Abstract
It is recommended that the adjuvant Montanide ISA 720 VG be used at a concentration of 70% v/v. At this concentration, Montanide causes at the site of immunization a local granuloma that can last for several weeks. To determine the safety and protective efficacy of a Chlamydia muridarum MOMP vaccine, formulated with CpG-1826 and four different concentrations of Montanide (70%, 50%, 30% and 10%), BALB/c (H-2d) female mice were immunized twice intramuscularly. Local reactogenicity was significant for vaccines formulated with 70% or 50% Montanide but not for those inoculated with 30% or 10% Montanide. Robust humoral and cell mediated memory immune responses were elicited by the 70%, 50% and 30% Montanide formulations. Mice were challenged intranasally with 104 C. muridarum inclusion forming units (IFU). Based on changes in body weight, lungs’s weight and number of IFU recovered, mice vaccinated with the 70%, 50% and 30% Montanide formulations were significantly protected, but not mice receiving 10% Montanide. To conclude, we recommend the 30% Montanide concentration to be tested in humans and animal models to determine its safety and efficacy, in comparison to the 70% Montanide concentration currently used. The 30% Montanide formulation could significantly facilitate licensing of this adjuvant for human use.
Similar content being viewed by others
Introduction
It is estimated that each year approximatly 130 million individuals worldwide are infected in the genitourinary tract with Chlamydia trachomatis1. In addition, C. trachomatis causes ocular, respiratory, and gastrointestinal infections2,3. In females, genital infections can result in long-term sequelae including pelvic inflammatory disease, chronic abdominal pain, ectopic pregnancy and infertility4,5. Genital C. trachomatis infections are also associated with other serious diseases such as cervical hypertrophy, squamous metaplasia and HIV or HPV infections6,7. Babies born from mothers with a genital chlamydial infection can develop conjunctivitis and pneumonia with negative long-term medical sequelae4. Immunocompromised individuals can also have respiratory infections with C. trachomatis8,9. Antibiotic therapy is available, but due to the high proportion of asymptomatic patients, this approach has not eradicated these infections10,11. Furthermore, treated patients may fail to develop natural immunity that can result in an increase in the prevalence of C. trachomatis infections11. Therefore, Chlamydia infections are a worldwide public health problem and a vaccine is needed to control them12,13,14,15,16,17.
Over the last four decades several Chlamydia proteins have been tested as vaccine candidates.12,13,14,15,16,17,18,19,20,21,22,23. Among them, the major outer membrane protein (MOMP) is the only antigen that has completed a Phase I Clinical Trial24. MOMP is a homotrimer porin that accounts for 60% of the mass of the outer membrane of Chlamydia and has four variable domains (VD) and five constant domains (CD)25,26,27. The VD contain multiple B-cell epitopes while the T-cell epitopes are mainly located in the CD28,29,30.
Most subunit vaccines, including MOMP, lack the adjuvant activity necessary to induce robust innate and adaptive immune responses31,32. In the mouse model, it has been shown that both humoral and cell mediated immune responses contribute to protection against a Chlamydia muridarum challenge33,34,35. Therefore, to formulate a vaccine with MOMP we need to identify single adjuvants, or adjuvants combinations, that induce innate responses resulting in mucosal and systemic humoral and cellular immune memory. A combination of MOMP with 70% (v/v) Montanide ISA 720 VG, that elicits Th2 responses and CpG, that induces Th1 responses, has been found to be very effective at protecting mice against genital and respiratory chlamydial challenges, and non-human primates against ocular infections15,20,36,37,38,39. A shortcoming of this vaccine formulation is the induction of local reactogenicity at the site of immunization.
Early studies using Freund’s incomplete adjuvant (FIA), a water in oil formulation like 70% Montanide ISA 720 VG, resulted in injection site granulomas in humans and experimental animals40. Montanide ISA 720 VG is part of a large family of Montanide’s that were developed as an alternative to FIA to reduce its local reactogenicity and thus, the name Incomplete Seppic Adjuvant (ISA)41,42. Although the 70% concentration, is recommended by Seppic Inc., we could not find published data to determine the reasons for this recommendation. It is possible that this concentration was found by Seppic Inc., to elicit antibody levels similar or higher than those induced by FIA and to produce a long-term depot effect41,43. Montanide ISA 720 VG at the 70% concentration has been used in experimental animals and also in Phase I and II clinical trials with vaccine candidates to protect against HIV-1, HPV, SARS-CoV-2, malaria, leishmania and cancer41,44,45,46,47,48. In spite of many years of use, Montanide ISA 720 VG is not licensed probably because its significant reactogenicity at the site of immunization.
Montanide ISA 720 VG is an emulsion of squalene, a natural, vegetable, biodegradable, non-toxic, non-mineral oil, and a surfactant from the mannide monooleate family43,49. The mechanisms underlying the effects of this adjuvant are not well understood but elicits Th2-skewed responses. Vaccines containing squalene induce high antibody titers, numerous long-lived plasma cells, enhance specific cytotoxic T-lymphocyte responses, and stimulate the recruitment of antigen presenting cells (APC)43,50. When Montanide ISA 720 VG is used at a 70%/30% oil/water v/v ratio, the main activity is thought to be due to the depot effect that allows for the slow release of antigen at the site of immunization43,49.
CpGs (cytosine phosphoguanine motifs), agonists of TLR-9, elicit Th1-biased immune responses with direct activation of monocytes, macrophages and dendritic cells (DC) that secrete IL-6, IL-12, IFN-γ and TNF-α and several chemokines51,52. Furthermore, CpGs stimulate B-cells to proliferate and secrete immunoglobulins IL-6 and IL-1253. The overall effect of CpGs is the induction of strong Th1 humoral and cellular immune responses and broadening of the B-cell epitope recognition51,52,54. FDA has recently approved the use of CpG-1018, an adjuvant optimized for stimulating robust immune responses in humans, for a hepatitis B virus vaccine55.
