Abstract
Loss of empathy is an early and central symptom of frontotemporal lobar degeneration spectrum diseases. We aimed to investigate the topographical distribution of morphometric brain changes associated with empathy in Progressive Supranuclear Palsy (PSP) and Corticobasal Syndrome (CBS) patients. Twenty-seven participants with CBS and 31 with PSP were evaluated using Interpersonal Reactivity Index scales in correlation with gray matter atrophy using a voxel-based morphometry approach. Lower levels of empathy were associated with an increased atrophy in fronto-temporal cortical structures. At subcortical level, empathy scores were positively correlated with gray matter volume in the amygdala, hippocampus and the cerebellum. These findings allow to extend the traditional cortico-centric view of cognitive empathy to the cerebellar regions in patients with neurodegenerative disorders and suggest that the cerebellum may play a more prominent role in social cognition than previously appreciated.
Similar content being viewed by others
Introduction
Empathy describes the ability to understand and share other’s emotional feelings and is fundamental to our emotional and social lives1. The two main neurocognitive components of empathic abilities are cognitive and emotional empathy. Cognitive empathy refers to cognitive ability of understanding of another person’s perspective and feelings (i.e. “I understanding what you feel”). Emotional empathy concerns the ability to feel and share the emotional experience of others (i.e. “I feel what you feel”). The neuroanatomical bases of these abilities in humans have been most consistently identified in a circuit comprising the insula, prefrontal cortices, superior temporal gyrus, temporoparietal junction and temporal poles, anterior cingulate cortex, and the inferior parietal lobules1,2,3, which are also regions that have been linked to the mirror neuron system4. However, whether socio-cognitive skills (including empathy) are functions independent from other nonsocial cognitive functions (e.g. memory or executive functions) with their own specialized brain modules is an open question in the literature.
While imaging of healthy subjects is necessary to identify the brain circuits involved in empathy5,6,7, complementary data from patients with neurodegeneration can add information about the relative importance of particular structures in those circuits, and can demonstrate which structures are required for normal empathy in real-life situations and which are not8. Notably, empathy impairment may be present across different neurodegenerative diseases with neuroanatomical underpinnings of that may be different across different disorders9,10. Loss of empathy can be a manifestation of several neurodegenerative disorders and can be an early and central symptom of frontotemporal lobar degeneration (FTLD), a neurodegenerative disorder characterized by the selective degeneration of the frontal and temporal cortices11,12. Emerging evidence indicates that difficulties in empathy abilities may also characterize both Progressive Supranuclear Palsy (PSP)13 and Corticobasal Syndrome (CBS)9,14, which are considered part of the FTLD spectrum. Interestingly, Arshad et al.15 investigated the empathy across different FLTD spectrum diseases, including behavioral variant Frontotemporal Dementia (bvFTD), primary progressive aphasia (PPA), amyotrophic lateral sclerosis (ALS) and PSP, finding that among these different neurodegenerative diseases, PSP patients manifest the maximal impairment in empathy abilities. However, the clinical and neuroanatomical correlates of empathy in PSP and CBS are not fully established and have been explored by only a limited number of studies9,13,16,17. Of particular interest, Ghosh et al. (2012) explored the social cognitive deficits and neural correlates for PSP patients by examine understanding of emotions in the voice13. They found that impairment of vocal emotion recognition was associated with atrophy of inferior frontal and cerebellar regions. Another previous study analyzed the relative neural correlates over 123 FTD patients, but only included a very small sample of CBS/PSP subjects18.
The purpose of the present study is to investigate the brain correlates of empathy deficits in a relatively large sample of PSP and CBS patients using a voxel-based morphometry (VBM) approach. To determine the specificity of these findings, we conducted a comparative analysis of the neural correlates of empathy deficits in behavioral variant Frontotemporal Dementia (bvFTD). We also explored whether empathy in these disorders is associated with general cognitive functions. We hypothesized that empathy deficits in PSP and CBS are independent of general cognitive functions and correlate with atrophy in frontotemporal regions.
