Abstract
Breast cancer is the most commonly diagnosed cancer in women worldwide. Approximately one-tenth of all patients with advanced breast cancer develop brain metastases resulting in an overall survival rate of fewer than 2 years. The challenges lie in developing new approaches to treat, monitor, and prevent breast cancer brain metastasis (BCBM). This review will provide an overview of BCBM from the integrated perspective of clinicians, researchers, and patient advocates. We will summarize the current management of BCBM, including diagnosis, treatment, and monitoring. We will highlight ongoing translational research for BCBM, including clinical trials and improved detection methods that can become the mainstay for BCBM treatment if they demonstrate efficacy. We will discuss preclinical BCBM research that focuses on the intrinsic properties of breast cancer cells and the influence of the brain microenvironment. Finally, we will spotlight emerging studies and future research needs to improve survival outcomes and preserve the quality of life for patients with BCBM.
Similar content being viewed by others
Introduction
Central nervous system (CNS) metastases are a devastating diagnosis for patients living with breast cancer. People living with breast cancer and CNS metastasis represent an understudied cohort of patients with unique challenges to manage their disease. CNS metastasis describes any metastases within the brain or the intramedullary spinal cord. This review will highlight the biology, current treatment strategies, and ongoing clinical trials for breast cancer that has metastasized specifically to the brain (BCBM). We will discuss unmet needs that leave patients living with BCBM feeling overlooked.
Breast cancer is the most common neoplasm among women and causes 500,000 deaths annually worldwide, with ~1.3 million new cases diagnosed each year1. Breast cancer is classified using pathological markers, TNM staging (tumor size, lymph node, and metastatic spread), and gene expression patterns1. Breast cancer is broadly classified by origin, either in the breast duct, in the case of intraductal carcinoma (IDC), or the breast lobule for intralobular carcinoma (ILC). Patients with ILC are reported to have a higher likelihood of bone, gastrointestinal and ovarian metastasis and less likely to have CNS, regional lymph nodes or lung metastasis as their first site of metastatic recurrence compared to patients with IDC.
In addition to characterization by origin, breast cancer has been molecularly characterized, initially by five main subtypes (Luminal A, Luminal B, Basal, HER2-enriched, Normal Breast-Like)2,3 which closely overlap with pathologically defined subtypes. Pathologists use immunohistochemical (IHC) staining to determine the presence or absence of two hormone receptors (HR), the progesterone receptor (PR) and the estrogen receptor (ER), as well as the human epidermal growth factor receptor 2 (HER2)1,4. IHC-defined tumor subtypes have been associated with a difference in a patients’ median survival at the time of a diagnosis of brain metastasis. Patients with HR+/HER2+, HR−/HER+, HR+/HER2−, and HR−/HER2− have a median survival of 21–27, 18–25, 10–14, and 6–9 months, respectively5. Molecular markers, like BRCA1 or BRCA2 (breast cancer gene 1 or 2) germline mutations, are indicators of possible basal-like breast cancer development and are used to determine potential risk and guide treatment6. Recent large-scale sequencing efforts have led to the identification of the genes with the highest mutation rates in breast cancer, including TP53, PIK3CA, AKT1, PTEN, ERBB2, ATM, CDH1, APC, KRAS, NRAS7. Ongoing research will determine whether these mutations are “actionable” in order to lay the foundation for personalized medicine approaches. The most common organ sites for breast cancer metastases are bone, brain, liver, and lungs8,9. Breast cancer is the second leading cause of all brain metastasis, and 10–16% of patients with advanced breast cancer have or develop brain metastasis10. National trials are underway to determine if early detection of brain metastasis would improve survival and quality of life, because current NCCN guidelines do not recommend brain imaging unless neurocognitive symptoms develop11,12. Patients are often not educated about the symptoms suggestive of cancer spread to the CNS. Failure to identify CNS metastasis early likely results in more invasive and toxic interventions such as whole-brain radiation therapy (WBRT) or surgical resection. There is increased support by the patient advocate and medical communities to include brain imaging at the time of a MBC diagnosis, especially in patients with an increased risk of developing BCBM. Koniali et al. provide an extensive review of the risk factors for BCBM13. The main risk factors include age (<49 years old), higher-grade cancer, prior visceral metastases, HER2-positive or triple-negative status, and mutations in the BRCA1 gene.
The incidence of BCBM is rising14. Several contributing factors include increased detection due to improved and more widely available radiological techniques and targeted therapies to treat systemic disease, which prolong survival14,15. The extended survival time has led to a 25–40% increased incidence in brain metastasis in patients with HER2-positive breast cancer and as high as 46% among patients with advanced TNBC14,16,17.
BCBM most commonly occurs in the brain’s parenchyma (neurons and glia). The spread of cells to the pia mater, arachnoid mater, subarachnoid spaces, and the cerebral spinal fluid (CSF) is known as leptomeningeal metastasis or disease (LMD)18. LMD remains understudied because it occurs at a lower rate, is difficult to diagnose19,20, and is associated with a median survival of 15 weeks20. Le Rhun et al. compared 50 patients with LMD to 50 patients with breast cancer and no CNS metastases including LMD. The cohorts were matched based on their age at time of diagnosis, the year of diagnosis, and the type of chemotherapy that they received. Factors associated with risk for LMD included: lobular histology, HR-negative status, and metastasis at time of breast cancer diagnosis21. In a separate study, patients with TNBC as well as patients with higher grade cancer developed LMD within a shorter time frame as compared to patients’ with receptor positive tumors and/or lower grade tumors22. There are no standards for neurological examinations, neuro-imaging assessment, or a specific CSF cytological to diagnose LMD. A Response Assessment in Neuro-Oncology (RANO) working group has been established to develop these criteria. Finding the means to overcome CNS metastasis is, undoubtedly, an unmet clinical need for which more research is required.