To determine if it is possible to decrease the local reactogenicity while maintaining its adjuvanticity, here Montanide ISA 720 VG was tested at four different ratios (v/v) of the total vaccine, 70%, 50%, 30% and 10%. Amounts of CpG-1826 and MOMP were kept constant in the four formulations. We hypothesized that the vaccine that elicits the highest levels of IFN-γ from CD4 + T-cells will be the most protective but also the most reactogenic. The results showed that vaccine formulations containing 70%, 50% and 30% of Montanide ISA 720 VG, elicited equivalent immune responses and protection in mice. Furthermore, the 30% concentration induced minimal reactogenicity at the site of immunization. To our knowledge, this is the first time that four Montanide ISA 720 VG formulations have been tested in parallel for safety and efficacy in an animal model.
Results
Assessment of the reactogenicity at the site of immunization
To evaluate the effects of the reactogenicity induced by the different vaccine formulations, mice were observed daily for a week after the two immunizations. No significant changes in the physical appearance or behaviour of the mice were observed. Pictures of the immunization site were taken at the end of the experiment. As shown in Fig. 1, in control mice that received PBS, or MOMP only, the site of immunization had a normal appearance. Similarly, no lesions were observed in mice immunized with 10% Montanide. In contrast, bullae ranging from ~ 2 – 5 mm in diameter were found in mice vaccinated with 70% and 50% Montanide while animals receiving 30% Montanide had no visible bullae, and only a few had indurations of ~ 1–2 mm in diameter.
Antibody responses in serum and vaginal washes following vaccination
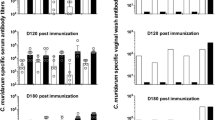
Serum samples, collected the day before the challenge, were used to evaluate the type of immune responses elicited by vaccination. Sera collected before immunization were used as negative controls. Levels of IgG2a and IgG1 were determined by ELISA using C. muridarum EB as the antigen. As shown in Fig. 2a, the highest IgG2a GMT was observed in mice vaccinated using 70% Montanide, 517,547, while mice immunized with 10% Montanide had the lowest IgG2a GMT, 73,530. The mice with the highest IgG1 GMT were immunized with 50% Montanide, 29,372, while mice receiving 10% Montanide had the lowest IgG1 GMT, 3195. Based on the IgG2a/IgG1 ratio the four groups of mice immunized using adjuvants had Th1-biased immune response. Mice inoculated only with MOMP had a higher IgG1 GMT, 7342 than IgG2a, 202, indicating Th2-skewed responses. Controls receiving PBS had no detectable levels of antibodies to C. muridarum EB (Limit of detection < 100).
Mice were vaccinated and blood and vaginal washes from each animal were collected the day before the i.n. challenge. a IgG1 and IgG2a ELISA GMT in serum. b In vitro neutralizing antibody GMT in serum and (c) IgG and IgA antibody GMT in vaginal washes. LOD Limit of detection. *P < 0.05 by Student’s t-test.
In vitro neutralization titers in serum were detected only in the three groups of mice immunized with 70% (119), 50% (59), or 30% (114) Montanide (Fig. 2b).
Mice vaccinated with Montanide had IgG GMT in vaginal washes ranging from 80–453 (Fig. 2c). Mice immunized with 10% Montanide had significantly lower IgG GMT than the other three groups (P < 0.05). The IgG GMT in vaginal washes for the negative controls immunized with MOMP only, or PBS were below the limits of detection (<10). Levels of IgA in vaginal washes were below the limit of detection for all groups of mice (<10).
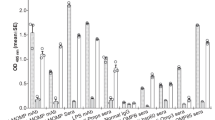
To determine the breadth of the antibody responses, 25 mer MOMP overlapping peptides were tested with sera using an ELISA. As shown in Fig. 3, the adjuvanted vaccines elicited antibodies to the four VDs, to peptides in CD1 and CD5 and to C. muridarum EB and MOMP. Antibody responses in mice immunized using 10% Montanide were weaker against peptides CD5 than in the other three groups. Mice immunized with MOMP had antibodies only to VD1, EB and MOMP. Sera from the negative control animals receiving PBS did not react with EB, MOMP or MOMP peptides.
Serum samples from vaccinated mice were collected the day before the i.n. challenge. Pooled serum samples from each group of mice were tested by an ELISA for their reactivity to 25-aa overlapping synthetic peptides corresponding to the C. muridarum mature MOMP. C. muridarum EB and MOMP were used as positive controls. C, a synthetic 25 mer non MOMP peptide, was used as negative control. Peptide 25, overlaps the N- and C- terminus of C. muridarum MOMP.
Cell mediated immune responses induced by vaccination
To evaluate the cellular memory immune responses, the day before the i.n. challenge, splenic T-cells were isolated using nylon wool from four mice/group and stimulated with C. muridarum EB. High levels of IFN-γ (pg/ml) were detected in the groups of mice vaccinated with 70% (781), 50% (996) and 30% (942) Montanide (Fig. 4a). T-cells from mice immunized with 10% Montanide secreted lower amounts of IFN-γ (565) (P < 0.05). Amounts of IFN-γ in mice immunized only with MOMP (131), or PBS (<20), were significantly lower than in the adjuvanted vaccines (P < 0.05). Levels of IL-4 in supernatants from T-cells stimulated with EB were low in all groups (Fig. 4b).
Mice were immunized and the day before the intranasal challenge they were euthanized, their spleens collected, T-cells isolated using nylon wool columns and stimulated with C. muridarum EB, or with Concanavalin A as a non-specific stimulant, or with medium as a negative control. a IFN-γ levels in T-cell supernatants and (b) Levels of IL-4 in T-cell supernatants. Mice per group four. LOD Limit of detection. *P < 0.05 by Student’s t-test.
Body weigh changes following the i.n. challenge with C. muridarum
Four weeks after the boost mice were challenged i.n. with 104 C. muridarum IFU and the body weight was determined daily for 10 days when all animals were euthanized (Fig. 5a). As determined by the repeated measures ANOVA test, all mice receiving adjuvants lost less body weight over the 10 d.p.c. when compared with the negative controls receiving MOMP only, or PBS (P < 0.05). All mice lost weight from D2 to D4 p.c. The two negative controls receiving PBS, or MOMP only, continued to lose weight until D10. Over the 10 days period, mice immunized with 10% Montanide lost more weight than the three other groups vaccinated using adjuvants (P < 0.05). Mice immunized with 30% Montanide recovered their body weight faster than the other three groups immunized with adjuvants.