Results
Clinical variables
The demographic and clinical characteristics of the PSP,CBS and bvFTD patients are summarized in Table 1. Significant differences were found in age distribution comparing PSP patients with CBS and bvFTD patients. PSP patients had higher PSPRS scores compared to patients with CBS (p = 0.018) and bvFTD (p < 0.001). Additionally, UPDRS III was higher for PSP respect to bvFTD (p < 0.001). IRI total score and IRI subscores (IRI-EC and IRI-PT) were similar among the three groups of patients (p = 0.360 and p = 0.603, respectively). Correlations between IRI and motor and cognitive variables are reported in Supplementary Table 1. We found no significant associations between IRI total score, IRI-EC, and IRI-PT subscores with any motor scores or cognitive tests.
Voxel-based morphometry
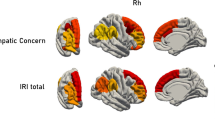
The associations between GM volume and IRI total score are reported in Fig. 1. Correlation analysis in PSP subjects, as reported in Fig. 1a, highlighted the left middle and superior temporal gyri as most correlated region (p-value < 0.05, FWE-corrected) with IRI total score, besides the left frontal orbital cortex. On the other hand, IRI total score in CBS subjects showed statistically significant correlations with GM volume in temporal pole bilaterally, also involving left hippocampus and amygdala(Fig. 1b, p-value < 0.05, FWE-corrected).
Correlation p-maps between IRI total scores and smoothed GM of PSP (FWE-corrected, p-value < 0.05) (a), CBS (FWE-corrected, p-value < 0.05) (b) and bvFTD subjects (uncorrected p-value < 0.05) (c). The design matrix for this whole-brain voxel-based morphometry analysis contained IRI total score, with sex, age, TIV and CDR Sum of Boxes included as nuisance covariates. Abbreviations: IRI Interpersonal Reactivity Index, GM Gray Matter, PSP Progressive Supranuclear Palsy, CBS Corticobasal Syndrome, FWE Family Wise Error.
Similarly, Fig. 2 and Fig. 3 reported correlations between GM volume and EC and PT subscores, respectively. In particular, EC subscore (Fig. 2) showed similar patterns of correlation as for total IRI evaluation for all analyses. Notably, empathic concern in both PSP (Fig. 2a) and CBS (Fig. 2b) patients reported an additional statistically significant cluster of correlation in the cerebellum, involving predominantly Crus II. In CBS patients, we also found the involvement of subcortical regions, mostly of hippocampus and amygdala bilaterally. Finally, PT subscore correlation’s maps (Fig. 3) reported only one significant cluster survived for PSP subjects in left middle temporal gyrus (Fig. 3a, p-value < 0.05, FWE-corrected), while no significative correlation was found between PT subscore and CBS patients (Fig. 3b, p-value < 0.05, uncorrected).
Correlation p-maps between EC subscores and smoothed GM of PSP (FWE-corrected, p-value < 0.05) (a), CBS (FWE-corrected, p-value < 0.05) (b) and bvFTD subjects (uncorrected p-value < 0.05) (c). The design matrix for this whole-brain voxel-based morphometry analysis contained only EC subscore, with sex, age, TIV and CDR Sum of Boxes included as nuisance covariates. Abbreviations: IRI Interpersonal Reactivity Index, EC Empathic Concern, GM Gray Matter, PSP Progressive Supranuclear Palsy, CBS Corticobasal Syndrome, FWE Family Wise Error.
Correlation p-maps between PT subscores and smoothed GM of PSP (FWE-corrected, p-value < 0.05) (a), CBS (uncorrected p-value < 0.05) (b) and bvFTD subjects (uncorrected p-value < 0.05) (c). The design matrix for this whole-brain voxel-based morphometry analysis contained only PT subscore, with sex, age,TIV and CDR Sum of Boxes included as nuisance covariates. Abbreviations: IRI Interpersonal Reactivity Index, PT Perspective Taking, GM Gray Matter, PSP Progressive Supranuclear Palsy, CBS Corticobasal Syndrome, FWE Family Wise Error.
To substantiate our finding, and to explore whether the neural correlates of empathy in PSP and CBS were different than in bvFTD, we examined brain correlates of empathy deficits in a sample of bvFTD subjects. As reported in Figs. 1c–2c–3c, no significant correlation was found between IRI scores and GM volume (only p-value < 0.05 uncorrected). However, sensitivity analysis discovered two main clusters in the right precentral and left postcentral gyri for the IRI total score and similarly for each subscore, with no involvement of subcortical regions. All information about clusters (e.g. number of voxels, coordinates) are reported in Supplementary Table 2.