BASIC science research
Animal models
Mouse models for breast cancer metastasis rely on the injection of human MBC cell lines or patient-derived organoids into immune-compromised mice. Several mouse cell lines derived from spontaneous mouse tumors are transplantable in a syngeneic murine background with a competent immune system23. Transgenic or knockout mice that develop spontaneous mammary carcinomas that metastasize have also been developed24,25. An alternative metastasis model was created using variants of human breast cancer cell lines serially injected via the heart or tail of mice and isolated from the bone, lung, or brain26. When tested in experimental metastasis models, by intracardiac injection, these variants showed preferential homing to the organ from which they were harvested27. BCBM experimental models can be established by injecting cells into the mouse brain or carotid artery28,29, and used to test the ability of therapies to treat CNS lesions effectively. The downside of such models is they circumvent the development of a primary tumor and bypass the initiating steps of metastasis28.
Clinically relevant models of LMD were established by Boire et al., who performed three serial rounds of the direct injection of human or mouse cancer cells into the cisterna magna and then collected the primed cells. The primed cells were intracardially injected into a separate cohort of animals, and the disseminated cells consistently formed LMD instead of CNS disease30. The group also discovered that cells in the CSF express complement component 3, promoting disruption of the brain-CSF barrier and predicting leptomeningeal relapse31. Kuruppu et al. established a model in which mice develop neurological symptoms that bear clinical resemblance to LMD. The model can be used to evaluate potential treatment strategies32. Preclinical models that replicate BCBM and LMD are improving, but the lack of spontaneous breast to brain models has stagnated research.
Organotropism
Metastasis studies have focused on the concept of “organotropism”, or the ability for a cancer cell to preferentially home to, and survive in, a specific organ. Organotropism could occur due to circulation patterns, a pre-metastatic niche33, a symbiotic relationship of cancer cells with resident cells, a specific gene expression profile, or even the immune microenvironment of the organ34,35. Whether breast cancer cells have inherent organotropism initially in the primary tumor or whether they adapt to the metastatic site continues to be debated in the field36.
In a recent study, circulating tumor cells (CTCs) acquired from four patients with breast cancer were injected into the left cardiac ventricle of NSG mice37. The metastases that formed were isolated, dissociated, and underwent multiple in vivo intracardiac injections to select for cells primed to colonize either the lung, bone, or brain37. These CTC-derived brain metastatic cells had high expression of semaphorin (SEM4AD), which increased transmigration in a simulated in vitro blood-brain barrier (BBB) assay and an in vivo mouse model37.
In separate studies, to elucidate mechanisms of extravasation in the brain, nude mice were subjected to serial intracarotid injections of MDA-MB-231 and CN34 cell lines to establish cell lines primed for brain metastasis38. The metastatic brain cells had increased COX2 and EGFR ligand (heparin-binding EGF) expression that promoted BBB permeability via prostaglandin production38. Also, the breast cancer cell surface expressed a brain-specific sialyltransferase (ST6GALNAC5)38. The next step is to combine the results of these studies to develop an assay that can be used to assess the risk of BCBM development.
Brain microenvironment
Using intravital imaging of intracranially injected MDA-MB-231 cells, Simon et al. observed microglia directly interacting with breast cancer cells, altering microglial morphology and disrupting normal brain electrophysiology39. Resident astrocytes and microglial cells express cytokines that promote breast cancer cell proliferation40. Conditioned media from rat neonatal and adult astrocytes enhanced cancer cell invasion in vitro due to secreted matrix metalloprotease-2 (MMP-2) and MMP-9, while inhibiting MMPs in the media decreased metastatic growth of breast cancer cells following intracardiac injection41. This suggests that secreted factors from astrocytes contribute to colonization within the CNS41,42.
Astrocyte-derived exosomes can promote chemokine production in breast cancer cells leading to enhanced proliferation and reduced apoptosis43. Cancer cells that adapt to the brain microenvironment by mimicking CNS cells have an increased chance of survival. For example, Neman et al. showed that some breast cancer cells express proteins typically expressed in neuronal cells and can even catabolize GABA into succinate to form NADH44. Furthermore, expression of reelin in HER2-positive cancer cell lines co-cultured with astrocytes had increased proliferation which was reversed with the knockdown of reelin and HER245. In both an intracardiac and intracranial injection models, TNBC and HER2-positive breast cancer cells activated astrocytes by expressing truncated glioma-associated oncogene homolog one, which enhanced brain colonization and increased the expression of genes associated with stemness (CD44, Nanog, Sox2, Oct4)46.
Microglia/macrophages can directly interact with breast cancer cells that have metastasized to the CNS. For example, Andreou et al. intracerebrally injected 4T-1 cells in BALB/c mice and identified subsets of activated pro-inflammatory and anti-inflammatory microglia/macrophages. The group then selectively depleted the anti-inflammatory microglia/macrophage population using mannosylated clodronate liposomes, reducing brain lesions47. Another study exploring the interaction of BCBM cells and macrophages demonstrated that cathepsin S is a regulator of BCBM. Inhibiting cathepsin S in both cancer cells as well as macrophages significantly reduced BCBM48. An analysis of metastasis-associated macrophages in the brain parenchyma of mice revealed upregulated cytokine and Lymphotoxin β production that promoted M2 polarization of macrophage cells49. Thus, astrocyte, microglia, and macrophage interactions with breast cancer cells promote metastasis through altered neuroinflammatory responses in the brain50. Basic research has led to the identification of potential targets that could be used to develop therapies or to predict BCBM but to be tested clinically, patients with BCBM need to be included in clinical trials.
Clinical and translational research
Patients with BCBM are often excluded from clinical trials because BCBM is linked with an increased mortality rate. Out of 1474 clinical trials for patients with breast cancer conducted from 1992 to 2016 in a review by Costa et al., only 29% included patients with CNS disease, and only 1% (16 studies) were designed to consider BCBM specifically51. In 2016, ASCO and Friends of Cancer Research (Friends) established a Brain Metastasis Working group to change the exclusionary nature of the current eligibility criteria. The group lobbied for the inclusion of patients with BCBM in trials suggesting that they could be stratified into three cohorts of patients during clinical trial design: (1) those with treated/stable BCBMs, (2) those who have active BCBMs, and (3) those who have LMD52. A fourth cohort consisting of patients that have not received prior treatment but have stable BCBM should be considered. While the ASCO-Friends guidance is welcomed by the patient advocate community, the majority of clinical trials restrict eligibility to those patients with stable BCBM whereas the majority of patients with BCBM have active disease and who are in dire need for a clinical trial. Retrospective and prospective exploratory analysis have been conducted within larger cohorts of patients enrolled in a clinical trial to identify the incidence rate, time to development, and overall survival time following a diagnosis of brain metastasis.