Immunized mice were challenge i.n. with 104 IFU of C. muridarum 4 weeks after the boost and changes in body weight recorded daily. At 10 days post-challenge mice were euthanized. Each group included 10 mice. a Percentage changes in daily mean body weight following the i.n. challenge with C. muridarum. *P < 0.05 by the Repeated Measures ANOVA. b % Changes in body weight at D10 p.c. *P < 0.05 by Student’s t-test. **P < 0.10 by Student’s t-test.
At D10 post challenge, control mice receiving MOMP, or PBS, had lost 21.1% and 23.1% of their initial body weight respectively, significantly more than the weight losses of mice vaccinated with adjuvanted formulations (P < 0.05) (Fig. 5b and Table 1). At D10 p.c., mice immunized with 70%, 50%, 30% and 10% Montanide had lost 4.3%, 3.0% 1.9% and 9.4% respectively, of their initial body weight. Mice vaccinated using 50% and 30% Montanide lost significantly less body weight than those immunized with 10% (P < 0.05).
Lung’s weights at D10 post C. muridarum challenge
As a parameter of the local inflammatory responses, the mean weights of the lungs (g) were determined following euthanasia (Fig. 6a and Table 1). Negative controls immunized with PBS, or MOMP only, had the heaviest lungs (0.28 and 0.29, respectively). These lungs’ weights were significantly heavier than those of the four groups receiving adjuvanted MOMP vaccines (P < 0.05). Mice vaccinated with 50% (0.20), or 30% Montanide (0.20) had lighter lungs than those immunized with 10% (0.23) (P < 0.05).
Vaccinated mice were challenged i.n. with 104 IFU of C. muridarum. At D10 p.c mice were euthanized, their lungs weighed, homogenized in 5 ml of SPG and cultured in HeLa-229 cell monolayers. Following staining with a monoclonal antibody to MOMP, the number of chlamydial inclusions was counted using light microscopy. Each group included 10 mice. a Lungs’ weights (g) at 10 days after the i.n. challenge. The mean is shown as a horizontal line. Each symbol represents an animal. *P < 0.05 by Student’s t-test. **P < 0.10 by Student’s t-test. b Number of C. muridarum IFU recovered from the lungs at D10 following the i.n. challenge. The median is shown as a horizontal line. Each symbol represents an animal. LOD Limit of detection. *P < 0.05 by the Mann–Whitney U-test.
Number of C. muridarum IFU recovered from the lungs at D10 p.c
The median number of C. muridarum IFU in mice, vaccinated only with MOMP, was 423,185 × 103, while in mice immunized with PBS it was 1,122,725 × 103 (Fig. 6b & Table 1). Both values were significantly different from the four groups of mice vaccinated using adjuvanted MOMP (P < 0.05). Mice vaccinated using 70% or 30% Montanide had the lowest median number of C. muridarum IFU in their lungs 14,300 and 19,800 respectively, while the group immunized with 10% had the highest median number of IFU 26,689 × 103 (P < 0.05) among the groups receiving adjuvants. No statistically significant differences were observed in the number of IFU recovered from the lungs when comparing the three groups of mice immunized with 70%, 50% or 30% Montanide (P > 0.05).
Levels of IFN-γ and C. muridarum specific IgA in lungs’ supernatants
It is expected that in mice that have cleared the infection, the mean levels of IFN-γ (pg/ml) in their lungs supernatants will be low, while in those that still have C. muridarum it will be high (Fig. 7a & Table 1). The negative controls immunized with MOMP alone, or PBS had high levels of IFN-γ, 2194 and 2991, respectively. Mice immunized with 70%, 50%, 30% or 10% Montanide, had significantly lower levels of IFN-γ, 174, 61, 48, and 628, respectively, than the negative controls (P < 0.05). Mice immunized with 10% Montanide had higher IFN-γ amounts than the other three groups that were vaccinated with MOMP plus the adjuvants (P < 0.05).
Vaccinated mice were challenged i.n. 4 weeks after the boost with 104 IFU of C. muridarum. At D10 p.c. the mice were euthanized, their lungs weighed and homogenized in 5 ml of SPG. Following homogenization, the lungs were centrifuged, the supernatants collected and used to determine the levels of IFN-γ and C. muridarum specific IgA. Each group included 10 mice. a IFN-γ levels in lungs’ supernatants at 10 d.p.c. The mean is shown as a horizontal line. Each symbol represents an animal. *P < 0.05 by Student’s t-test. **P < 0.1 by Student’s t-test. b C. muridarum-specific IgA levels in lungs’ supernatants at 10 d.p.c. The mean is shown as a horizontal line. Each symbol represents an animal. *P < 0.05 by Student’s t-test. **P < 0.1 by Student’s t-test.
Levels of C. muridarum-specific IgA (OD450) were also determined in the lung’s supernatants. Mice that mount robust immune responses to vaccination are expected to have high levels of IgA. As shown in Fig. 7b and Table 1, the three groups of mice immunized with 70%, 50% and 30% Montanide had significantly higher levels of C. muridarum IgA 0.206, 0.147, and 0.143, respectively, than mice vaccinated with 10%, 0.120, or the two negative control groups receiving MOMP, 0.120 or PBS, 0.096 (P < 0.05).
Discussion
The aim of this study was to compare the safety and efficacy of a vaccine formulated with MOMP, CpG-1826 and Montanide ISA 720 VG to induce in mice protective humoral and cell mediated immune responses against a C. muridarum respiratory challenge. Montanide ISA 720 VG was used at four different concentrations 70%, 50%, 30% and 10% (v/v) while the quantities of MOMP and CpG-1826 were kept constant in the four vaccines. When compared with the 30% and 10% concentrations, i.m. vaccination with the 70% and 50% formulations of Montanide ISA 720 VG produced higher reactogenicity at the site of immunization that lasted for the length of the experiment. Humoral and cellular immune memory responses were similar in mice vaccinated with the 70%, 50% and 30% Montanide ISA 720 VG but were weaker in the group immunized with 10%. Following the i.n. challenge, based on changes in body weight, weights of the lungs, and the number of C. muridarum IFU recovered from the lungs, vaccines containing 70%, 50% and 30% Montanide ISA 720 VG elicited similar robust protection while the 10% did not. Our results indicate that vaccines using 30% Montanide ISA 720 VG should be compared with the 70% formulation in humans and animal models for their safety and efficacy at inducing protection against pathogens.
