Abstract
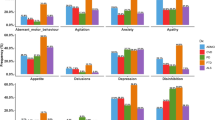
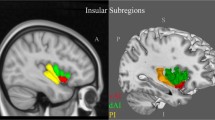
Change in empathy is an increasingly recognised symptom of neurodegenerative diseases and contributes to caregiver burden and patient distress. Empathy impairment has been associated with brain atrophy but its relationship to white matter hyperintensities (WMH) is unknown. We aimed to investigate the relationships amongst WMH, brain atrophy, and empathy deficits in neurodegenerative and cerebrovascular diseases. Five hundred thirteen participants with Alzheimer’s disease/mild cognitive impairment, amyotrophic lateral sclerosis, frontotemporal dementia (FTD), Parkinson’s disease, or cerebrovascular disease (CVD) were included. Empathy was assessed using the Interpersonal Reactivity Index. WMH were measured using a semi-automatic segmentation and FreeSurfer was used to measure cortical thickness. A heterogeneous pattern of cortical thinning was found between groups, with FTD showing thinning in frontotemporal regions and CVD in left superior parietal, left insula, and left postcentral. Results from both univariate and multivariate analyses revealed that several variables were associated with empathy, particularly cortical thickness in the fronto-insulo-temporal and cingulate regions, sex (female), global cognition, and right parietal and occipital WMH. Our results suggest that cortical atrophy and WMH may be associated with empathy deficits in neurodegenerative and cerebrovascular diseases. Future work should consider investigating the longitudinal effects of WMH and atrophy on empathy deficits in neurodegenerative and cerebrovascular diseases.




Similar content being viewed by others
Data availability
The datasets presented in this article are not readily available because the ONDRI data will be made publicly available through an application process. For more information on the ONDRI project, please visit http://ondri.ca/. Requests to access the datasets should be directed to http://ondri.ca/.
Abbreviations
- AD:
-
Alzheimer’s disease
- ALS:
-
Amyotrophic lateral sclerosis
- bvFTD:
-
Behavioural variant frontotemporal dementia
- CVD:
-
Cerebrovascular disease
- CBS:
-
Corticobasal syndrome
- CT:
-
Computed tomography
- dWMH:
-
Deep white matter hyperintensities
- dx:
-
Diagnostic
- EC:
-
Emotional concern
- FTD:
-
Frontotemporal dementia
- IRI:
-
Interpersonal Reactivity Index
- MCI:
-
Mild cognitive impairment
- MRI:
-
Magnetic resonance imaging
- MoCA:
-
Montreal Cognitive Assessment
- MSE:
-
Mean square error
- nfvPPA:
-
Non-fluent primary progressive aphasia
- ONDRI:
-
Ontario Neurodegenerative Disease Research Initiative
- OFC:
-
Orbitofrontal cortex
- PeD:
-
Personal distress
- PLSc:
-
Partial least square correlation
- PT:
-
Perspective taking
- PD:
-
Parkinson’s disease
- PSP:
-
Progressive supranuclear palsy
- pWMH:
-
Periventricular white matter hyperintensities
- svPPA:
-
Semantic variant primary progressive aphasia
- ST-TIV:
-
Supratentorial total intracranial volume
- SVD:
-
Small vessel disease
- WMH:
-
White matter hyperintensities
References
Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–26.
Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J Autism Dev Disord. 2004;34:163–75.
Christidi F, Migliaccio R, Santamaría-García H, Santangelo G, Trojsi F. Social cognition dysfunctions in neurodegenerative diseases: neuroanatomical correlates and clinical implications. Behav Neurol. 2018;2018:1–18.
Pick E, Kleinbub JR, Mannarini S, Palmieri A. Empathy in neurodegenerative diseases: a systematic review. Neuropsychiatr Dis Treat. 2019;15:3287–304.
Multani N, Taghdiri F, Anor CJ, Varriano B, Misquitta K, Tang-Wai DF, Keren R, Fox S, Lang AE, Vijverman AC, Marras C, Tartaglia MC. Association between social cognition changes and resting state functional connectivity in frontotemporal dementia, Alzheimer’s disease, Parkinson’s disease, and healthy controls. Front Neurosci. 2019;13:1–14.
Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. J Neuropsychiatry Clin Neurosci. 2011;23:74–82.
Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW, Miller BL. Structural anatomy of empathy in neurodegenerative disease. Brain. 2006;129:2945–56.
Baez S, Manes F, Huepe D, Torralva T, Fiorentino N, Richter F, Huepe-Artigas D, Ferrari J, Montaes P, Reyes P, Matallana D, Vigliecca NS, Decety J, Ibanez A. Primary empathy deficits in frontotemporal dementia. Front Aging Neurosci. 2018;6:1–11.
Cosentino S, Zahodne LB, Brandt J, Blacker D, Albert M, Dubois B, Stern Y. Social cognition in Alzheimer’s disease: a separate construct contributing to dependence. Alzheimer’s Dement. 2014;10:818–26.