Several treatment approaches are available to patients with BCBM, including local and systemic therapies. The majority of patients diagnosed with BCBM will have one or more local treatments but will likely continue their systemic therapy or transition to a different systemic approach.
Localized therapy
Localized treatments for BCBM include surgery, WBRT, and radiosurgery. WBRT is preferred when there are many metastatic lesions but does not come without risk. WBRT can cause neurocognitive complications (e.g., sensory deficits, headache, changes in mental status, cognitive disturbances, seizures, ataxia, and motor loss) and does not improve overall survival unless combined with surgery or chemotherapy53. Stereotactic radiosurgery (SRS) is a favorable alternative because of reduced cognitive impairments. Recent Phase III results presented at ASTRO 2020 demonstrated that SRS led to less cognitive decline than conventional WBRT even in patients with multiple lesions (more than 4) without compromising disease control. This serves as the foundation of an ongoing clinical trial comparing SRS with hippocampal-avoidant WBRT plus memantine for 5–15 BM (NCT03550391)54. The study includes patients with active brain metastases but excludes any patients with LMD, >15 BM on a volumetric T1 contrast MRI within the past 14-days or >10 metastasis on non-volumetric MRI. Emerging data suggest that SRS remains a reasonable alternative even for patients with a large number of BCBMs, with one series reporting utilization of SRS in a patient with as many as 34 brain metastases55. A clinical trial of patients with BCBM from non-small cell lung carcinoma, melanoma, and renal cell carcinoma demonstrated a benefit in overall survival from concurrent SRS with immune checkpoint inhibitors, suggesting more studies regarding the timing of SRS in BCBM could provide insight56. Surgery is reserved for patients who present with a limited number of large or symptomatic brain lesion(s). Surgery is typically followed by adjuvant radiation therapy (RT), either in the form of SRS or WBRT57. Following a randomized, controlled, phase III trial (n = 194 patients), post-operative SRS has become the standard of care due to reduced cognitive decline but similar survival benefit when compared to WBRT58. Another treatment option for metastatic brain lesions is laser interstitial thermal therapy (LITT), which remains highly experimental though the technology was established in 1990. It was not until the late 2000s that FDA approved two ablation systems used to treat primary or recurrent tumors and radiation-induced necrosis, which are only available at large medical institutions. LITT is used on deep-seated tumors but its use is limited by the high cost of the procedure59,60.
Current clinical studies are focused on improving the effectiveness of RT and assessing specific neurological impairments caused by RT. Radiosensitizers like motexafin gadolinium (produces reactive oxygen species), efaproxiral (induces low oxygen by binding to hemoglobin), and RRx-001, which dilates blood vessels and improves oxygenation to the tumor site, have demonstrated efficacy in the prevention of neurocognitive impairment61. Drugs like memantine, an Alzheimer’s prescription drug, have been utilized to block vascular damage and reduce side effects like dementia that result from WBRT treatment of the hippocampal region62. To assess the neurological impairments that result from radiation treatment, specific cognitive examinations that establish a baseline and evaluate changes should be developed63.
Since patients with HER2-positive disease and TNBC have a higher rate of BCBM, prophylactic cranial irradiation (PCI), which has historically been utilized for patients with small-cell lung cancer and brain metastasis, is now being considered14,64. Some clinicians are concerned that PCI may be associated with too many adverse effects, including a risk to the patient’s quality of life65. Thus, the application of PCI remains controversial in the field, and clinical trials would be warranted. A partial list of active clinical trials that include a RT component are highlighted in Table 1.
Targeted therapies
The systemic treatments often used to manage BCBM include corticosteroids to reduce cerebral edema and standard chemotherapy agents such as capecitabine, carboplatin, gemcitabine, and methotrexate66. Doxorubicin, cyclophosphamide, fluorouracil, paclitaxel, docetaxel, and vinorelbine may also be used but have poor blood-brain barrier penetrance11.
Systemic treatments for patients with BCBM beyond chemotherapy now include a small but quickly growing arsenal of targeted therapies. For example, small molecule inhibitors are being used to treat patients with HER2-positive cancer, and endocrine therapy combined with the cyclin-dependent kinase (CDK)4/6 inhibitor, abemaciclib, for patients with hormone receptor-positive disease.
A retrospective analysis of EMILIA trial data showed that the rate of CNS progression was similar (extracranial ORR), but the median overall survival was significantly improved in patients with asymptomatic CNS metastasis that were treated with trastuzumab emtansine (T-DM1) compared to lapatinib plus capecitabine67. Likewise, in the CLEOPATRA trial, an exploratory analysis demonstrated no difference in incidence, but the time to develop CNS metastases was prolonged from 11.9 to 15 months when pertuzumab was added to a trastuzumab and docetaxel treatment regimen68. The NALA trial compared the progression-free survival (PFS) of 101 patients with stable BCBM treated with either neratinib (N = 51) or lapatinib (N = 50) in combination with capecitabine. Patients with BCBM treated with neratinib had a median PFS of 7.8 months compared to 5.5 months for patients treated with lapatinib69. Results from the HER2CLIMB trial show that the median PFS in patients with active BCBM at baseline that received tucatinib, trastuzumab, and capecitabine was 7.6 months compared to 5.4 months in patients who did not receive tucatinib70. In a subgroup analysis of the DESTINY-Breast01 trial, a phase 1 dose-finding study for trastuzumab deruxtecan (T-DXd), 24 of the 184 patients enrolled had stable CNS metastasis. The objective response rate was 58.3%, and median PFS was 18.1 months for these patients71. T-DM1, T-DXd, neratinib, and tucatinib are FDA-approved treatments for patients with HER2-positive MBC that has progressed on prior HER2-targeted therapy(ies).