The intensity of the reactogenicity of a vaccine is dependent on several factors including the site of immunization, volume of the vaccine, and type of antigen and adjuvant used56. The immunological status of the vaccinee will also impact the level of reactogenicity. When formulated as “a water-in-oil” emulsion (70% v/v), a shortcoming of Montanide ISA 720 VG is the production of a granuloma at the site of immunization that can last for weeks or months43. The Montanide ISA 720 VG induced granuloma, by creating a depot effect, helps to slowly release the antigen and maintain local immune responses over a long period of time. In addition to the depot effect, other mechanisms appear to be involved in the induction of immune responses by Montanide ISA 720 VG. For example, injection of the antigen in one site, and of Montanide ISA 720 VG at a different location, still has an adjuvant effect although it is weaker when compared with delivery at the same site43. Our results confirm that the depot effect of Montanide ISA 720 VG is only one of the components that affect its adjuvanticity. A similar phenomenon occurs with Alum. The depot effect is not necessary for induction of innate immune responses57. While the 70% formulation generated bullae, that measured ~ 2–5 mm in diameter, and were still present at the end of the experiment, the 30% Montanide ISA 720 VG formulation resulted in a ~1–2 mm indurations that disappeared over a period of 3–4 weeks. In spite of the differences in local reactogenicity between the Montanide ISA 720 VG at a 70% versus a 30% concentration both elicited the same immune responses indicative that they are independent of the depot effect. Attraction of APC to the immunization site could be a mechanism by which Alum and Montanide ISA 720 VG enhance immunity43.
Most studies in animal models, and data collected from patients, indicate that CD4 + T-cells producing IFN-γ, are required to protect against C. trachomatis infections, while CD8 + T-cells play a secondary role34,58,59. Although the role of antibodies in protection is still controversial, they are probably important particularly during the early stages of the infection33,60,61. For example, Brunham et al.11 demonstrated that levels of C. trachomatis secretory IgA specific antibodies in the cervix inversely correlated with the number of recoverable EB. Therefore, adjuvants combinations that elicit robust Th1 and a Th2 responses may be required for a chlamydia vaccine to optimize protection.
CpG-1826, delivered alone with MOMP, has a limited adjuvant effect likely because it readily diffuses systemically62,63. Enhancement of the immune responses has been observed when CpGs, or other TLR agonists, are delivered with Montanide ISA 720 VG36,39,41,64. These adjuvants combinations elicit more robust APC activation, resulting in enhanced expression of CCR7, MHC class II and co-stimulatory molecules that lead to strong T-cell activation in the lymph nodes42,65.
It is known that delivering antigens and adjuvants to the same APC helps to enhance immune responses51,66. The negative charges of MOMP likely interact with the positively charged polar head of cationic lipids on Montanide ISA 720 VG. CpG-1826 also carries negative electric charges and has strong electrostatic attraction to the surface of Montanide ISA 720 VG. The complex of MOMP+Montanide ISA 720+CpG-1826 can then bind to the positive charged surface of APC. As a result, colocalized delivery of antigen and adjuvants can enhance immune responses at the vaccination site42,64,66,67,68,69. Furthermore, by trapping multiple molecules, the adjuvant increases the density of the antigen leading to enhanced B-cell responses67,70,71,72.
Following immunization, the C. muridarum EB-specific lgG2a/IgG ratio in serum showed that the Montanide ISA 720 VG+CpG-1826 vaccines all elicited Th1-biased responses while MOMP, without adjuvants, induced Th2-skewed responses. Neutralizing antibodies in serum were present in the three groups of mice immunized with high concentrations of Montanide ISA 720 VG but were negative in the 10% group. These findings were supported by the high levels of IFN-γ present in the supernatants of T-cells stimulated with EB from mice vaccinated with 70%, 50% and 30% Montanide ISA 720 VG, but not with the 10% formulation.
Levels of protection against the i.n. challenge with C. muridarum paralleled the immune responses. Mice vaccinated with 70%, 50% or 30% Montanide ISA 720 VG lost less body weight than those immunized with the 10% Montanide ISA 720 VG. A similar trend was observed when the lungs’ weights of mice were determined at 10 days following i.n. challenge. Similarly, the number of C. muridarum IFU in the lungs were significantly higher in mice receiving the 10% Montanide ISA 720 VG than in the other three groups of mice. The high levels of C. muridarum specific of IFN-γ in T-cell supernatants, the presence of neutralizing antibodies in serum and of C. muridarum specific IgA in lung’s supernatants induced by formulations containing the 70%, 50% or 30% Montanide ISA 720 VG, likely are responsible for the protection observed against the respiratory challenge.
A limitation of this study is the use of the respiratory tract model rather than the genital tract model for infection with C. muridarum73. Testing for local and systemic reactogenicity is not a limitation since the i.m. route will likely be implemented when a vaccine for C. trachomatis genital infections becomes available. Furthermore, these results maybe can directly be applied to vaccines developed for respiratory bacterial and viral pathogens such as Chlamydia pneumoniae, Mycobacterium tuberculosis, Streptococcus pneumoniae, Haemophilus influenzae, Bordetella pertussis, influenza viruses, coronaviruses, and respiratory syncytial virus.
We have used the respiratory model extensively to test chlamydial antigens, adjuvants, and routes of immunization16,23,74. The respiratory and the genital tract have a mucosal and a systemic component and therefore, we can evaluate immune responses in both compartments. Immunization and effects on protection can be tested in the respiratory model in <3 months while experiments with the genital tract model take 7 months to complete. This is a major difference that significantly affects supplies and personnel costs. We have performed several experiments, and our conclusion is that, if we cannot induce protection in the respiratory model, that vaccine formulation and delivery system, likely will not protect against a genital tract challenge.
Another shortcoming of this experiment is that the immune responses and protective activity of these vaccines formulations were tested only 4 weeks following the boost. It will be important to test these vaccination protocols for their ability to induce long-term protection. It is possible that the 70% Montanide ISA 720 VG formulation, by having a longer depot effect, may induce extended memory immune responses when compared to the 30% Montanide ISA 720 VG vaccine. However, it is also possible that a long exposure to the antigen will result in tolerance with changes in the immune responses that will lead to increase susceptibility to infection75.
