiol. 93, 20200298 (2020).
Khoo, C. C. et al. Likert vs PI-RADS v2: a comparison of two radiological scoring systems for detection of clinically significant prostate cancer. BJU Int. 125, 49–55 (2020).
Latifoltojar, A., Appayya, M. B., Barrett, T. & Punwani, S. Similarities and differences between Likert and PIRADS v2.1 scores of prostate multiparametric MRI: a pictorial review of histology-validated cases. Clin. Radiol. 74, 895.e1–895.e15 (2019).
Hansen, N. L. et al. Multiparametric prostate magnetic resonance imaging and cognitively targeted transperineal biopsy in patients with previous abdominoperineal resection and suspicion of prostate cancer. Urology 96, 8–14 (2016).
Puech, P. et al. Multiparametric MRI-targeted TRUS prostate biopsies using visual registration. Biomed Res. Int. 2014, (2014).
Beyersdorff, D. et al. MR imaging-guided prostate biopsy with a closed MR unit at 1.5 T: initial results. Radiology 234, 576–581 (2005).
Wegelin, O. et al. Comparing three different techniques for magnetic resonance imaging-targeted prostate biopsies: a systematic review of in-bore versus magnetic resonance imaging-transrectal ultrasound fusion versus cognitive registration. Is there a preferred technique? Eur. Urol. 71, 517–531 (2017).
Simmons, L. A. M. et al. Accuracy of transperineal targeted prostate biopsies, visual estimation and image fusion in men needing repeat biopsy in the PICTURE trial. J. Urol. 200, 1227–1234 (2018).
Hamid, S. et al. The SmartTarget biopsy trial: a prospective, within-person randomised, blinded trial comparing the accuracy of visual-registration and magnetic resonance imaging/ultrasound image-fusion targeted biopsies for prostate cancer risk stratification. Eur. Urol. 75, 733–740 (2019).
Watts, K. L. et al. Systematic review and meta-analysis comparing cognitive vs. image-guided fusion prostate biopsy for the detection of prostate cancer. Urol. Oncol. Semin. Orig. Investig. 38, 734.e19–734.e25 (2020).
Venderink, W., Govers, T. M., De Rooij, M., Futterer, J. J. & Sedelaar, J. P. M. Cost-effectiveness comparison of imaging-guided prostate biopsy techniques: systematic transrectal ultrasound, direct in-bore MRI, and image fusion. Am. J. Roentgenol. 208, 1058–1063 (2017).
Hale, G. R. et al. Comparison of elastic and rigid registration during magnetic resonance imaging/ultrasound fusion-guided prostate biopsy: a multi-operator phantom study. J. Urol. 200, 1114–1121 (2018).
Ukimura, O. et al. 3-Dimensional elastic registration system of prostate biopsy location by real-time 3-dimensional transrectal ultrasound guidance with magnetic resonance/transrectal ultrasound image fusion. J. Urol. 187, 1080–1086 (2012).
Valerio, M. et al. Detection of clinically significant prostate cancer using magnetic resonance imaging-ultrasound fusion targeted biopsy: a systematic review. Eur. Urol. 68, 8–19 (2015).
Tamhankar, A. S. et al. The clinical and financial implications of a decade of prostate biopsies in the NHS: analysis of hospital episode statistics data 2008–2019. BJU Int. 126, 133–141 (2020).
Gorin, M. A. et al. Transperineal prostate biopsy with cognitive magnetic resonance imaging/biplanar ultrasound fusion: description of technique and early results. World J. Urol. 38, 1943–1949 (2020).
Pepdjonovic, L. et al. Zero hospital admissions for infection after 577 transperineal prostate biopsies using single-dose cephazolin prophylaxis. World J. Urol. 35, 1199–1203 (2017).
Hossack, T. et al. Location and pathological characteristics of cancers in radical prostatectomy specimens identified by transperineal biopsy compared to transrectal biopsy. J. Urol. 188, 781–785 (2012).
Israël, B. et al. Clinical implementation of pre-biopsy magnetic resonance imaging pathways for the diagnosis of prostate cancer. BJU Int. https://doi.org/10.1111/BJU.15562 (2021).
Xiang, J. et al. Transperineal versus transrectal prostate biopsy in the diagnosis of prostate cancer: a systematic review and meta-analysis. World J. Surg. Oncol. 17, 31 (2019).
Kuru, T. H. et al. Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for enhanced prostate diagnostics. BJU Int. 112, 568–577 (2013).
Onik, G. & Barzell, W. Transperineal 3D mapping biopsy of the prostate: an essential tool in selecting patients for focal prostate cancer therapy. Urol. Oncol. Semin. Orig. Investig. 26, 506–510 (2008).
Hansen, N. L. et al. Multicentre evaluation of targeted and systematic biopsies using magnetic resonance and ultrasound image-fusion guided transperineal prostate biopsy in patients with a previous negative biopsy. BJU Int. 120, 631–638 (2017).
Hansen, N. L. et al. Multicentre evaluation of magnetic resonance imaging supported transperineal prostate biopsy in biopsy-naïve men with suspicion of prostate cancer. BJU Int. 122, 40–49 (2018).
Das, C. J., Razik, A., Netaji, A. & Verma, S. Prostate MRI–TRUS fusion biopsy: a review of the state of the art procedure. Abdom. Radiol. 45, 2176–2183 (2020).
Moore, C. M. et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an international working group. Eur. Urol. 64, 544–552 (2013).