At the time of publication, globally, 230 ongoing clinical trials include patients with BCBM of which 36 are open to patients with LMD. The majority of the studies include targeted therapy such as antibody drug conjugates (ADCs), immunotherapies, novel chemotherapeutics, and small molecule inhibitors (Table 2). Table 3 highlights a partial list of recently reported BCBM clinical trial results. Table 4 highlights a partial list of active trials at the time of publication. For an up-to-date list of recruiting and active trials, please see both the patient-managed clinical trial database at TheStormRiders.org and the US-based Metastatic Breast Cancer Trial Search at BreastCancerTrials.org.
Immunotherapy
There is a broadening interest in using immune checkpoint inhibitors as therapeutics for BCBM after preliminary studies showed efficacy in other solid tumor types72. A retrospective study of 84 BCBM biopsies demonstrated that PD-L1 and PD-L2 were expressed in 53 and 36% patients, respectively, suggesting immune checkpoint inhibitors could provide a therapeutic benefit73. Another viable immunotherapy uses chimeric antigen receptor-engineered T (CAR T) cells that have been optimized in a xenograft mouse model of BCBM. The HER2-CAR T cells reduced T-cell exhaustion in vivo and intracranial delivery demonstrated antitumor efficacy74. This research has led to an ongoing clinical trial (NCT03696030) for patients with active brain or leptomeningeal metastases. Other immunotherapies include vaccines that introduce neoantigens that are specific to glioma. When viable neoantigens are identified for BCBM, the same strategy could be employed75.
Therapeutics that cross the blood-brain barrier (BBB)
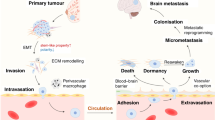
One main obstacle for identifying efficacious BCBM therapies is the BBB. BCBM development occurs when cancer cells detach from the primary tumor, invade, cross the endothelial barrier, survive in the bloodstream, extravasate, and grow at the secondary site76. The capillaries that make up the BBB are different from the endothelium in other organs. The BBB is composed of endothelial cells with tight junctions, no fenestrations or pinocytic vesicles, and are encased in a basal membrane and extracellular matrix barrier77. Apart from size, polarity (nonpolar preference) and lipophilicity contribute to the restrictions for passive diffusion77. We refer the readers to a review by Deeken and Loscher that discusses ways to overcome the BBB using transporter inhibition, nanoparticles, immunoliposomes, peptide vectors, or carrier-mediated active transport mechanisms77. We also note a retrospective review of animal and human studies of HER2-positive BCBMs by Kabraji et al. that revealed a drug’s ability to cross the BBB did not necessarily correlate with efficacy78, suggesting additional factors contribute to the inadequate response.
Local administration of drugs
The direct injection of therapeutics into the spinal canal or subarachnoid space has improved drug efficacy in some instances79. For example, a case study reported on a patient with HER2-positive breast cancer and multiple brain lesions treated with intrathecally-delivered trastuzumab, resulting in stabilizing brain and epidural metastases80. A phase 1 clinical trial (NCT01325207) was initiated to test intrathecal delivery of trastuzumab and identify a maximum tolerated dose for patients with LMD and stable systemic disease in HER2+ breast cancer81. Another method of direct CNS delivery is convection-enhanced delivery (CED) via a pressure gradient at the tip of an infusion catheter. Still, this method has not been largely successful due to the dependence on volume and rate of gradient infusion, resulting in an uneven distribution of drugs or potential drug efflux from the injection site and toxicity from treatment82. One final strategy for increasing CNS drug delivery is ultrasound-induced BBB opening that has shown some preclinical efficacy as well as efficacy for CNS diseases such as gliomas82. Intrathecal delivery is largely limited to patients with HER2+ disease as other agents (e.g., chemotherapy) can cause debilitating toxic side effects with limited benefit and are unsustainable for indefinite use.
Imaging
MRI is most commonly used to monitor disease progression and side effects from treatment, detect recurrence, and identify new metastases post-treatment. Still, current imaging techniques lack the power to differentiate pseudo-progression from actual progression. Improving imaging techniques to diagnose and monitor BCBM earlier could enhance the quality of life for patients11. A recent study using AMT-PET imaging successfully differentiated primary brain tumors and metastatic brain tumors with greater than 90% accuracy launching a clinical study for patients with BCBM to improve diagnoses by enhancing the ability to differentiate abnormal and normal tissue (NCT01302821)83. Artificial intelligence (AI) will likely play a future role in assessing treatment response of brain tumors; Machine learning methods carefully trained on standard MRI could be more reliable and precise than established methods84.
Liquid biopsies
To identify patients most at risk to develop BCBM, researchers are advancing the capabilities of liquid biopsies since actual BCBM biopsies are an impossibility in most cases85. Riebensahm et al. showed a significant association of decreased overall survival when CTCs were detectable86. Cell-free DNA (cfDNA) has been used to assess genomic alterations. To identify genetic mutations, cfDNA was isolated and sequenced in blood samples from 13 patients with BCBM and 36 patients without BCBM87. There was a high correlation of mutations in APC, BRCA1, and CDKN2A associated with BCBM, which provided supportive evidence for cfDNA biomarkers88. In a study of 194 patients with MBC cfDNA and CTCs were compared for their ability to predict PFS, OS, and response to treatment. cfDNA was simpler to isolate, more informative, and less expensive than isolating and quantifying the number of CTCs89. Proteins in the serum or CSF of patients have also been considered as predictive biomarkers of metastasis. For example, Dao et al. identified increased (>0.1 ng/mL) levels of the angiopoietin-like fibrinogen-like domain (cANGPTL4) in the serum of patients with breast cancer, which was associated with increased risk for BCBM90.