To summarize, for the first time, we have shown that a C. muridarum MOMP vaccine, formulated with 30% Montanide ISA 720 VG, combined with CpG-1826, elicits minimal local reactogenicity at the site of immunization, while inducing robust protective immune responses, similar to the 70% formulation, against a respiratory C. muridarum challenge. Our next step is to determine, in the genital tract mouse model, if the protective immune responses, elicited by the vaccine formulation containing 30% Montanide ISA 720 VG, induce an equivalent protection to that obtained with the 70% concentration. Studies in humans should then validate the results obtained with animal models. Positive data could help to move forwards the licensing of Montanide ISA 720 VG for clinical use.
Methods
Stocks of C. muridarum
C. muridarum (strain Nigg II; American Type Culture Collection) was grown in HeLa-229 cell using high glucose Dulbecco’s medium, plus cycloheximide (1 µg/ml) and gentamycin 10 µg/ml, without foetal bovine serum. Elementary bodies (EB) were purified and stored in sucrose phosphate glutamate buffer (SPG) at -80 °C as described76. The number of C. muridarum inclusion forming units (IFU) in the stock was assessed in HeLa-229 cells using immune-peroxidase staining with a C. muridarum-MOMP specific mAb (MoPn-40) produced in our laboratory18.
Cloning, expression and purification of C. muridarum MOMP
The method to clone, express and purify the C. muridarum MOMP has been published19. By the Limulus amoebocyte assay (Associates of Cape Cod Inc.; East Falmouth, MA), MOMP had <0.05 EU of endotoxin/mg of protein.
Vaccination of female BALB/c mice
Four-to-five-week-old female BALB/c (H-2d) mice (Charles River Laboratories) were vaccinated with C. muridarum MOMP (10 μg/mouse/immunization), twice at a 4 week interval by the intramuscular (i.m.) route in the quadriceps muscle. The following adjuvants combinations were used: CpG-1826 (Tri-Link) (10 µg/mouse/immunization) + Montanide ISA 720 VG (Seppic Inc.) at four different concentrations (70%, 50%; 30% and 10% v/v)18. Montanide ISA 720 VG was mixed with MOMP and CpG-1826 using a vortex (Fisher Scientific). Each formulation was vortexed for 1 min followed by 1 min rest at room temperature. The cycle was repeated five times. A negative immunization control received PBS, and an adjuvant negative control was injected only with MOMP. Mice were euthanized by injecting ketamine and xylazine followed by cervical dislocation. The University of California, Irvine, Animal Care and Use Committee (IACUC) approved the experimental vertebrate protocol.
Evaluation of the humoral immune responses following immunization
Blood was collected from the periorbital plexus the day before vaccination and the day before the challenge. Using 96-well plates, ELISA antibody titers to C. muridarum EB (1 μg/well) were determined as described21. Goat anti-mouse IgG1 and IgG2a (BD Bioscience, San Diego, CA; Catalogue # 559626 and #553391, respectively) diluted 1:1000 for the two isotypes, were used. To stain, the substrate ABTS [2,2’-azino-bis-(3-ethylbenzthiazoline-6-sulfonate)] (Sigma-Aldrich, St. Louis, MO) was utilized and the plates were scanned in an ELISA reader at 405 nm. Titers were calculated using pre-immune sera ± 2 SD and reported as geometric mean titers (GMT). The limit of detection was 100.
The in vitro neutralization assays were performed in triplicate as described77. Two-fold serial dilutions of mouse serum were made in Ca+2/Mg+2 free PBS. Guinea pig serum (5%) was the source of complement. Samples were incubated with 104 C. muridarum IFU for 45 min at 37 °C. Samples were then centrifuged onto HeLa-229 monolayers grown in flat bottom 96-well plates. Following incubation at 37 °C for 30 h in culture medium with cycloheximide (1 µg/ml), the cells were fixed with methanol. IFU were stained with mAb MoPn-40 and counted with a light microscope77. Neutralization was defined as greater than, or equal to, a 50% decrease in the number of IFU when compared with the controls incubated with pre-immunization serum18. The limit of detection was a titer of 50 or less.
Antibodies to linear epitopes induced by immunization were determined using overlapping 25 mers (SynBioSci Corp.; Livermore, CA), corresponding to the mature C. muridarum MOMP amino acid sequence78,79. Peptide 25 (p25) overlaps the N- and C-terminus of C. muridarum MOMP. EB and MOMP were used as positive controls and C, a non-MOMP 25-mer peptide, as negative control. Peptides were adsorbed onto high binding affinity ELISA microtiter plates (1 µg/well of a 96-well plate). Antibody binding was determined in triplicates using anti-mouse IgG as above80.
Humoral immune responses in the genital mucosa to C. muridarum EB, were assessed in vaginal washes collected the day before the i.n. challenge using samples collected before immunization as negative controls. Levels of IgG and IgA were determined as above in pooled samples. The limit of detection was 10 for IgG and IgA.
Determination of cell mediated immune responses following immunization
Splenic T-cells, purified using nylon wool (>85% purity), collected the day before the challenge, from four mice/group, were stimulated with EB in the presence of irradiated (3300 rads, 137Cs) antigen presenting cells (APC)81. T-cells and APC were incubated in flat bottom 48-well plates (1.25 × 105/well of each cell) at 37 °C for 48 h. with C. muridarum EB at a 1:1 ratio. Concanavalin A (5 μg/ml) was used as a positive stimulant, and cell culture medium (RPMI with 10% FBS) was the negative control. Quantities of IFN-γ and IL-4 in T-cells supernatants, were determined with commercial kits (BD Pharmingen, San Diego, CA)21.
Intranasal challenge with C. muridarum and evaluation of the course of the infection in mice
Anesthetized mice were challenged i.n. with 104 IFU of C. muridarum 4 weeks after the second immunization82. Daily body weight changes were assessed for 10 days post-challenge (d.p.c.) when mice were euthanized, their lungs weighed and homogenized (Seward Stomacher 80; Lab System) in 5 ml of SPG. To establish the number of C. muridarum IFU, six serial dilutions of the lungs’ homogenates were used to infect Hela-229 cells grown in 48 well plates. Following incubation for 30 h at 37 °C in a CO2 incubator, the IFU were visualized with mAb MoPn-40, and counted using a light microscope81. The limit of detection (LD) was < or less 50 C. muridarum IFU/lungs/mouse.