Discussion
In this study, VBM analysis was used to investigate correlations between gray matter volumes and measures of empathy in patients with PSP and CBS. We demonstrated that empathy deficits are independent from general cognitive functions in PSP and CBS. The primary finding was that lower levels of empathy were significantly associated with atrophy of fronto-temporal cortical structures, particularly the left superior and middle temporal gyrus and temporal pole bilaterally. We also found that atrophy in subcortical structures, and in particular the cerebellum may underlie empathy deficit in PSP and CBS patients. Additionally, we observed different neural correlates in patients with behavioral variant Frontotemporal Dementia (bvFTD), suggesting that those findings are specific to 4R-tauopathies.
We found that empathy scores correlated with volumes of several cortical areas, including the middle and superior temporal gyri, the inferior frontal gyrus and the temporal pole. The middle temporal gyrus is a core region of emotion generation and processing19,20, participating in processing cognitive empathy21,22 and theory of mind23. One study in healthy individuals found that, compared with the control stimuli, emotional exclamations of others’ pain elicited increased activation in the superior and middle temporal gyri, suggesting that these regions are involved in empathy24. Another study5, investigating the functional correlated of empathy using functional MRI (fMRI), found activations in the middle temporal gyrus and inferior frontal gyri when respondents had to make empathic judgments in a verbal task. More recently, Jie et al.25 showed that both empathy and counter-empathy, which refers to emotional reactions that are incongruent (or even at odds with the emotional states of others) shared a common neural mechanism in the middle temporal gyrus.
The superior temporal gyrus, which is connected to the limbic system via the insula, is another brain region that is important for numerous aspects of social cognition, and it has been shown to be involved in emotional empathy26,27. Evidence suggests that the superior temporal sulcus region is involved in social cognitive tasks, such as the passive perception of social scenes28,29, and in emotional content of the facial stimuli30. Interestingly, inferior temporal gyrus is involved in visual processing and is associated with the representation of objects, places, faces, and colors31. Moreover, a fMRI study showed that activity in a region of the posterior inferior temporal lobe was related to cognitive empathy32.
The role of temporal pole in social and emotional processing has been extensively explored in the literature. This region lies between the orbital frontal cortex and the amygdala and receives and sends connections to both regions, coupling emotional responses to highly processed sensory stimuli. One of the first hints that the temporal pole was involved in socioemotional processing came from studies of Klüver–Bucy syndrome33. Many functional neuroimaging studies34,35 found the temporal poles implied in theory of mind, as the ability to infer the desires, intentions, or beliefs of others. Vollm and colleagues21 also found that activation clusters for a theory of mind task and an empathy task overlapped in the temporal pole. Interestingly, a VBM study on empathy over FTLD spectrum found that the total empathy score, measured using the IRI score, the same measure of empathy that we have used in the current study, were correlated with structural MRI brain volume in the temporal pole and the fusiform gyrus18.
We have also found that subcortical structures, such as the amygdala, hippocampus and the cerebellum, correlates with empathy scores in patients with PSP and CBS. Previous studies have highlighted how volume loss in the amygdala and hippocampus may play a role in empathy deficits36,37,38. More interesting, and somewhat unexpected finding concerns the possible involvement of the cerebellum in empathic processes of these neurodegenerative disease. Previous studies on PSP and CBS patients had just found a possible involvement of cerebellar regions in ability to understand and share other’s emotions14. In particular for PSP, Ghosh et al.13 demonstrates a correlation between atrophy of the cerebellum and the task of understanding of emotions in the voice. However, the cerebellum has only recently been recognized as one of the major structures involved in empathy16,39. Meta-analyses of functional MRI studies of emotional empathy, particularly for other people’s feelings of pain, have shown activation of cerebellum in association with the emotional empathy task40. The cerebellar involvement in cognitive empathy fits with the significant covariation of the cerebellum with self-rated individual differences in empathy for pain described by Singer and colleagues40, and with the reported cerebellar involvement in social cognition41,42. On the other hand, mounting evidence suggests an association between cerebellar atrophy and cognitive impairment in the main frontotemporal dementia syndromes. A recent study43 aimed to comprehensively chart profiles of cognitive impairment in relation to cerebellar atrophy in 49 demented patients with PSP and CBS, revealing distinct cerebellar subregions differentially implicated across cognitive domains in each patient group and offering insights into the cerebellum’s contribution to cognitive processing in these neurodegenerative disorders. Furthermore, an association between alterations in cerebellum and deficit in empathy and in theory of mind have been showed in behavioural variant FTD and AD44,45. Overall, our findings indicate that the cerebellum may be of one of the main brain regions involved in empathic processes in both PSP and CBS highlighting its involvement in socio-cognitive processes (the “social cerebellum”39). This is also in line with the concept that a damage of the cerebellum can cause the cerebellar cognitive affective syndrome (CCAS), also called Schmahmann’s syndrome, which is characterized by deficits in executive function, linguistic processing, spatial cognition, affect and social regulation46.