Patient perspectives: care gaps and future research needs
Patients living with central nervous system (CNS) metastasis
Due to a lack of guidelines for the treatment of CNS metastasis, once a diagnosis is confirmed, treatment is at the discretion of a patient’s oncology team. Most cancer patients are treated locally in community hospitals. They are not likely to have access to a breast oncologist specializing in CNS metastasis or a multidisciplinary team who form a consensus on treatment decisions. Even with access to a multidisciplinary team, which often includes a medical oncologist, neuro-radiation oncologist, neuro-oncologist, and neurosurgeon, the continuity of care is often a problem for patients because the burden of facilitating communication between the three specialties falls on the patient themselves. Treatment options are limited to invasive interventions that can cause debilitating side effects and seriously impact the quality of life. Despite having access to the “best” care, patients with CNS metastasis still have a worse prognosis, disproportionate treatment response, and lower overall survival than patients with metastasis in other organs. The disparities in the quality of care are even more significant among patients with low socioeconomic status as well as patients identified as racial/ethnic minorities—African Americans, American Indians, and Alaskan Natives, Asians, Native Hawaiians/other Pacific Islanders, and Hispanics/Latinos91.
Patients living with CNS metastasis represent a vulnerable cohort, and when medicine and research fail this understudied community, they often turn to other patients and advocates for solutions. Therefore, patient advocates have been charged with leading the effort to address the care gaps and research needs of breast cancer patients who develop CNS metastasis. Though many assume patient advocacy is synonymous with support (e.g., emotional, financial, and educational) patients have become increasingly valuable to the medical and research community, because they offer a unique perspective as experts living with cancer. As the ultimate end users of products developed through research, patients can, and have, helped drive more impactful research that improves survival outcomes.
Future research needs
In 2020, the MBC Alliance patient advocates recognized an opportunity to capitalize on the momentum gained from the approval of tucatinib (Tukysa®), the first and only drug approved for BCBM. The Alliance launched the patient-led BCBM Initiative: Marina Kaplan Project, in memory of Marina Pomare Kaplan, with the overarching goal to identify the unmet research needs of patients living with CNS metastasis. The project includes members with representation from industry, research organizations, and individual patients. Nearly one-third of the group is comprised of patients living with brain metastases or LMD. The 17-member scientific advisory board, comprised of a multidisciplinary array of experts in the field of brain metastasis and LMD from breast cancer, advised on the identification of the following gaps in CNS-metastasis research: 1) a poor understanding of the unique brain microenvironment, 2) the absence of sufficient preclinical in vivo animal models that mimic multiple aspects of brain metastasis in a clinical setting, 3) the inability of many anticancer agents to cross either the blood-brain or blood-tumor barrier, 4) the lack of clinically meaningful endpoints that measure survival and quality of life, and 5) the lack of representation of patients with brain metastasis in clinical trials due to restrictive eligibility criteria. Future work addressing each of these gaps will be essential to reduce deaths due to brain metastasis.
Concluding remarks
Over the past 20 years, basic research in breast cancer metastasis has led to identifying candidate genes whose expression is predictive of metastasis to specific organs. Prospective studies are warranted to develop assays that detect biomarkers linked to metastatic outcomes. Such improvements would be invaluable for decisions regarding clinical treatment and monitoring.
Advances in clinical trial design have allowed subgroup analyses to determine the effectiveness of new therapies of smaller patient subpopulations over the last decade. These analyses have led to the FDA approval of several HER2-targeted treatments that have efficacy for patients with BCBM. Still, much work is needed, particularly to extend outcomes beyond months into years and to consider TNBC. An integrated approach to cancer research that includes the voice of patient advocates will allow us to tackle the remaining challenges while improving the lives and outcomes for patients with breast cancer.
References
Taherian-Fard, A., Srihari, S. & Ragan, M. A. Breast cancer classification: linking molecular mechanisms to disease prognosis. Brief. Bioinform. 16, 461–474 (2014).
Sørlie, T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur. J. Cancer 40, 2667–2675 (2004).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Harbeck, N. et al. Breast cancer. Nat. Rev. Dis. Prim. 5, 1–31 (2019).
Sperduto, P. W. et al. Beyond an updated graded prognostic assessment (Breast GPA): a prognostic index and trends in treatment and survival in breast cancer brain metastases from 1985 to today. Int. J. Radiat. Oncol. Biol. Phys. 107, 334–343 (2020).
Merino Bonilla, J. A., Torres Tabanera, M. & Ros Mendoza, L. H. Breast cancer in the 21st century: from early detection to new therapies. Radiologia 59, 368–379 (2017).
Ghosh, M. et al. Landscape of clinically actionable mutations in breast cancer ‘A cohort study’. Transl. Oncol. 14, 100877 (2021).
Wang, R. et al. The clinicopathological features and survival outcomes of patients with different metastatic sites in stage IV breast cancer. BMC Cancer https://doi.org/10.1186/s12885-019-6311-z (2019).
Patanaphan, V., Salazar, O. M. & Risco, R. Breast cancer: metastatic patterns and their prognosis. South. Med. J. 81, 1109–1112 (1988).
Yuan, P. & Gao, S.-L. Management of breast cancer brain metastases: focus on human epidermal growth factor receptor 2-positive breast cancer. Chronic Dis. Transl. Med. 3, 21–32 (2017).
Lin, N. U., Bellon, J. R. & Winer, E. P. CNS metastases in breast cancer. J. Clin. Oncol. 22, 3608–3617 (2004).
Assi, H. I., Mahmoud, T., Saadeh, F. S. & El Darsa, H. Management of leptomeningeal metastasis in breast cancer. Clin. Neurol. Neurosurg. 172, 151–159 (2018).
Koniali, L. et al. Risk factors for breast cancer brain metastases: a systematic review. Oncotarget 11, 650–669 (2020).
Clayton, A. J. et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br. J. Cancer 91, 639–643 (2004).
Pesapane, F., Downey, K., Rotili, A., Cassano, E. & Koh, D. M. Imaging diagnosis of metastatic breast cancer. Insights Imaging https://doi.org/10.1186/s13244-020-00885-4 (2020).
Bendell, J. C. et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer 97, 2972–2977 (2003).
Lv, Y., Ma, X., Du, Y. & Feng, J. Understanding patterns of brain metastasis in triple-negative breast cancer and exploring potential therapeutic targets. OncoTargets Ther. 14, 589–607 (2021).
Chamberlain, M. C. Leptomeningeal metastasis. Curr. Opin. Oncol. 22, 627–635 (2010).
Franzoi, M. A. & Hortobagyi, G. N. Leptomeningeal carcinomatosis in patients with breast cancer. Crit. Rev. Oncol. Hematol. 135, 85–94 (2019).