Gray HM, Tickle-Degnen L. A meta-analysis of performance on emotion recognition tasks in Parkinson’s disease. Neuropsychology. 2010;24:176–91.
Kleinbub JR, Palmieri A, Broggio A, Pagnini F, Benelli E, Sambin M, Soraru G. Hypnosis-based psychodynamic treatment in ALS: a longitudinal study on patients and their caregivers. Front Psychol. 2015; 6:1–14.
Bodden ME, Mollenhauer B, Trenkwalder C, Cabanel N, Eggert KM, Unger MM, Oertel WH, Kessler J, Dodel R, Kalbe E. Affective and cognitive theory of mind in patients with Parkinson’s disease. Parkinsonism Relat Disord. 2010;16:466–70.
Dermody N, Wong S, Ahmed R, Piguet O, Hodges JR, Irish M. Uncovering the neural bases of cognitive and affective empathy deficits in Alzheimer’s disease and the behavioral-variant of frontotemporal dementia. J Alzheimer’s Dis. 2016;53:801–16.
Ariatti A, Benuzzi F, Nichelli P. Recognition of emotions from visual and prosodic cues in Parkinson’s disease. Neurol Sci. 2008;29:219–27.
Assogna F, Pontieri FE, Cravello L, Peppe A, Pierantozzi M, Stefani A, Stanzione P, Pellicano C, Caltagirone C, Spalletta G. Intensity-dependent facial emotion recognition and cognitive functions in Parkinson’s disease. J Int Neuropsychol Soc. 2010;16:867–76.
Kan Y, Kawamura M, Hasegawa Y, Mochizuki S, Nakamura K. Recognition of emotion from facial, prosodic and written verbal stimuli in Parkinson’s disease. Cortex. 2002;38:623–30.
Martinez M, Multani N, Anor CJ, Misquitta K, TangWai DF, Keren R, Fox S, Lang AE, Marras C, Tartaglia MC. Emotion detection deficits and decreased empathy in patients with Alzheimer’s disease and Parkinson’s disease affect caregiver mood and burden. Front Aging Neurosci. 2018;10:1–9.
Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci. 2009;13:27–35.
Ibarretxe-Bilbao N, Junque C, Tolosa E, Marti M-J, Valldeoriola F, Bargallo N, Zarei M. Neuroanatomical correlates of impaired decision-making and facial emotion recognition in early Parkinson’s disease. Eur J Neurosci. 2009;30:1162–71.
Baggio HC, Segura B, Ibarretxe-Bilbao N, Valldeoriola F, Marti MJ, Compta Y, Tolosa E, Junqué C. Structural correlates of facial emotion recognition deficits in Parkinson’s disease patients. Neuropsychologia. 2012;50:2121–8.
Cerami C, Dodich A, Canessa N, Crespi C, Iannaccone S, Corbo M, Lunetta C, Consonni M, Scola E, Falini A, Cappa SF. Emotional empathy in amyotrophic lateral sclerosis: a behavioural and voxel-based morphometry study. Amyotroph Lateral Scler Front Degener. 2014;15:21–9.
Leigh R, Oishi K, Hsu J, Lindquist M, Gottesman RF, Jarso S, Crainiceanu C, Mori S, Hillis AE. Acute lesions that impair affective empathy. Brain. 2013;136:2539–49.
De Groot JC, De Leeuw F-E, Oudkerk M, Van Gijn J, Hofman A, Jolles J, Breteler MMB. Periventricular cerebral white matter lesions predict rate of cognitive decline. Ann Neurol. 2002;52:335–41.
Garde E, Mortensen EL, Krabbe K, Rostrup E, Larsson HB. Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet. 2000;356:628–34.
Wardlaw JM, Valdés Hernández MC, Muñoz‐Maniega S. What are white matter hyperintensities made of? J Am Heart Assoc. 2015; 4:1–19.
Barber R, Scheltens P, Gholkar A, Ballard C, McKeith I, Ince P, Perry R, O’Brien J. White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J Neurol Neurosurg Psychiatry. 1999;67:66–72.
Park KH, Lee J-Y, Na DL, Kim SY, Cheong H-K, Moon SY, Shim YS, Park KW, Ku BD, Choi SH, Joo H, Lee JS, Go SM, Kim SH, Kim SangYun, Cha KR, Lee J, Seo SW. Different associations of periventricular and deep white matter lesions with cognition, neuropsychiatric symptoms, and daily activities in dementia. J Geriatr Psychiatry Neurol. 2011;24:84–90.
Starkstein SE, Mizrahi R, Capizzano AA, Acion L, Brockman S, Power BD. Neuroimaging correlates of apathy and depression in Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2009;21:259–65.
Soennesyn H, Oppedal K, Greve OJ, Fritze F, Auestad BH, Nore SP, Beyer MK, Aarsland D. White matter hyperintensities and the course of depressive symptoms in elderly people with mild dementia. Dement Geriatr Cogn Dis Extra. 2012;2:97–111.