In this study we also investigated the possible association of non-social cognitive functions with the empathic capabilities. Ghosh et al.13 stressed that the deficit of theory of mind in PSP appear not to be affected by concomitant deficits in executive function. Here, we assess the correlations between IRI and performance in wide range of neuropsychological tasks of non-social cognitive functions. We did not find any correlation between empathy and other non-social cognitive skills (i.e. memory, executive function). This evidence suggests that empathy in PSP and CBS may be independent by other non-social cognitive functions.
There are some limitations and future directions to be considered. First, understanding of the precise structural contributions to empathic dysfunction in both CBS and PSP would require the inclusion of more subjects and multimodal MRI approaches that measures of structural and functional connectivity between brains regions. Secondly, Alzheimer’s Disease (AD) is found to be the primary underlying pathology in ~25% of patients presenting with CBS47. In this study, “in vivo” biomarkers of AD and neuropathology were unavailable, therefore we could not identify CBS patients with underlying AD. Future neuroimaging studies assessing empathy should be performed in CBS patients with AD and non-AD pathology and should include cohorts of patient’s with typical AD and healthy controls to further confirm the specificity of neural correlates of empathy. Furthermore, a variety of measures have been developed in order to quantify empathy in neurodegenerative disorders, such as Multifaceted Empathy Test (MET) or Empathy Quotient (EQ)48. In this study we used the Interpersonal Reactivity Index as the most common questionnaire to quantify empathy. However, our results may be influenced by reporter bias and must be replicated through the use of different types of empathy measurement.
In conclusion, we sought to characterize empathy deficit and their neuroanatomic correlates in both PSP and CBS. We found that empathy score was independent of general cognitive functions and that correlated with of several cortical areas, including the middle temporal gyrus, inferior temporal gyrus, the temporal pole, the fusiform and lingual gyrus. We also found that atrophy in subcortical structures, and in particular the cerebellum, may underlie empathy deficit in PSP and CBS patients. These findings allow to extend the traditional cortico-centric view of cognitive empathy to the cerebellar regions in patients with neurodegenerative disorders and suggest that the cerebellum may play a more prominent role in social cognition than previously appreciated.
Methods
Participants
4-Repeat Tauopathies
Data used in the preparation of this manuscript were obtained from the 4-Repeat Neuroimaging Initiative (4RTNI) database (http://4rtni-ftldni.ini.usc.edu/). 4RTNI was launched in early 2011 and is funded through the National Institute of Aging and The Tau Research Consortium. The primary goal of 4RTNI is to identify neuroimaging and biomarker indicators for disease progression in the 4-repeat tauopathy neurodegenerative diseases, progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). The Principal Investigator of 4RTNI is Dr. Adam Boxer, MD, PhD, at the University of California, San Francisco. Twenty-seven participants with CBS and 31 with PSP were recruited at University of California, San Francisco (UCSF) as part of the 4 Repeat Tauopathy Neuroimaging Initiative (4RTNI) study. The study was approved by the Institutional Review Board of UCSF site and all subjects, or their legal guardians gave informed written consent. Patients with PSP met the National Institute of Neurological Disorders and Stroke/Society for PSP-Richardson syndrome as modified for the AL-108–231 study49,50. Participants with CBS met Armstrong criteria for possible or probable CBS-CBD subtype (CBS)51. All subjects were evaluated at baseline with clinical rating scales, and MRI scans. The Mini-Mental State Examination (MMSE)52, the Unified Parkinson’s Disease Rating Scale (UPDRS), the Progressive Supranuclear Palsy Rating Scale (PSPRS), the Clinical Dementia Rating (CDR) score, the Functional Activity Questionnaire (FAQ), the Schwab and England Activities of Daily Living (SEADL) scale and 15-item Geriatric Depression Scale (GDS-15) were administered. The participants also performed a neuropsychological evaluation, including forward and backward digit span tests from the Wechsler Memory Scale III, the letter fluency and category fluency tests modified trail-making task, the Boston naming test, the California Verbal Learning Test and the modified Rey-Osterrieth copy performance. As measure of empathy, we used the Interpersonal Reactivity Index (IRI), a self-reporting questionnaire consisting of four subscales53 previously used for dementia and traumatic brain injury patients8,54. Two subscales were used to measure cognitive and emotional aspects of empathy, respectively the Perspective Taking (PT, the tendency to spontaneously imagine the cognitive perspective of another person) and the Empathic Concern (EC, the other-centered emotional response resulting from the perception of another’s emotional state). A total score of empathy was also obtained summing PT and EC subscales.