Scott, B. J., Oberheim-Bush, N. A. & Kesari, S. Leptomeningeal metastasis in breast cancer—a systematic review. Oncotarget 7, 3740–3747 (2016).
Le Rhun, E. et al. Clinicopathological features of breast cancers predict the development of leptomeningeal metastases: a case–control study. J. Neurooncol. 105, 309–315 (2011).
Yust-Katz, S. et al. Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J. Neurooncol. 114, 229–235 (2013).
Pulaski, B. A. & Ostrand‐Rosenberg, S. Mouse 4T1 breast tumor model. Curr. Protoc. Immunol. https://doi.org/10.1002/0471142735.im2002s39 (2000).
Menezes, M. E. et al. Genetically engineered mice as experimental tools to dissect the critical events in breast cancer. Adv. Cancer Res. 121, 331–382 (2014).
Borowsky, A. D. Choosing a mouse model: experimental biology in context-the utility and limitations of mouse models of breast cancer. Cold Spring Harb. Perspect. Biol. https://doi.org/10.1101/cshperspect.a009670 (2011).
Francia, G., Cruz-Munoz, W., Man, S., Xu, P. & Kerbel, R. S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nat. Rev. Cancer 11, 135–141 (2011).
Minn, A. J. et al. Distinct organ-specific metastatic potential of individual breast cancer cells and primary tumors. J. Clin. Invest. 115, 44–55 (2005).
Soto, M. S. & Sibson, N. R. Mouse models of brain metastasis for unravelling tumour progression. Adv. Exp. Med. Biol. 899, 231–244 (2016).
Zhang, C., Lowery, F. J. & Yu, D. Intracarotid cancer cell injection to produce mouse models of brain metastasis. J. Vis. Exp. https://doi.org/10.3791/55085 (2017).
Boire, A., DeAngelis, L. & Massagué, J. Development of a mouse model of leptomeningeal metastasis (P7.014). Neurology https://n.neurology.org/content/82/10_Supplement/P7.014 (2014).
Boire, A. et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell 168, 1101–1113.e13 (2017).
Kuruppu, D. et al. A model of breast cancer meningeal metastases: characterization with in vivo molecular imaging. Cancer Gene Ther. 26, 145–156 (2019).
Liu, Y. & Cao, X. Characteristics and significance of the pre-metastatic Niche. Cancer Cell 30, 668–681 (2016).
Chen, W., Hoffmann, A. D., Liu, H. & Liu, X. Organotropism: new insights into molecular mechanisms of breast cancer metastasis. npj Precis. Oncol. https://doi.org/10.1038/s41698-018-0047-0 (2018).
Yuzhalin, A. E. & Yu, D. Brain metastasis organotropism. Cold Spring Harb. Perspect. Med. https://doi.org/10.1101/cshperspect.a037242 (2019).
Cacho-Díaz, B. et al. Tumor microenvironment differences between primary tumor and brain metastases. J. Transl. Med. https://doi.org/10.1186/s12967-019-02189-8 (2020).
Klotz, R. et al. Circulating tumor cells exhibit metastatic tropism and reveal brain metastasis drivers. Cancer Discov. 10, 86–103 (2020).
Bos, P. D. et al. Genes that mediate breast cancer metastasis to the brain. Nature 459, 1005–1009 (2009).
Simon, A. et al. Metastatic breast cancer cells induce altered microglial morphology and electrical excitability in vivo. J. Neuroinflamm. https://doi.org/10.1186/s12974-020-01753-0 (2020).
Termini, J., Neman, J. & Jandial, R. Role of the neural niche in brain metastatic cancer. Cancer Res. 74, 4011–4015 (2014).
Wang, L. et al. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS ONE https://doi.org/10.1371/journal.pone.0080933 (2013).
Shumakovich, M. A. et al. Astrocytes from the brain microenvironment alter migration and morphology of metastatic breast cancer cells. FASEB J. 31, 5049–5067 (2017).
Zhang, L. et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature 527, 100–104 (2015).
Neman, J. et al. Human breast cancer metastases to the brain display GABAergic properties in the neural niche. Proc. Natl Acad. Sci. USA 111, 984–989 (2014).
Jandial, R., Choy, C., Levy, D. M., Chen, M. Y. & Ansari, K. I. Astrocyte-induced Reelin expression drives proliferation of Her2+ breast cancer metastases. Clin. Exp. Metastasis 34, 185–196 (2017).
Sirkisoon, S. R. et al. TGLI1 transcription factor mediates breast cancer brain metastasis via activating metastasis-initiating cancer stem cells and astrocytes in the tumor microenvironment. Oncogene 39, 64–78 (2020).
Andreou, K. E. et al. Anti-inflammatory microglia/macrophages as a potential therapeutic target in brain metastasis. Front. Oncol. https://doi.org/10.3389/fonc.2017.00251 (2017).
Sevenich, L. et al. Analysis of tumour- and stroma-supplied proteolytic networks reveals a brain-metastasis-promoting role for cathepsin S. Nat. Cell Biol. 16, 876–888 (2014).
Rippaus, N. et al. Metastatic site-specific polarization of macrophages in intracranial breast cancer metastases. Oncotarget 7, 41473–41487 (2016).
Doron, H., Pukrop, T. & Erez, N. A blazing landscape: Neuroinflammation shapes brain metastasis. Cancer Res. 79, 423–436 (2019).
Costa, R. et al. Systematic analysis of early phase clinical studies for patients with breast cancer: inclusion of patients with brain metastasis. Cancer Treat. Rev. 55, 10–15 (2017).
Lin, N. U. et al. Modernizing clinical trial eligibility criteria: recommendations of the American society of clinical oncology-friends of cancer research brain metastases working group. J. Clin. Oncol. 35, 3760–3773 (2017).
De Ieso, P. B., Schick, U., Rosenfelder, N., Mohammed, K. & Ross, G. M. Breast cancer brain metastases—A 12 year review of treatment outcomes. Breast 24, 426–433 (2015).
Hong, A. M. et al. Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: a multicenter, randomized phase III trial. J. Clin. Oncol. 37, 3132–3141 (2019).