Anor CJ, O’Connor S, Saund A, Tang-Wai DF, Keren R, Tartaglia MC. Neuropsychiatric symptoms in Alzheimer disease, vascular dementia, and mixed dementia. Neurodegener Dis. 2017;17:127–34.
Kim HJ, Kang SJ, Kim C, Kim GH, Jeon S, Lee JM, Oh SJ, Kim JS, Choe YS, Lee KH, Noh Y, Cho H, Yoon CW, Chin J, Cummings JL, Lee JH, Na DL, Seo SW. The effects of small vessel disease and amyloid burden on neuropsychiatric symptoms: a study among patients with subcortical vascular cognitive impairments. Neurobiol Aging. 2013;34:1913–20.
Desmarais P, Gao AF, Lanctôt K, Rogaeva E, Ramirez J, Herrmann N, Stuss DT, Black SE, Keith J, Masellis M. White matter hyperintensities in autopsy-confirmed frontotemporal lobar degeneration and Alzheimer’s disease. Alzheimers Res Ther. 2021;13:129.
Farhan SMK, Bartha R, Black SE, Corbett D, Finger E, Freedman M, Greenberg B, Grimes DA, Hegele RA, Hudson C, Kleinstiver PW, Lang AE, Masellis M, McIlroy WE, McLaughlin PM, Montero-Odasso M, Munoz DG, Munoz DP, Strother S, Swartz RH, Symons S, Tartaglia MC, Zinman L, Strong MJ. The Ontario Neurodegenerative Disease Research Initiative (ONDRI). Can J Neurol Sci. 2017;44:196–202.
Sunderland KM, Beaton D, Arnott SR, Kleinstiver P, Kwan D, Lawrence-Dewar JM, Ramirez J, Tan B, Bartha R, Black SE, Borrie M, Brien D, Casaubon LK, Coe B, Cornish B, Dilliott AA, Dowlatshahi D, Finger E, Fischer C, Frank A, Fraser J, Freedman M, Greenberg B, Grimes DA, Hassan A, Hatch W, Hegele RA, Hudson C, Jog M, Kumar S, Lang A, Levine B, Lou W, Mandzia J, Marras C, McIlroy W, Montero-Odasso M, Munoz DG, Munoz DP, Orange JB, Park DS, Pasternak SH, PierucciniFaria F, Rajji TK, Roberts AC, Robinson JF, Rogaeva E, Sahlas DJ, Saposnik G, Scott CJM, Seitz D, Shoesmith C, Steeves TDL, Strong MJ, Strother SC, Swartz RH, Symons S, Tang-Wai DF, Tartaglia MC, Troyer AK, Turnbull J, Zinman L, McLaughlin PM, Masellis M, Binns MA. The Ontario Neurodegenerative Disease Research Initiative. medRxiv. 2020: 1–41.
Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, Gamst A, Holtzman DM, Jagust WJ, Petersen RC, Snyder PJ, Carrillo MC, Thies B, Phelps CH. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270–9.
McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263–9.
Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. Subcommittee on Motor Neuron Diseases/Amyotrophic Lateral Sclerosis of the World Federation of Neurology Research Group on Neuromuscular Diseases and th. J Neurol Sci. 1994;124(Suppl):96–107.
Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77.
Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, Ogar JM, Rohrer JD, Black S, Boeve BF, Manes F, Dronkers NF, Vandenberghe R, Rascovsky K, Patterson K, Miller BL, Knopman DS, Hodges JR, Mesulam MM, Grossman M. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14.
Hauw J-J, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994;44:2015–2015.
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Tröster AI, Vidailhet M, Weiner WJ. Criteria for the diagnosis of corticobasal degeneration. Neurology. 2013;80:496–503.
Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–52.
Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, Powers WJ, Decarli C, Merino JG, Kalaria RN, Vinters HV, Holtzman DM, Rosenberg GA, Wallin A, Dichgans M, Marler JR, Leblanc GG. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37:2220–41.
Rankin KP, Kramer JH, Miller BL. Patterns of cognitive and emotional empathy in frontotemporal lobar degeneration. Cogn Behav Neurol. 2005;18:28–36.
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9.
Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–86.
Scott CJM, Arnott SR, Chemparathy A, Dong F, Solovey I, Gee T, Schmah T, Lobaugh N, Nanayakkara N, Liang S, Zamyadi M, Ozzoude M, Holmes MF, Szilagyi GM, Ramirez J, Symons S, Black SE, Bartha R, Strother S. An overview of the quality assurance and quality control of magnetic resonance imaging data for the Ontario Neurodegenerative Disease Research Initiative (ONDRI): pipeline development and neuroinformatics. bioRxiv. 2020: 1–16.