Behavioral variant frontotemporal dementia
Data used in the preparation of this retrospective study were obtained from the Frontotemporal Lobar Degeneration Neuroimaging Initiative (FTLDNI) database (for up-to-date information on participation and protocol https://4rtni-ftldni.ini.usc.edu). We considered 15 patients with bvFTD who had valid baseline T1-weighted MR images. To avoid potential bias derived from different imaging protocols, we selected exclusively images acquired at the University of California, San Francisco (UCSF), i.e., the largest recruiting center. All the patients underwent comprehensive neurological, neuropsychological, and functional assessments and were diagnosed according to the current diagnostic criteria55.
Subjects with bvFTD that form this cohort received the same clinical assessment than 4-Repeat Tauopathies cohort, including Interpersonal Reactivity Index evaluation.
MRI acquisition and processing
Structural T1-weighted (T1w) MR images were collected using 3 T Siemens Trio Tim system equipped with a 12-channel head coil. Acquisition parameters of structural MR images, using a three-dimensional magnetization-prepared rapid gradient echo (3D-MPRAGE) scheme, were TR/TE/TI = 2300/3/900 ms, flip angle of 9°, sagittal orientation with 256×240×160 matrix size, 1 mm3 isotropic voxel resolution. Each MRI volume was visually inspected for gross structural alterations and artifacts. Voxel-based morphometry (VBM) analysis was performed with CAT12 toolbox (Structural Brain Mapping Group, Jena University Hospital, Jena, Germany) implemented in SPM12 (Statistical Parametric Mapping, Institute of Neurology, London, UK). The structural MR imaging data were preprocessed with default settings of the CAT12 toolbox, including corrections for bias-field inhomogeneities, segmentation into gray matter (GM), white matter, and cerebrospinal fluid, followed by spatial normalization to the DARTEL template in MNI space (voxelsize: 1.5 mm×1.5 mm×1.5 mm). Normalized images were modulated to guarantee that relative volumes were preserved following the spatial normalization procedure. Next, the preprocessed GM data were smoothed with an 8 mm full-width-half-maximum (FWHM) isotropic Gaussian kernel. An optimal GM mask was also generated from all normalized images using the SPM12 Masking toolbox and the Luo–Nichols anti-mode method of automatic thresholding56.
Statistical analysis
Demographic, clinical and neuropsychological data analysis was performed with IBM SPSS Statistics 23 (IBM Corporation, New York, EUA). Normality of the data was tested using the Shapiro–Wilk test, followed by ANOVA or Kruskal-Wallis test, and Benjamini-Hochberg correction for multiple comparison, accordingly. Categorical variables were compared with Chi-squared test. The associations between empathy score and motor and clinical variables were explored using Spearman’s correlations, corrected for age, sex, education and disease duration. Statistical significance was considered when p-value < 0.003, after Bonferroni correction for multiple comparisons.