Schouten, M. G. et al. Why and where do we miss significant prostate cancer with multi-parametric magnetic resonance imaging followed by magnetic resonance-guided and transrectal ultrasound-guided biopsy in biopsy-naïve men? Eur. Urol. 71, 896–903 (2017).
Tracy, C. R. et al. Optimizing MRI-targeted prostate biopsy: the diagnostic benefit of additional targeted biopsy cores. Urol. Oncol. Semin. Orig. Investig. 39, 193.e1–193.e6 (2021).
Ploussard, G. et al. Assessment of the minimal targeted biopsy core number per MRI lesion for improving prostate cancer grading prediction. J. Clin. Med. 9, 225 (2020).
Lu, A. J. et al. Role of core number and location in targeted magnetic resonance imaging-ultrasound fusion prostate biopsy. Eur. Urol. 76, 14–17 (2019).
Meng, X. et al. The institutional learning curve of magnetic resonance imaging-ultrasound fusion targeted prostate biopsy: temporal improvements in cancer detection in 4 years. J. Urol. 200, 1022–1029 (2018).
Bevill, M. D. et al. Number of cores needed to diagnose prostate cancer during MRI targeted biopsy decreases after the learning curve. Urol. Oncol. Semin. Orig. Investig. https://doi.org/10.1016/j.urolonc.2021.05.029 (2021).
Costa, D. N. et al. Gleason grade group concordance between preoperative targeted biopsy and radical prostatectomy histopathologic analysis: a comparison between in-bore MRI-guided and MRI–transrectal US fusion prostate biopsies. Radiol. Imaging Cancer 3, e200123 (2021).
Gnanapragasam, V. J. et al. Using prognosis to guide inclusion criteria, define standardised endpoints and stratify follow-up in active surveillance for prostate cancer. BJU Int. 124, 758–767 (2019).
Kench, J. G. et al. Dataset for the reporting of prostate carcinoma in radical prostatectomy specimens: updated recommendations from the International Collaboration on Cancer Reporting. Virchows Arch. 475, 263–277 (2019).
Egevad, L. et al. Standardization of Gleason grading among 337 European pathologists. Histopathology 62, 247–256 (2013).
Chen, S. D., Fava, J. L. & Amin, A. Gleason grading challenges in the diagnosis of prostate adenocarcinoma: experience of a single institution. Virchows Arch. 468, 213–218 (2016).
Siedow, M. et al. Impact of prostate biopsy secondary pathology review on radiotherapy management. Prostate 82, 210–215 (2022).
Smith, E. B., Frierson, H. F., Mills, S. E., Boyd, J. C. & Theodorescu, D. Gleason scores of prostate biopsy and radical prostatectomy specimens over the past 10 years: is there evidence for systematic upgrading? Cancer 94, 2282–2287 (2002).
Allsbrook, W. C. et al. Interobserver reproducibility of Gleason grading of prostatic carcinoma: urologic pathologists. Hum. Pathol. 32, 74–80 (2001).
Short, E., Warren, A. Y. & Varma, M. Gleason grading of prostate cancer: a pragmatic approach. Diagn. Histopathol. 25, 371–378 (2019).
Egevad, L., Delahunt, B., Yaxley, J. & Samaratunga, H. Evolution, controversies and the future of prostate cancer grading. Pathol. Int. 69, 55–66 (2019).
Epstein, J. I., Feng, Z., Trock, B. J. & Pierorazio, P. M. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy: incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur. Urol. 61, 1019–1024 (2012).
Ozkan, T. A. et al. Interobserver variability in Gleason histological grading of prostate cancer. Scand. J. Urol. 50, 420–424 (2016).
Kweldam, C. F., van Leenders, G. J. & van der Kwast, T. Grading of prostate cancer: a work in progress. Histopathology 74, 146–160 (2019).
Egevad, L. et al. Utility of pathology imagebase for standardisation of prostate cancer grading. Histopathology 73, 8–18 (2018).
Harnden, P. et al. Evaluation of the use of digital images for a national prostate core external quality assurance scheme. Histopathology 59, 703–709 (2011).
Bulten, W. et al. Artificial intelligence assistance significantly improves Gleason grading of prostate biopsies by pathologists. Mod. Pathol. 34, 660–671 (2021).
Nagpal, K. et al. Development and validation of a deep learning algorithm for improving Gleason scoring of prostate cancer. npj Digit. Med. 2, 1–10 (2019).
Bulten, W. et al. Automated deep-learning system for Gleason grading of prostate cancer using biopsies: a diagnostic study. Lancet Oncol. 21, 233–241 (2020).
Author information
Authors and Affiliations
Contributions
T.B., M.D.R., F.G. and C.A. researched data for the article. All authors contributed substantially to discussion of the content. T.B., M.D.R. and F.G. wrote the article. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks C.K. Kim; P. Pinto, who co-reviewed with M. Rothberg; V. Panebianco; and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Pathology imagebase: https://isupweb.org/pib-start/
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barrett, T., de Rooij, M., Giganti, F. et al. Quality checkpoints in the MRI-directed prostate cancer diagnostic pathway. Nat Rev Urol 20, 9–22 (2023). https://doi.org/10.1038/s41585-022-00648-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-022-00648-4
- Springer Nature Limited
This article is cited by
-
Interactive training workshop to improve prostate mpMRI knowledge: results from the ESOR Nicholas Gourtsoyiannis teaching fellowship
Insights into Imaging (2024)
-
Expect the unexpected: investigating discordant prostate MRI and biopsy results
European Radiology (2024)
-
Artificial intelligence in liver imaging: methods and applications
Hepatology International (2024)