Yamamoto, M. et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 15, 387–395 (2014).
Chen, L. et al. Concurrent immune checkpoint inhibitors and stereotactic radiosurgery for brain metastases in non-small cell lung cancer, melanoma, and renal cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 100, 916–925 (2018).
Mehrabian, H., Detsky, J., Soliman, H., Sahgal, A. & Stanisz, G. J. Advanced magnetic resonance imaging techniques in management of brain metastases. Front. Oncol. https://doi.org/10.3389/fonc.2019.00440 (2019).
Brown, P. D. et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 18, 1049–1060 (2017).
Holste, K. G. & Orringer, D. A. Laser interstitial thermal therapy. Neurooncol. Adv. https://doi.org/10.1093/NOAJNL/VDZ035 (2020).
Mirza, F. A., Mitha, R. & Shahzad Shamim, M. Current role of laser interstitial thermal therapy in the current role of laser interstitial thermal therapy in the treatment of intracranial tumors treatment of intracranial tumors. https://doi.org/10.4103/ajns.ajns_185_20 (2020).
Patel, R. R. & Mehta, M. P. Targeted therapy for brain metastases: improving the therapeutic ratio. Clin. Cancer Res. 13, 1675–1683 (2007).
Gondi, V. et al. NRG Oncology CC001: a phase III trial of hippocampal avoidance (HA) in addition to whole-brain radiotherapy (WBRT) plus memantine to preserve neurocognitive function (NCF) in patients with brain metastases (BM). J. Clin. Oncol. https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.2009 (2019).
Noll, K. R. et al. Monitoring of neurocognitive function in the care of patients with brain tumors. Curr. Treat. Opt. Neurol. https://doi.org/10.1007/s11940-019-0573-2 (2019).
Dawood, S. et al. Survival among women with triple receptor-negative breast cancer and brain metastases. Ann. Oncol. 20, 621–627 (2009).
Lin, N. U. & Winer, E. P. Brain metastases: the HER2 paradigm. Clin. Cancer Res. 13, 1648–1655 (2007).
Kim, J. S. & Kim, I. A. Evolving treatment strategies of brain metastases from breast cancer: current status and future direction. Ther. Adv. Med. Oncol. https://doi.org/10.1177/1758835920936117 (2020).
Krop, I. E. et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Ann. Oncol. 26, 113–119 (2015).
Swain, S. M. et al. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: results from the randomized phase III study CLEOPATRA. Ann. Oncol. 25, 1116–1121 (2014).
Saura, C. et al. Impact of neratinib plus capecitabine on outcomes in HER2-positive metastatic breast cancer patients with central nervous system disease at baseline: findings from the phase 3 NALA trial. J. Clin. Oncol. https://doi.org/10.1200/JCO.20.00147 (2020).
Murthy, R. K. et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382, 597–609 (2020).
Batra, A., Kong, S. & Cheung, W. Y. Eligibility of real-world patients with metastatic breast cancer for clinical trials. Breast 54, 171–178 (2020).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Duchnowska, R. et al. Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res. https://doi.org/10.1186/s13058-016-0702-8 (2016).
Priceman, S. J. et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2+ breast cancer metastasis to the brain. Clin. Cancer Res. 24, 95–105 (2018).
Keskin, D. B. et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 565, 234–239 (2019).
Wanleenuwat, P. & Iwanowski, P. Metastases to the central nervous system: molecular basis and clinical considerations. J. Neurol. Sci. https://doi.org/10.1016/j.jns.2020.116755 (2020).
Deeken, J. F. & Löscher, W. The blood-brain barrier and cancer: Transporters, treatment, and trojan horses. Clin. Cancer Res. 13, 1663–1674 (2007).
Kabraji, S. et al. Drug resistance in HER2-positive breast cance brain metastases: blame the barrier or the brain? Clin. Cancer Res. 24, 1795–1804 (2018).
Bailleux, C., Eberst, L. & Bachelot, T. Treatment strategies for breast cancer brain metastases. Br. J. Cancer 124, 142–155 (2021).
Bousquet, G. et al. Intrathecal trastuzumab halts progression of CNS metastases in breast cancer. J. Clin. Oncol. https://doi.org/10.1200/JCO.2012.44.8894 (2016).
Bonneau, C. et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur. J. Cancer 95, 75–84 (2018).
Chen, K. T., Wei, K. C. & Liu, H. L. Theranostic strategy of focused ultrasound induced blood-brain barrier opening for CNS disease treatment. Front. Pharmacol. https://doi.org/10.3389/fphar.2019.00086 (2019).
Kamson, D. O. et al. Differentiation of glioblastomas from metastatic brain tumors by tryptophan uptake and kinetic analysis: a Pet Study with MRI comparison. Mol. Imaging 12, 327 (2013).
Kickingereder, P. et al. Automated quantitative tumour response assessment of MRI in neuro-oncology with artificial neural networks: a multicentre, retrospective study. Lancet Oncol. 20, 728–740 (2019).
Boire, A. et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro-Oncology 21, 571–583 (2019).
Riebensahm, C. et al. Clonality of circulating tumor cells in breast cancer brain metastasis patients. Breast Cancer Res. 21, 101 (2019).
Seoane, J. et al. Cerebrospinal fluid cell-free tumour DNA as a liquid biopsy for primary brain tumours and central nervous system metastases. Ann. Oncol. 30, 211–218 (2019).
Vidula, N. et al. Comparison of the cell-free DNA genomics in patients with metastatic breast cancer (MBC) who develop brain metastases versus those without brain metastases. J. Clin. Oncol. https://ascopubs.org/doi/10.1200/JCO.2020.38.15_suppl.1094 (2020).
Fernandez-Garcia, D. et al. Plasma cell-free DNA (cfDNA) as a predictive and prognostic marker in patients with metastatic breast cancer. Breast Cancer Res. 21, 149 (2019).
Van Tu, D. et al. Expression of angiopoietin-like 4 fibrinogen-like domain increases risk of brain metastases in women with breast cancer. Ann. Oncol. https://doi.org/10.18632/oncotarget.27553 (2019).