Ramirez J, Holmes MF, Scott CJM, Ozzoude M, Adamo S, Szilagyi GM, Gao F, Arnott SR, Dewar JML, Beaton D, Strother SC, Douglas P, Masellis M, Swartz RH, Bartha R, Symons S, Black SE, Investigators O. Ontario Neurodegenerative Disease Research Initiative (ONDRI): structural MRI methods & outcome measures. Front Neurol. 2020; 17: 1–11.
Duchesne S, Chouinard I, Potvin O, Fonov VS, Khademi A, Bartha R, Bellec P, Collins DL, Descoteaux M, Hoge R, McCreary CR, Ramirez J, Scott CJM, Smith EE, Strother SC, Black SE, CIMA-Q group and the CCNA group,. The Canadian dementia imaging protocol: harmonizing national cohorts. J Magn Reson Imaging. 2019;49:456–65.
Dade LA, Gao FQ, Kovacevic N, Roy P, Rockel C, O’Toole CM, Lobaugh NJ, Feinstein A, Levine B, Black SE. Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. Neuroimage. 2004;22:1492–502.
Gibson E, Gao F, Black SE, Lobaugh NJ. Automatic segmentation of white matter hyperintensities in the elderly using FLAIR images at 3T. J Magn Reson. 2010;31:1311–22.
Kovacevic N, Lobaugh NJ, Bronskill MJ, Levine B, Feinstein A, Black SE. A robust method for extraction and automatic segmentation of brain images. Neuroimage. 2002;17:1087–100.
Ramirez J, McNeely AA, Scott CJM, Masellis M, Black SE. White matter hyperintensity burden in elderly cohort studies. The Sunnybrook Dementia Study. Alzheimer Disease Neuroimaging Initiative, and Three-City Study. Alzheimers Dement. 2015;12:203–10.
Ramirez J, McNeely AA, Scott CJ, Stuss DT, Black SE. Subcortical hyperintensity volumetrics in Alzheimer’s disease and normal elderly in the Sunnybrook Dementia Study: correlations with atrophy, executive function, mental processing speed, and verbal memory. Alzheimers Res Ther. 2014;6:49.
Ramirez J, McNeely A, Scott CJM, Stuss DT, Black SE. Strategic regional subcortical hyperintensity volumetrics in Alzheimer’s disease and normal elderly: correlations with executive function, mental processing speed, and verbal memory. Alzheimers Res Ther. 2014;49:1–12.
Ramirez J, Gibson E, Quddus A, Lobaugh NJ, Feinstein A, Levine B, Scott CJM, Levy-Cooperman N, Gao FQ, Black SE. Lesion explorer: a comprehensive segmentation and parcellation package to obtain regional volumetrics for subcortical hyperintensities and intracranial tissue. Neuroimage. 2011;54:963–73.
Sunderland KM, Beaton D, Fraser J, Kwan D, McLaughlin PM, Montero-Odasso M, Peltsch AJ, Pieruccini-Faria F, Sahlas DJ, Swartz RH, Strother SC, Binns MA, Binns MA. The utility of multivariate outlier detection techniques for data quality evaluation in large studies: an application within the ONDRI project. BMC Med Res Methodol. 2019;19:102.
Fischl B. FreeSurfer Neuroimage. 2012;62:774–81.
Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80.
Ozzoude M, Ramirez J, Raamana PR, Holmes MF, Walker K, Scott CJM, Gao F, Goubran M, Kwan D, Tartaglia MC, Beaton D, Saposnik G, Hassan A, LawrenceDewar J, Dowlatshahi D, Strother SC, Symons S, Bartha R, Swartz RH, Black SE. Cortical thickness estimation in individuals with cerebral small vessel disease, focal atrophy, and chronic stroke lesions. Front Neurosci. 2020;14:1–12.
Wickham H. ggplot2: Elegant Graphics for Data Analysis. 2009;35:1–3.
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80.
Zou H, Hastie T. Regularization and variable selection via the elastic net. J R Stat Soc Ser B Statistical Methodol. 2005;67:301–20.
Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22.
Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial least squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage. 2011;56:455–75.
McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3:143–57.
Beaton D, Chin Fatt CR, Abdi H. An ExPosition of multivariate analysis with the singular value decomposition in R. Comput Stat Data Anal. 2014;72:176–89.
De Vos M, Prince J, Buchanan T, FitzGerald JJ, Antoniades CA. Discriminating progressive supranuclear palsy from Parkinson’s disease using wearable technology and machine learning. Gait Posture. 2020;77:257–63.
Bouts MJRJ, Möller C, Hafkemeijer A, van Swieten JC, Dopper E, van der Flier WM, Vrenken H, Wink AM, Pijnenburg YAL, Scheltens P, Barkhof F, Schouten TM, de Vos F, Feis RA, van der Grond J, de Rooij M, Rombouts SARB. Single subject classification of Alzheimer’s disease and behavioral variant frontotemporal dementia using anatomical, diffusion tensor, and resting-state functional magnetic resonance imaging. J Alzheimer’s Dis. 2018;62:1827–39.