Whole-brain multiple-regression analyses were computed by using modulated GM images as dependent variables, the IRI total scores and IRI subscores as regressors. Age and sex were used as covariate, together with the total intracranial volume to correct for individual differences in head size and CDR Sum of Boxes to control for disease severity. Statistical analyses were performed using the Threshold-Free Cluster Enhancement (TFCE) from FSL-randomise, a nonparametric permutation-based approach performed with 5000 permutations57 Only clusters with p < 0.05 after correcting for family-wise error (FWE) were considered statistically significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
Data used in the preparation of this manuscript were obtained from the 4-Repeat Neuroimaging Initiative (4RTNI) database and the Frontotemporal Lobar Degeneration Neuroimaging Initiative (FTLDNI) (http://4rtni-ftldni.ini.usc.edu/). 4RTNI was launched in early 2011 and is funded through the National Institute of Aging and The Tau Research Consortium. The primary goal of 4RTNI is to identify neuroimaging and biomarker indicators for disease progression in the 4-repeat tauopathy neurodegenerative diseases, progressive supranuclear palsy (PSP) and corticobasal degeneration (CBD). FTLDNI is also founded through the National Institute of Aging, and started in 2010. The primary goals of FTLDNI are to identify neuroimaging modalities and methods of analysis for tracking frontotemporal lobar degeneration (FTLD) and to assess the value of imaging versus other biomarkers in diagnostic roles. The Principal Investigator of 4RTNI is Dr. Adam Boxer, MD, PhD, at the University of California, San Francisco. The data is the result of collaborative efforts at four sites in North America. For more information on 4RTNI, please visit: http://memory.ucsf.edu/research/studies/4rtni.
Code availability
The underlying code for this study is not publicly available but may be made available to qualified researchers on reasonable request from the corresponding author.
References
Bernhardt, B. C. & Singer, T. The neural basis of empathy. Annu. Rev. Neurosci. 35, 1–23 (2012).
Molenberghs, P., Johnson, H., Henry, J. D. & Mattingley, J. B. Understanding the minds of others: a neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 65, 276–291 (2016).
Shamay-Tsoory, S. G. The neural bases for empathy. Neuroscientist 17, 18–24 (2011).
Rizzolatti, G., Fabbri-Destro, M. & Cattaneo, L. Mirror neurons and their clinical relevance. Nat. Clin. Pract. Neurol. 5, 24–34 (2009).
Farrow, T. F. et al. Investigating the functional anatomy of empathy and forgiveness. Neuroreport 12, 2433–2438 (2001).
Decety, J. & Jackson, P. L. The functional architecture of human empathy. Behav. Cogn. Neurosci. Rev. 3, 71–100 (2004).
Shamay-Tsoory, S. G. et al. The neural correlates of understanding the other’s distress: a positron emission tomography investigation of accurate empathy. NeuroImage 27, 468–472 (2005).
Rankin, K. P., Kramer, J. H. & Miller, B. L. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn. Behav. Neurol. 18, 28–36 (2005).
Magno, M. A., Canu, E., Filippi, M. & Agosta, F. Social cognition in the FTLD spectrum: evidence from MRI. J. Neurol. 269, 2245–2258 (2022).
Poletti, M., Enrici, I. & Adenzato, M. Cognitive and affective Theory of Mind in neurodegenerative diseases: neuropsychological, neuroanatomical and neurochemical levels. Neurosci. Biobehav. Rev. 36, 2147–2164 (2012).
Bang, J., Spina, S. & Miller, B. L. Frontotemporal dementia. Lancet Lond. Engl. 386, 1672–1682 (2015).
Pick, E., Kleinbub, J. R., Mannarini, S. & Palmieri, A. Empathy in neurodegenerative diseases: a systematic review. Neuropsychiatr. Dis. Treat. 15, 3287–3304 (2019).
Ghosh, B. C. P. et al. Social cognitive deficits and their neural correlates in progressive supranuclear palsy. Brain J. Neurol. 135, 2089–2102 (2012).
Southi, N., Honan, C. A., Hodges, J. R., Piguet, O. & Kumfor, F. Reduced capacity for empathy in corticobasal syndrome and its impact on carer burden. Int. J. Geriatr. Psychiatry 34, 497–503 (2019).
Arshad, F. et al. Social cognition deficits are pervasive across both classical and overlap frontotemporal dementia syndromes. Dement. Geriatr. Cogn. Disord. Extra 10, 115–126 (2020).
Brown, C. L. et al. Comparing two facets of emotion perception across multiple neurodegenerative diseases. Soc. Cogn. Affect. Neurosci. 15, 511–522 (2020).