To evaluate the local cellular immune responses, quantities of IFN-γ in lungs’ supernatants at 10 d.p.c. were determined by an ELISA as described63. To assess the humoral immune responses in the lungs, levels of C. muridarum specific IgA were determined in the lung’s supernatants63. All animal experiments were replicated once.
Statistical analyses
The two-tailed Student’s t-test was employed to evaluate differences between changes in body weight at day 10 post-challenge, lungs’ weights, levels of IFN-γ and IL-4 in T-cell recall assays, and levels of IFN-γ and IgA in lungs’ supernatants. Two-way repeated measures ANOVA with Sidak’s multiple comparison test was employed to compare changes in mean body weight over the 10 days of observation following the C. muridarum i.n. challenge. The Mann–Whitney U-Test was used to compare the antibody titers and the number of C. muridarum IFU in the lungs. A P-value of <0.05 was considered to be significant. A P-value of <0.1 was regarded as approaching significance. Statistical analyses were performed as discussed by Goodman83 and Rubin84.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
All experimental data, related to this manuscript, will be provided upon reasonable request by the corresponding author.
References
CDC. Division of STD Prevention, 1–168 (U.S. Department of Health and Human Services, Atlanta, 2021).
Schachter, J. & Dawson, C. R. Human Chlamydial Infections, 273 (PSG Pub. Co., 1978).
Beem, M. O. & Saxon, E. M. Respiratory-tract colonization and a distinctive pneumonia syndrome in infants infected with Chlamydia trachomatis. N. Engl. J. Med 296, 306–310 (1977).
O’Connell, C. M. & Ferone, M. E. Chlamydia trachomatis genital Infections. Micro. Cell 3, 390–403 (2016).
Westrom, L., Joesoef, R., Reynolds, G., Hagdu, A. & Thompson, S. E. Pelvic inflammatory disease and fertility. A cohort study of 1844 women with laparoscopically verified disease and 657 control women with normal laparoscopic results. Sex. Transm. Dis. 19, 185–192 (1992).
Plummer, F. A. et al. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J. Infect. Dis. 163, 233–239 (1991).
Silva, J., Cerqueira, F. & Medeiros, R. Chlamydia trachomatis infection: implications for HPV status and cervical cancer. Arch. Gynecol. Obstet. 289, 715–723 (2014).
Ito, J. I. et al. Pneumonia due to chlamydia trachomatis in an immunocompromised adult. N. Engl. J. Med. 307, 95–98 (1982).
Harrison, H. R., Taussig, L. M. & Fulginiti, V. A. Chlamydia trachomatis and chronic respiratory disease in childhood. Pediatr. Infect. Dis. 1, 29–33 (1982).
Burton, M. J. et al. Re-emergence of Chlamydia trachomatis infection after mass antibiotic treatment of a trachoma-endemic Gambian community: a longitudinal study. Lancet 365, 1321–1328 (2005).
Brunham, R. C., Pourbohloul, B., Mak, S., White, R. & Rekart, M. L. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J. Infect. Dis. 192, 1836–1844 (2005).
de la Maza, M. A. & de la Maza, L. M. A new computer model for estimating the impact of vaccination protocols and its application to the study of Chlamydia trachomatis genital infections. Vaccine 13, 119–127 (1995).
Rockey, D. D., Wang, J., Lei, L. & Zhong, G. Chlamydia vaccine candidates and tools for chlamydial antigen discovery. Expert Rev. Vaccines 8, 1365–1377 (2009).
Farris, C. M. & Morrison, R. P. Vaccination against Chlamydia genital infection utilizing the murine C muridarum model. Infect. Immun. 79, 986–996 (2011).
Carmichael, J. R., Pal, S., Tifrea, D. & de la Maza, L. M. Induction of protection against vaginal shedding and infertility by a recombinant Chlamydia vaccine. Vaccine 29, 5276–5283 (2011).
de la Maza, L. M., Zhong, G. & Brunham, R. C. Update on Chlamydia trachomatis vaccinology. Clin. Vaccine Immunol. 24, e00543 (2017).
Phillips, S., Quigley, B. L. & Timms, P. Seventy years of Chlamydia vaccine research - Limitations of the past and directions for the future. Front. Microbiol. 10, 70 (2019).
Pal, S., Peterson, E. M. & de la Maza, L. M. Vaccination with the Chlamydia trachomatis major outer membrane protein can elicit an immune response as protective as that resulting from inoculation with live bacteria. Infect. Immun. 73, 8153–8160 (2005).
Sun, G., Pal, S., Weiland, J., Peterson, E. M. & de la Maza, L. M. Protection against an intranasal challenge by vaccines formulated with native and recombinant preparations of the Chlamydia trachomatis major outer membrane protein. Vaccine 27, 5020–5025 (2009).
Kari, L. et al. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J. Immunol. 182, 8063–8070 (2009).
Tifrea, D. F., Pal, S., Popot, J. L., Cocco, M. J. & de la Maza, L. M. Increased immunoaccessibility of MOMP epitopes in a vaccine formulated with amphipols may account for the very robust protection elicited against a vaginal challenge with Chlamydia muridarum. J. Immunol. 192, 5201–5213 (2014).
Teng, A. et al. Proteomic identification of immunodominant chlamydial antigens in a mouse model. J. Proteom. 77, 176–186 (2012).
de la Maza, L. M., Pal, S., Olsen, A. W., and Follmann, F. Chlamydia Vaccines, 339–383 (Caister Academic Press, 2020).
Abraham, S. et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect. Dis. 19, 1091–1100 (2019).
Caldwell, H. D. & Schachter, J. Antigenic analysis of the major outer membrane protein of Chlamydia spp. Infect. Immun. 35, 1024–1031 (1982).
Stephens, R. S., Sanchez-Pescador, R., Wagar, E. A., Inouye, C. & Urdea, M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J. Bacteriol. 169, 3879–3885 (1987).