Tosun D, Schuff N, Rabinovici GD, Ayakta N, Miller BL, Jagust W, Kramer J, Weiner MM, Rosen HJ. Diagnostic utility of ASL-MRI and FDG-PET in the behavioral variant of FTD and AD. Ann Clin Transl Neurol. 2016;3:740–51.
Teipel SJ, Grothe MJ, Metzger CD, Grimmer T, Sorg C, Ewers M, Franzmeier N, Meisenzahl E, Klöppel S, Borchardt V, Walter M, Dyrba M. Robust detection of impaired resting state functional connectivity networks in Alzheimer’s disease using elastic net regularized regression. Front Aging Neurosci. 2017;8:1–9.
James G, Witten D, Hastie T, Tibshirani R. An introduction to statistical learning. New York, New York, NY: Springer; 2013.
Berry KJ, Johnston JE, Mielke PW. Permutation methods. Wiley Interdiscip Rev Comput Stat. 2011;3:527–42.
Peres-Neto PR, Jackson DA, Somers KM. How many principal components? Stopping rules for determining the number of non-trivial axes revisited. Comput Stat Data Anal. 2005;49:974–97.
Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1:54–75.
Hesterberg T. Bootstrap. Wiley Interdiscip Rev. Comput Stat. 2011;3:497–526.
Piguet O, Hornberger M, Mioshi E, Hodges JR. Behavioural-variant frontotemporal dementia: diagnosis, clinical staging, and management. Lancet Neurol. 2011;10:162–72.
Bartochowski Z, Gatla S, Khoury R, Al-Dahhak R, Grossberg GT. Empathy changes in neurocognitive disorders: a review. Ann Clin Psychiatry. 2018;30:220–32.
Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR. Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia. 2006;44:950–8.
Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3:71–100.
Narme P, Mouras H, Roussel M, Devendeville A, Godefroy O. Assessment of socioemotional processes facilitates the distinction between frontotemporal lobar degeneration and Alzheimer’s disease. J Clin Exp Neuropsychol. 2013;35:728–44.
Sturm VE, Yokoyama JS, Seeley WW, Kramer JH, Miller BL, Rankin KP. Heightened emotional contagion in mild cognitive impairment and Alzheimer’s disease is associated with temporal lobe degeneration. Proc Natl Acad Sci. 2013;110:9944–9.
Fernandez-Duque D, Hodges SD, Baird JA, Black SE. Empathy in frontotemporal dementia and Alzheimer’s disease. J Clin Exp Neuropsychol. 2009;32:1–12.
Narme P, Mouras H, Roussel M, Duru C, Krystkowiak P, Godefroy O. Emotional and cognitive social processes are impaired in Parkinson’s disease and are related to behavioral disorders. Neuropsychology. 2013;27:182–92.
van der Hulst E-J, Bak TH, Abrahams S. Impaired affective and cognitive theory of mind and behavioural change in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry. 2015;86:1208–15.
Schmidt N, Paschen L, Deuschl G, Witt K. Reduced empathy scores in patients with Parkinson’s disease: a non-motor symptom associated with advanced disease stages. J Parkinsons Dis. 2017;7:713–8.
Coundouris SP, Adams AG, Henry JD. Empathy and theory of mind in Parkinson’s disease: a meta-analysis. Neurosci Biobehav Rev. 2020;109:92–102.
Shamay-Tsoory SG, Tomer R, Goldsher D, Berger BD, Aharon-Peretz J. Impairment in cognitive and affective empathy in patients with brain lesions: anatomical and cognitive correlates. J Clin Exp Neuropsychol. 2004;26:1113–27.
Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007;45:3054–67.
Di Tella M, Miti F, Ardito RB, Adenzato M. Social cognition and sex: are men and women really different? Pers Individ Dif. 2020;162:110045.
Martínez-Velázquez ES, Ahuatzin González AL, Chamorro Y, Sequeira H. The influence of empathy trait and gender on empathic responses. A study with dynamic emotional stimulus and eye movement recordings. Front Psychol. 2020;11:1–11.
Eisenberg N, Lennon R. Sex differences in empathy and related capacities. Psychol Bull. 1983;94:100–31.
Baez S, Flichtentrei D, Prats M, Mastandueno R, García AM, Cetkovich M, Ibáñez A.Men, women…who cares? A population-based study on sex differences and gender roles in empathy and moral cognition. PLoS One. 2017;12, e0179336.
Hillis AE. Inability to empathize: brain lesions that disrupt sharing and understanding another’s emotions. Brain. 2014;137:981–97.
Kim EJ, Son J-W, Park SK, Chung S, Ghim H-R, Lee S, Lee S-I, Shin C-J, Kim S, Ju G, Park H, Lee J. Cognitive and emotional empathy in young adolescents: an fMRI study. Soa--ch’ongsonyon chongsin uihak = J child Adolesc psychiatry. 2020;31:121–30.