Shdo, S. M. et al. Deconstructing empathy: neuroanatomical dissociations between affect sharing and prosocial motivation using a patient lesion model. Neuropsychologia 116, 126–135 (2018).
Rankin, K. P. et al. Structural anatomy of empathy in neurodegenerative disease. Brain J. Neurol. 129, 2945–2956 (2006).
Critchley, H. et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: a functional magnetic resonance imaging study. Hum. Brain Mapp. 9, 93–105 (2000).
Seehausen, M. et al. Talking about social conflict in the MRI scanner: neural correlates of being empathized with. NeuroImage 84, 951–961 (2014).
Völlm, B. A. et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. NeuroImage 29, 90–98 (2006).
Carrington, S. J. & Bailey, A. J. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Hum. Brain Mapp. 30, 2313–2335 (2009).
Kandylaki, K. D. et al. Processing of false belief passages during natural story comprehension: An fMRI study. Hum. Brain Mapp. 36, 4231–4246 (2015).
Lang, S., Yu, T., Markl, A., Müller, F. & Kotchoubey, B. Hearing others’ pain: neural activity related to empathy. Cogn. Affect. Behav. Neurosci. 11, 386–395 (2011).
Jie, J. et al. Establishing a counter-empathy processing model: evidence from functional magnetic resonance imaging. Soc. Cogn. Affect. Neurosci. 17, 273–289 (2021).
Carr, L., Iacoboni, M., Dubeau, M.-C., Mazziotta, J. C. & Lenzi, G. L. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc. Natl Acad. Sci. Usa. 100, 5497–5502 (2003).
Chakrabarti, B., Bullmore, E. & Baron-Cohen, S. Empathizing with basic emotions: common and discrete neural substrates. Soc. Neurosci. 1, 364–384 (2006).
Krämer, U. M., Mohammadi, B., Doñamayor, N., Samii, A. & Münte, T. F. Emotional and cognitive aspects of empathy and their relation to social cognition–an fMRI-study. Brain Res 1311, 110–120 (2010).
Allison, T., Puce, A. & McCarthy, G. Social perception from visual cues: role of the STS region. Trends Cogn. Sci. 4, 267–278 (2000).
de Greck, M. et al. Neural substrates underlying intentional empathy. Soc. Cogn. Affect. Neurosci. 7, 135–144 (2012).
Baldauf, D. & Desimone, R. Neural mechanisms of object-based attention. Science 344, 424–427 (2014).
Hooker, C. I., Verosky, S. C., Germine, L. T., Knight, R. T. & D’Esposito, M. Neural activity during social signal perception correlates with self-reported empathy. Brain Res 1308, 100–113 (2010).
Olson, I. R., Plotzker, A. & Ezzyat, Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain J. Neurol. 130, 1718–1731 (2007).
Fletcher, P. C. et al. Other minds in the brain: a functional imaging study of ‘theory of mind’ in story comprehension. Cognition 57, 109–128 (1995).
Saxe, R. & Powell, L. J. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 17, 692–699 (2006).
Stern, J. A., Botdorf, M., Cassidy, J. & Riggins, T. Empathic responding and hippocampal volume in young children. Dev. Psychol. 55, 1908 (2019).
Rushby, J. A. et al. Brain volume loss contributes to arousal and empathy dysregulation following severe traumatic brain injury. NeuroImage Clin. 12, 607–614 (2016).
Bruneau, E. G., Jacoby, N. & Saxe, R. Empathic control through coordinated interaction of amygdala, theory of mind and extended pain matrix brain regions. NeuroImage 114, 105–119 (2015).
Picerni, E. et al. Macro- and micro-structural cerebellar and cortical characteristics of cognitive empathy towards fictional characters in healthy individuals. Sci. Rep. 11, 8804 (2021).
Singer, T. et al. Empathy for pain involves the affective but not sensory components of pain. Science 303, 1157–1162 (2004).
Van Overwalle, F., Baetens, K., Mariën, P. & Vandekerckhove, M. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. NeuroImage 86, 554–572 (2014).
Schmahmann, J. D., Guell, X., Stoodley, C. J. & Halko, M. A. The theory and neuroscience of cerebellar cognition. Annu. Rev. Neurosci. 42, 337–364 (2019).