Sun, G. et al. Structural and functional analyses of the major outer membrane protein of Chlamydia trachomatis. J. Bacteriol. 189, 6222–6235 (2007).
Baehr, W. et al. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc. Natl Acad. Sci. USA 85, 4000–4004 (1988).
Stephens, R. S., Wagar, E. A. & Schoolnik, G. K. High-resolution mapping of serovar-specific and common antigenic determinants of the major outer membrane protein of Chlamydia trachomatis. J. Exp. Med 167, 817–831 (1988).
Ortiz, L. et al. Chlamydia trachomatis major outer membrane protein (MOMP) epitopes that activate HLA class II-restricted T cells from infected humans. J. Immunol. 157, 4554–4567 (1996).
Pulendran, B., Arunachalam, P. S. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454–475 (2021).
Plotkin, S. A., Orenstein, W. A. & Offit, P. A. Plotkin’s Vaccines, 7th edn (Elsevier, 2018).
Morrison, S. G. & Morrison, R. P. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J. Immunol. 175, 7536–7542 (2005).
Morrison, R. P., Feilzer, K. & Tumas, D. B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 63, 4661–4668 (1995).
Farris, C. M., Morrison, S. G. & Morrison, R. P. CD4 + T cells and antibody are required for optimal major outer membrane protein vaccine-induced immunity to Chlamydia muridarum genital infection. Infect. Immun. 78, 4374–4383 (2010).
Tifrea, D. F. et al. Improved protection against Chlamydia muridarum using the native major outer membrane protein trapped in Resiquimod-carrying amphipols and effects in protection with addition of a Th1 (CpG-1826) and a Th2 (Montanide ISA 720) adjuvant. Vaccine 38, 4412–4422 (2020).
Pal, S. et al. Vaccination with the recombinant major outer membrane protein elicits long-term protection in mice against vaginal shedding and infertility following a Chlamydia muridarum genital challenge. NPJ Vaccines 5, 90 (2020).
Pal, S., Theodor, I., Peterson, E. M. & de la Maza, L. M. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein can elicit a protective immune response against a genital challenge. Infect. Immun. 69, 6240–6247 (2001).
Pal, S., Davis, H. L., Peterson, E. M. & de la Maza, L. M. Immunization with the Chlamydia trachomatis mouse pneumonitis major outer membrane protein by use of CpG oligodeoxynucleotides as an adjuvant induces a protective immune response against an intranasal chlamydial challenge. Infect. Immun. 70, 4812–4817 (2002).
Stills, H. F. Jr. Adjuvants and antibody production: dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 46, 280–293 (2005).
Huijbers, E. J. et al. The non-toxic and biodegradable adjuvant Montanide ISA 720/CpG can replace Freund’s in a cancer vaccine targeting ED-B–a prerequisite for clinical development. Vaccine 30, 225–230 (2012).
Melssen, M. M., Fisher, C. T., Slingluff, C. L. & Melief, C. J. M. Peptide emulsions in incomplete Freund’s adjuvant create effective nurseries promoting egress of systemic CD4(+) and CD8( + ) T cells for immunotherapy of cancer. J. Immunother. Cancer 10, 9 (2022).
Aucouturier, J., Dupuis, L., Deville, S., Ascarateil, S. & Ganne, V. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev. Vaccines 1, 111–118 (2002).
Toledo, H. et al. A phase I clinical trial of a multi-epitope polypeptide TAB9 combined with Montanide ISA 720 adjuvant in non-HIV-1 infected human volunteers. Vaccine 19, 4328–4336 (2001).
Saul, A. et al. A human phase 1 vaccine clinical trial of the plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in montanide ISA720 adjuvant. Vaccine 23, 3076–3083 (2005).
Motavalli Khiavi, F. et al. A dual-type L2 11-88 peptide from HPV Types 16/18 formulated in montanide ISA 720 induced strong and balanced Th1/Th2 immune responses, associated with high titers of broad sectrum cross-reactive antibodies in vaccinated mice. J. Immunol. Res. 2018, 9464186 (2018).
Alleva, D. G. et al. Development of an IgG-Fc fusion COVID-19 subunit vaccine, AKS-452. Vaccine 39, 6601–6613 (2021).
Shokri, M., Roohvand, F., Alimohammadian, M. H., Ebrahimirad, M. & Ajdary, S. Comparing Montanide ISA 720 and 50-V2 adjuvants formulated with LmSTI1 protein of Leishmania major indicated the potential cytokine patterns for induction of protective immune responses in BALB/c mice. Mol. Immunol. 76, 108–115 (2016).
Miles, A. P. et al. Montanide ISA 720 vaccines: quality control of emulsions, stability of formulated antigens, and comparative immunogenicity of vaccine formulations. Vaccine 23, 2530–2539 (2005).
Fox, C. B., Baldwin, S. L., Duthie, M. S., Reed, S. G. & Vedvick, T. S. Immunomodulatory and physical effects of oil composition in vaccine adjuvant emulsions. Vaccine 29, 9563–9572 (2011).
Wagner, H. The immunogenicity of CpG-antigen conjugates. Adv. Drug Deliv. Rev. 61, 243–247 (2009).
Krieg, A. M. Immune effects and mechanisms of action of CpG motifs. Vaccine 19, 618–622 (2000).
de Gruijter, N. M., Jebson, B. & Rosser, E. C. Cytokine production by human B cells: role in health and autoimmune disease. Clin. Exp. Immunol. 210, 253–262 (2022).
Krieg, A. M. CpG motifs in bacterial DNA and their immune effects. Annu Rev. Immunol. 20, 709–760 (2002).
Girndt, M. et al. Immunogenicity and safety of a booster dose of the hepatitis B vaccine HepB-CpG (HEPLISAV-B(R)) compared with HepB-Eng (Engerix-B(R)) and HepB-AS04 (Fendrix(R)) in adults receiving hemodialysis who previously received hepatitis B vaccination and are not seroprotected: results of a randomized, multicenter phase 3 study. Hum. Vaccines Immunother. 18, 2136912 (2022).
van Doorn, E., Liu, H., Huckriede, A. & Hak, E. Safety and tolerability evaluation of the use of Montanide ISA51 as vaccine adjuvant: a systematic review. Hum. Vaccines Immunother. 12, 159–169 (2016).