Naor N, Rohr C, Schaare LH, Limbachia C, Shamay-Tsoory S, Okon-Singer H. The neural networks underlying reappraisal of empathy for pain. Soc Cogn Affect Neurosci. 2020;15:733–44.
Seeley WW, Allman JM, Carlin DA, Crawford RK, Macedo MN, Greicius MD, Dearmond SJ, Miller BL. Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: Reciprocal networks and neuronal evolution. Alzheimer Dis Assoc Disord. 2007;21:S50–7.
Couto B, Sedeño L, Sposato LA, Sigman M, Riccio PM, Salles A, Lopez V, Schroeder J, Manes F, Ibanez A. Insular networks for emotional processing and social cognition: comparison of two case reports with either cortical or subcortical involvement. Cortex. 2013;49:1420–34.
Gu X, Gao Z, Wang X, Liu X, Knight RT, Hof PR, Fan J. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135:2726–35.
Yeh Z-T, Tsai C-F. Impairment on theory of mind and empathy in patients with stroke. Psychiatry Clin Neurosci. 2014;68:612–20.
Zhong Y, Utriainen D, Wang Y, Kang Y, Haacke EM. Automated white matter hyperintensity detection in multiple sclerosis using 3D T2 FLAIR. Int J Biomed Imaging. 2014;2014:1–7.
Zhou T, Ahmad TK, Gozda K, Truong J, Kong J, Namaka M. Implications of white matter damage in amyotrophic lateral sclerosis. Mol Med Rep. 2017;16:4379–92.
Matsusue E, Sugihara S, Fujii S, Kinoshita T, Nakano T, Ohama E, Ogawa T. Cerebral cortical and white matter lesions in amyotrophic lateral sclerosis with dementia: correlation with MR and pathologic examinations. Am J Neuroradiol. 2007;28:1505–10.
Mascalchi M. Neurodegenerative diseases with associated white matter pathology. MR imaging in white matter diseases of the brain and spinal cord. 2006;27:377–388.
Dadar M, Manera AL, Ducharme S, Louis CD. White matter hyperintensities, grey matter atrophy, and cognitive decline in Alzheimer's disease and frontotemporal dementia. Neurobiology of aging. 2022;111:54–63.
Woollacott IOC, Bocchetta M, Sudre CH, Ridha BH, Strand C, Courtney R, Ourselin S, Cardoso MJ, Warren JD, Rossor MN, Revesz T, Fox NC, Holton JL, Lashley T, Rohrer JD. Pathological correlates of white matter hyperintensities in a case of progranulin mutation associated frontotemporal dementia. Neurocase. 2018;24:166–74.
McAleese KE, Walker L, Graham S, Moya ELJ, Johnson M, Erskine D, Colloby SJ, Dey M, Martin-Ruiz C, Taylor J-P, Thomas AJ, McKeith IG, De Carli C, Attems J. Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 2017;134:459–73.
Kynast J, Lampe L, Luck T, Frisch S, Arelin K, Hoffmann K-T, Loeffler M, Riedel-Heller SG, Villringer A, Schroeter ML. White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory. J Cereb Blood Flow Metab. 2018;38:996–1009.
Dvash J, Shamay-Tsoory SG. Theory of mind and empathy as multidimensional constructs. Top Lang Disord. 2014;34:282–95.
Oishi K, Faria AV, Hsu J, Tippett D, Mori S, Hillis AE. Critical role of the right uncinate fasciculus in emotional empathy. Ann Neurol. 2015;77:68–74.
Parkinson C, Wheatley T. Relating anatomical and social connectivity: white matter microstructure predicts emotional empathy. Cereb Cortex. 2014;24:614–25.
Multani N, Galantucci S, Wilson SM, Shany-Ur T, Poorzand P, Growdon ME, Jang JY, Kramer JH, Miller BL, Rankin KP, Gorno-Tempini ML, Tartaglia MC. Emotion detection deficits and changes in personality traits linked to loss of white matter integrity in primary progressive aphasia. NeuroImage Clin. 2017;16:447–54.
Crespi C, Cerami C, Dodich A, Canessa N, Iannaccone S, Corbo M, Lunetta C, Falini A, Cappa SF. Microstructural correlates of emotional attribution impairment in non-demented patients with amyotrophic lateral sclerosis. PLoS One. 2016;11:e0161034.
Comes-Fayos J, Romero-Martinez A, Moya-Albiol L. Role of major long fiber tracts association in empathy. Rev Neurol. 2018;67:263–72.
Crespi C, Cerami C, Dodich A, Canessa N, Arpone M, Iannaccone S, Corbo M, Lunetta C, Scola E, Falini A, Cappa SF. Microstructural white matter correlates of emotion recognition impairment in amyotrophic lateral sclerosis. Cortex. 2014;53:1–8.
Shamay-Tsoory SG, Lester H, Chisin R, Israel O, Bar-Shalom R, Peretz A, Tomer R, Tsitrinbaum Z, Aharon-Peretz J. The neural correlates of understanding the other’s distress: a positron emission tomography investigation of accurate empathy. Neuroimage. 2005;27:468–72.
Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017;134:171–86.
Hua AY, Sible IJ, Perry DC, Rankin KP, Kramer JH, Miller BL, Rosen HJ, Sturm VE. Enhanced positive emotional reactivity undermines empathy in behavioral variant frontotemporal dementia. Front Neurol. 2018;9:1–14.
Sollberger M, Stanley CM, Wilson SM, Gyurak A, Beckman V, Growdon M, Jang J, Weiner MW, Miller BL, Rankin KP. Neural basis of interpersonal traits in neurodegenerative diseases. Neuropsychologia. 2009;47:2812–27.
Miller LA, Mioshi E, Savage S, Lah S, Hodges JR, Piguet O. Identifying cognitive and demographic variables that contribute to carer burden in dementia. Dement Geriatr Cogn Disord. 2013;36:43–9.
Brown CL, Lwi SJ, Goodkind MS, Rankin KP, Merrilees J, Miller BL, Levenson RW. Empathic accuracy deficits in patients with neurodegenerative disease: association with caregiver depression. Am J Geriatr Psychiatry. 2018;26:484–93.
Acknowledgements
We would like to thank the ONDRI participants for the time, consent, and participation in our study. The manuscript has been submitted to bioRxiv preprint.
CONSORTIUM NAME: ONDRI Investigators; Members: Michael Strong1, Peter Kleinstiver2, Jane Lawrence-Dewar3, Natalie Rashkovan4, Susan Bronskil5,6,7, Julia Fraser8, Bill McIlroy8, Ben Cornish8, Karen Van Ooteghem8, Frederico Faria9, Yanina Sarquis-Adamson9, Alanna Black9, Barry Greenberg10, Wendy Hatch11, Chris Hudson11,12, Elena Leontieva12,13, Ed Margolin11, Efrem Mandelcorn11, Faryan Tayyari12, Sherif Defrawy11, Don Brien14, Ying Chen14, Brian Coe14, Doug Munoz14, Alisia Southwell4, Dennis Bulman15,16,17, Allison Ann Dilliott18,19, Mahdi Ghani20, Rob Hegele21, John Robinson1, Ekaterina Rogaeva20, Sali Farhan22, Seyyed Mohammad Hassan Haddad1, Nuwan Nanayakkara1, Courtney Berezuk23, Sabrina Adamo23,24, Malcolm Binns25,26, Wendy Lou26, Athena Theyers25, Abiramy Uthirakumaran25, Guangyong (GY) Zou27, Sujeevini Sujanthan28, Mojdeh Zamyadi25, David Munoz29, Roger A. Dixon30, John Woulfe31, Brian Levine25,32, JB Orange33,34, Alicia Peltsch35, Angela Troyer36,37, Marvin Chum38,39.
Affiliations:
1Robarts Research Institute, Western University, London, ON, Canada
2Schulich School of Medicine & Dentistry, Western University, London, ON, Canada
3Thunder Bay Regional Research Institute, ON, Canada
4Sunnybrook Health Sciences Centre, Toronto, ON, Canada.
5Institute of Clinical Evaluative Science, Toronto, ON, Canada
6Institute of Health Policy, Management & Evaluation, Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
7Sunnybrook Research Institute, Toronto, ON, Canada
8Department of Kinesiology, University of Waterloo, Waterloo, ON, Canada
9Gait and Brain Lab, Western University, London, ON, Canada
10Toronto Dementia Research Alliance, University of Toronto, Toronto, ON, Canada
11Department of Ophthalmology and Vision Sciences, University of Toronto, ON, Canada
12School of Optometry and Vision Science, University of Waterloo, Waterloo, ON, Canada
13Kensington Eye Institute, Toronto, ON, Canada
14Centre for Neuroscience Studies, Queen’s University, Kingston, ON, Canada
15University of Ottawa Brain and Mind Research Institute,, Ottawa, ON, Canada
16Department of Pediatrics, Faculty of Medicine, University of Ottawa, ON, Canada
17Regenerative Medicine, Ottawa Health Research Institute, ON, Canada
18Department of Biochemistry, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada
19Robarts Research Institute, Western University, London, ON, Canada
20Tanz Centre for Research in Neurodegenerative Diseases, University of Toronto, Toronto, ON, Canada
21Department of Medicine and Robarts Research Institute, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada
22Department of Neurology and Neurosurgery, Department of Human Genetics, The Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada
23Department of Psychological Clinical Science, University of Toronto Scarborough, Scarborough, ON, Canada
24Toronto Western Hospital Neuropsychology Research, University Health Network, Toronto, ON, Canada
25Rotman Research Institute of Baycrest Centre, Toronto, ON, Canada
26Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada
27Department of Epidemiology and Biostatistics, Western University, London, ON, Canada
28Department of Ophthalmology and Visual Sciences, Research Institute of the McGill University Health Center, Quebec, Canada
29Department of Laboratory medicine, St Michael's Hospital, Unity Health, Toronto, ON, Canada
30Department of Psychology (Science), Neuroscience and Mental Health Institute, University of Alberta, Edmonton, Alberta, Canada
31Ottawa Hospital Research Institute and University of Ottawa, Ottawa, ON, Canada
32Department of Psychology and Medicine (Neurology), University of Toronto, Toronto, ON, Canada
33School of Communication Sciences and Disorders, Faculty of Health Sciences, Western University, London, ON, Canada