Tse, N. Y. et al. Cerebellar contributions to cognition in corticobasal syndrome and progressive supranuclear palsy. Brain Commun. 2, fcaa194 (2020).
Synn, A. et al. Mental states in moving shapes: distinct cortical and subcortical contributions to theory of mind impairments in dementia. J. Alzheimers Dis. JAD 61, 521–535 (2018).
Van den Stock, J. et al. Reduced tendency to attribute mental states to abstract shapes in behavioral variant frontotemporal dementia links with cerebellar structural integrity. NeuroImage Clin. 22, 101770 (2019).
Hoche, F., Guell, X., Vangel, M. G., Sherman, J. C. & Schmahmann, J. D. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain J. Neurol. 141, 248–270 (2018).
Lee, S. E. et al. Clinicopathological correlations in corticobasal degeneration. Ann. Neurol. 70, 327–340 (2011).
Bartochowski, Z., Gatla, S., Khoury, R., Al-Dahhak, R. & Grossberg, G. T. Empathy changes in neurocognitive disorders: a review. Ann. Clin. Psychiatry 30, 220–232 (2018).
Litvan, I. et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology 47, 1–9 (1996).
Boxer, A. L. et al. Davunetide in patients with progressive supranuclear palsy: a randomised, double-blind, placebo-controlled phase 2/3 trial. Lancet Neurol. 13, 676–685 (2014).
Armstrong, M. J. et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 80, 496–503 (2013).
Folstein, M. F., Folstein, S. E. & McHugh, P. R. Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198 (1975).
Davis, M. H. Measuring individual differences in empathy: evidence for a multidimensional approach. J. Pers. Soc. Psychol. 44, 113–126 (1983).
Shamay-Tsoory, S. G., Tomer, R., Berger, B. D. & Aharon-Peretz, J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J. Cogn. Neurosci. 15, 324–337 (2003).
Rascovsky, K. et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain J. Neurol. 134, 2456–2477 (2011).
Luo, W.-L. & Nichols, T. E. Diagnosis and exploration of massively univariate neuroimaging models. NeuroImage 19, 1014–1032 (2003).
Smith, S. M. & Nichols, T. E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44, 83–98 (2009).
Acknowledgements
Data collection and sharing for this project was funded by the 4-Repeat Tauopathy Neuroimaging Initiative (4RTNI) (National Institutes of Health Grant R01 AG038791) and through generous contributions from the Tau Research Consortium. The study is coordinated through the University of California, San Francisco, Memory and Aging Center. 4RTNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. Data collection and sharing for this project was also funded by the Frontotemporal Lobar Degeneration Neuroimaging Initiative (National Institutes of Health Grant R01 AG032306). The study is coordinated through the University of California, San Francisco, Memory and Aging Center. FTLDNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California. This study was supported in part by the National Institutes of Health grants (R01AG038791, R01AG032306); The Frontotemporal Lobar Degeneration Clinical Research Consortium (U54NS092089); Tau Consortium; and with resources of the Veterans Affairs Medical Center, San Francisco, California. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work has been supported with the founding of Regione Puglia and CNR for Tecnopolo per la Medicina di Precisione. D.G.R. n. 2117 of 21.11.2018 (CUPB84I18000540002)—C.I.R.E.M.I.C. (Research Center of Excellence for Neurodegenerative Diseases and Brain Aging)—University of Bari “Aldo Moro”.
Author information
Authors and Affiliations
Contributions
B.T. and D.U. contributed equally and share co-first authorship; B.T., D.U., S.N., L.M., and G.L. conceived the idea, planned, and designed the study; B.T., D.U., and S.N. planned the data management and statistical analysis; B.T. and D.U. wrote the first draft; L.M., R.D.B., K.R.C., and G.L. provided critical insights; B.T., D.U., S.N., L.M., R.D.B., K.R.C., and G.L. reviewed the manuscript. All the authors have approved and contributed to the final written manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tafuri, B., Urso, D., Nigro, S. et al. Grey-matter correlates of empathy in 4-Repeat Tauopathies. npj Parkinsons Dis. 9, 138 (2023). https://doi.org/10.1038/s41531-023-00576-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41531-023-00576-z
- Springer Nature Limited