Hutchison, S. et al. Antigen depot is not required for alum adjuvanticity. FASEB J. 26, 1272–1279 (2012).
Igietseme, J. U., Magee, D. M., Williams, D. M. & Rank, R. G. Role for CD8 + T cells in antichlamydial immunity defined by Chlamydia-specific T-lymphocyte clones. Infect. Immun. 62, 5195–5197 (1994).
Poston, T. B. et al. Cervical cytokines associated With Chlamydia trachomatis susceptibility and protection. J. Infect. Dis. 220, 330–339 (2019).
Russell, A. N. et al. Analysis of factors driving incident and ascending infection and the role of serum antibody in Chlamydia trachomatis genital tract infection. J. Infect. Dis. 213, 523–531 (2016).
Naglak, E. K., Morrison, S. G. & Morrison, R. P. IFNgamma is required for optimal antibody-mediated immunity against genital Chlamydia infection. Infect. Immun. 84, 3232–42 (2016).
Jiang, P. et al. Evaluation of tandem Chlamydia trachomatis MOMP multi-epitopes vaccine in BALB/c mice model. Vaccine 35, 3096–3103 (2017).
Cheng, C., Pal, S., Tifrea, D., Jia, Z. & de la Maza, L. M. A vaccine formulated with a combination of TLR-2 and TLR-9 adjuvants and the recombinant major outer membrane protein elicits a robust immune response and significant protection against a Chlamydia muridarum challenge. Microbes Infect. 16, 244–252 (2014).
Jones, T. R. et al. Synthetic oligodeoxynucleotides containing CpG motifs enhance immunogenicity of a peptide malaria vaccine in aotus monkeys. Vaccine 17, 3065–3071 (1999).
Sallusto, F. & Lanzavecchia, A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 4, S127–132 (2002).
Blander, J. M. & Medzhitov, R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature 440, 808–812 (2006).
Jegerlehner, A. et al. Regulation of IgG antibody responses by epitope density and CD21-mediated costimulation. Eur. J. Immunol. 32, 3305–3314 (2002).
Fischer, N. O. et al. Colocalized delivery of adjuvant and antigen using nanolipoprotein particles enhances the immune response to recombinant antigens. J. Am. Chem. Soc. 135, 2044–2047 (2013).
de Titta, A. et al. Nanoparticle conjugation of CpG enhances adjuvancy for cellular immunity and memory recall at low dose. Proc. Natl Acad. Sci. USA 110, 19902–19907 (2013).
Mohsen, M. O. et al. Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. J. Control Release 251, 92–100 (2017).
Bachmann, M. F. et al. The influence of antigen organization on B cell responsiveness. Science 262, 1448–1451 (1993).
Mohsen, M. O. & Bachmann, M. F. Virus-like particle vaccinology, from bench to bedside. Cell Mol. Immunol. 19, 993–1011 (2022).
de la Maza, L. M., Pal, S., Khamesipour, A. & Peterson, E. M. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect. Immun. 62, 2094–2097 (1994).
de la Maza, L. M., Darville, T. L. & Pal, S. Chlamydia trachomatis vaccines for genital infections: where are we and how far is there to go? Expert Rev. Vaccines 20, 421–35 (2021).
Mestecky, J., Russell, M. W. & Elson, C. O. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J. Immunol. 179, 5633–5638 (2007).
Caldwell, H. D., Kromhout, J. & Schachter, J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect. Immun. 31, 1161–1176 (1981).
Peterson, E. M., Zhong, G. M., Carlson, E. & de la Maza, L. M. Protective role of magnesium in the neutralization by antibodies of Chlamydia trachomatis infectivity. Infect. Immun. 56, 885–891 (1988).
Su, H., Morrison, R. P., Watkins, N. G. & Caldwell, H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J. Exp. Med. 172, 203–212 (1990).
Cheng, C. et al. Assessment of the role in protection and pathogenesis of the Chlamydia muridarum V-type ATP synthase subunit A (AtpA) (TC0582). Microbes Infect. 16, 123–133 (2014).
Pal, S., Cheng, X., Peterson, E. M. & de la Maza, L. M. Mapping of a surface-exposed B-cell epitope to the variable sequent 3 of the major outer-membrane protein of Chlamydia trachomatis. J. Gen. Microbiol. 139, 1565–1570 (1993).
Pal, S., Fielder, T. J., Peterson, E. M. & de la Maza, L. M. Protection against infertility in a BALB/c mouse salpingitis model by intranasal immunization with the mouse pneumonitis biovar of Chlamydia trachomatis. Infect. Immun. 62, 3354–3362 (1994).
Pal, S., Tifrea, D. F., Follmann, F., Andersen, P. & de la Maza, L. M. The cationic liposomal adjuvants CAF01 and CAF09 formulated with the major outer membrane protein elicit robust protection in mice against a Chlamydia muridarum respiratory challenge. Vaccine 35, 1705–1711 (2017).
Goodman, S. N. Statistics. Aligning statistical and scientific reasoning. Science 352, 1180–1181 (2016).
Rubin, M. When to adjust alpha during multiple testing: a consideration of disjunction, conjunction, and individual testing. Synthese 199, 10969–11000 (2021).
Acknowledgements
This work was supported by the National Institutes of Health grant U19 AI144184 from the National Institute of Allergy and Infectious Diseases.
Author information
Authors and Affiliations
Contributions
L.M.d.l.M. designed and supervised the study, analyzed results, and wrote the manuscript with contributions from all co-authors. A.S and S.P. performed all in vitro and in vivo experiments, compiled and analyzed data, performed statistical analyses, prepared tables and figures. M.A.C. and A.R. contributed to the designed of the study and the analysis of the results. L.M.d.l.M. and M.A.C. funding acquisition. All authors approved the completed version of the manuscript.All authors are accountable for all the aspects of the work including accuracy and integrity of all the data.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Slepenkin, A., Pal, S., Rasley, A. et al. Safety and efficacy of C. muridarum vaccines adjuvanted with CpG-1826 and four concentrations of Montanide-ISA-720-VG. npj Vaccines 9, 104 (2024). https://doi.org/10.1038/s41541-024-00880-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41541-024-00880-6
- Springer Nature Limited