34Canadian Centre for Activity and Aging, Western University, London, ON, Canada
35Faculty of Engineering and Applied Science, Queen's University, Kingston, ON, Canada
36Neuropsychology and Cognitive Health Program, Baycrest Health Sciences, Toronto, ON, Canada
37Department of Psychology, University of Toronto, Toronto, ON, Canada
38Faculty of Health Sciences, McMaster University, Hamilton, ON, Canada
39Department of Medicine, McMaster University, Hamilton, ON, Canada
Funding
This research was conducted with the support of the Ontario Brain Institute, an independent non-profit corporation, funded partially by the Ontario government. The opinions, results, and conclusions are those of the authors and no endorsement by the Ontario Brain Institute is intended or should be inferred. Matching funds were provided by participant hospital and research foundations, including the Baycrest Foundation, Bruyere Research Institute, Centre for Addiction and Mental Health Foundation, London Health Sciences Foundation, McMaster University Faculty of Health Sciences, Ottawa Brain and Mind Research Institute, Queen’s University Faculty of Health Sciences, the Thunder Bay Regional Health Sciences Centre, the University of Ottawa Faculty of Medicine, University Health Network, Sunnybrook, and the Windsor/Essex County ALS Association. The Temerty Family Foundation provided the major infrastructure matching funds.
Author information
Authors and Affiliations
Consortia
Contributions
MO: conceptualisation, data curation, formal analysis, investigation, methodology, project administration, software, visualisation, and writing (draft, review, and editing). BV: conceptualisation, investigation, methodology, project administration, writing (review and editing). DB: data curation, formal analysis, supervision, methodology, software, visualisation, and writing (draft, review, and editing). JR: data curation, supervision, and writing (review and editing). MFH, PM, AR, CJMS, and BT: data curation and writing (review and editing). KS: data curation, software, writing (review and editing). JR, SA, and MG: writing (review and editing). DK: data curation, project administration, writing (review and editing). FG: data curation, validation, resources, writing (review and editing). RB: data curation, resources, funding acquisition, writing (review and editing). AR, SS, RHS, AA, GS, MM, AEL, CM, SEB, LZ, CS, MM, CF, AF, MF, MMO, SK, SP, BP, TKR, DS, DT, MC, JT, DD, AH, LC, JM, DS, DPB, DG, MJ, TS, and EF: resources, funding acquisition, writing (review and editing). MCT: conceptualisation, investigation, methodology, supervision, resources, funding acquisition, writing (review and editing). All authors contributed to the article and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by ONDRI. Study participants were recruited at various health centres across Ontario, Canada: London Health Science Centre and Parkwood Institute in London; Hamilton General Hospital and McMaster Medical Centre in Hamilton; The Ottawa Civic Hospital in Ottawa; Thunder Bay Regional Health Sciences Centre in Thunder Bay; and St. Michael’s Hospital, Sunnybrook Health Sciences Centre, Baycrest Health Sciences, Centre for Addiction and Mental Health, and Toronto Western Hospital (University Health Network) in Toronto. Ethics approval was obtained from all participating institutions and performed in accordance with the Declaration of Helsinki. All participants and study partners provided informed consent. The patients/participants provided their written informed consent to participate in this study.
Conflict of interest
TKR has received research support from Brain Canada, Brain and Behavior Research Foundation, BrightFocus Foundation, Canada Foundation for Innovation, Canada Research Chair, Canadian Institutes of Health Research, Centre for Aging and Brain Health Innovation, National Institutes of Health, Ontario Ministry of Health and Long-Term Care, Ontario Ministry of Research and Innovation, and the Weston Brain Institute. TKR also received in-kind equipment support for an investigator-initiated study from Magstim, and in-kind research accounts from Scientific Brain Training Pro. DPB is supported by a Wellcome Clinical Research Career Development Fellowship (214571/Z/18/Z). Other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ONDRI Investigators and their affiliations are listed under the CONSORTIUM NAME section.
Supplementary Information
Below is the link to the electronic supplementary material.
About this article
Cite this article
Ozzoude, M., Varriano, B., Beaton, D. et al. Investigating the contribution of white matter hyperintensities and cortical thickness to empathy in neurodegenerative and cerebrovascular diseases. GeroScience 44, 1575–1598 (2022). https://doi.org/10.1007/s11357-022-00539-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11357-022-00539-x