Zavala, V. A. et al. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Cancer 124, 315–332 (2021).
Hoj, J. P., Mayro, B. & Pendergast, A. M. A TAZ-AXL-ABL2 feed-forward signaling axis promotes lung adenocarcinoma brain metastasis. Cell Rep. 29, 3421–3434.e8 (2019).
Chen, Q. et al. Effectiveness and safety of pyrotinib, and association of biomarker with progression-free survival in patients with HER2-Positive metastatic breast cancer: a real-world, multicentre analysis. Front. Oncol. https://doi.org/10.3389/fonc.2020.00811 (2020).
Shen, W. et al. THER-01. Preclinical development of EO1001, a novel irreversible brain penetrating PAN-ErbB inhibitor. Neuro-Oncol. Adv. https://doi.org/10.1093/noajnl/vdz014.044 (2019).
Tanaka, Y. et al. Distribution analysis of epertinib in brain metastasis of HER2-positive breast cancer by imaging mass spectrometry and prospect for antitumor activity. Sci. Rep. https://doi.org/10.1038/s41598-017-18702-2 (2018).
Nguyen, L. V., Searle, K. & Jerzak, K. J. Central nervous system-specific efficacy of CDK4/6 inhibitors in randomized controlled trials for metastatic breast cancer. Oncotarget 10, 6317–6322 (2019).
Niehr, F. et al. Combination therapy with vemurafenib (PLX4032/RG7204) and metformin in melanoma cell lines with distinct driver mutations. J. Transl. Med. https://doi.org/10.1186/1479-5876-9-76 (2011).
Exman, P., Mallery, R. M., Lin, N. U. & Parsons, H. A. Response to olaparib in a patient with germline BRCA2 mutation and breast cancer leptomeningeal carcinomatosis. npj Breast Cancer https://doi.org/10.1038/s41523-019-0139-1 (2019).
Han, H. S. et al. Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic breast cancer: randomized phase II study. Ann. Oncol. 29, 154–161 (2018).
Anders, C. et al. TBCRC 018: phase II study of iniparib in combination with irinotecan to treat progressive triple negative breast cancer brain metastases. Breast Cancer Res. Treat. 146, 557–566 (2014).
Vinayak, S. et al. Open-label clinical trial of niraparib combined with pembrolizumab for treatment of advanced or metastatic triple-negative breast cancer. JAMA Oncol. 5, 1132–1140 (2019).
Mohammad, A. S. et al. Liposomal irinotecan accumulates in metastatic lesions, crosses the blood-tumor barrier (BTB), and prolongs survival in an experimental model of brain metastases of triple negative breast cancer. Pharm. Res. 35, 31 (2018).
Zimmer, A. S. et al. Temozolomide in secondary prevention of HER2-positive breast cancer brain metastases. Future Oncol. 16, 899–909 (2020).
O’Shaughnessy, J. et al. CONTESSA: a multinational, multicenter, randomized, phase III registration study of tesetaxel plus a reduced dose of capecitabine in patients (pts) with HER2-, hormone receptor+ (HR+) locally advanced or metastatic breast cancer (LA/MBC) who have previously received a taxane. J. Clin. Oncol. https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.TPS1107 (2019).
Keith, K. C., Lee, Y., Ewend, M. G., Zagar, T. M. & Anders, C. K. Activity of trastuzumab-emtansine (TDM1) in HER2-positive breast cancer brain metastases: a case series. Cancer Treat. Commun. 7, 43–46 (2016).
Bardia, A. et al. Efficacy & safety of anti-Trop-2 antibody drug conjugate sacituzumab govitecan (IMMU-132) in heavily pretreated patients with metastatic triple-negative breast cancer. J. Clin. Oncol. 35, 2141–2148 (2017).
Modi, S. et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N. Engl. J. Med. 382, 610–621 (2020).
Freedman, R. A. et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J. Clin. Oncol. 37, 1081–1089 (2019).
Saura, C. et al. Neratinib+ capecitabine versus lapatinib+ capecitabine in patients with HER2+ metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Findings from the multinational, randomized, phase III NALA trial. J. Clin. Oncol. https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.1002 (2019).
Anders, C. K. et al. A phase II study of abemaciclib in patients (pts) with brain metastases (BM) secondary to HR+, HER2− metastatic breast cancer (MBC). J. Clin. Oncol. https://ascopubs.org/doi/abs/10.1200/JCO.2019.37.15_suppl.1017 (2019).
Tolaney, S. M. et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin. Cancer Res. 26, 5310–5319 (2020).
Zimmerman, S. et al. 2017–2018 Scientific advances in thoracic oncology: small cell lung cancer. J. Thorac. Oncol. 14, 768–783 (2019).
Lin, N. U. et al. Pertuzumab plus high-dose trastuzumab in patients with progressive brain metastases and HER2-positive metastatic breast cancer: primary analysis of a phase II study. J. Clin. Oncol., https://doi.org/10.1200/JCO.20.02822 (2021).
Morikawa, A. et al. Phase I study of intermittent high-dose lapatinib alternating with capecitabine for HER2-positive breast cancer patients with central nervous system metastases. Clin. Cancer Res. 25, 3784–3792 (2019).
Metzger Filho, O. et al. Phase I dose-escalation trial of tucatinib in combination with trastuzumab in patients with HER2-positive breast cancer brain metastases. Ann. Oncol. 31, 1231–1239 (2020).
Acknowledgements
The work in the Gilkes laboratory is supported by U54-CA210173 (NCI), Susan G. Komen Foundation (CCR17483484), The Jayne Koskinas Ted Giovanis Foundation for Health and Policy, The Emerson Collective, and the SKCCC Core Grant (P50CA006973; NCI). Vered Stearns acknowledges Centers for Disease Control and Prevention (NU58DP006673) grant which focuses on supporting needs of women with metastatic breast cancer as well as the SKCCC Core Grant (P50CA006973; NCI).
Author information
Authors and Affiliations
Contributions
All authors contributed to the overall outline of the review. N.J. prepared the first draft. V.S. wrote the targeted therapeutics section. K.R. wrote the radiation oncology section. C.H. and K.R. wrote the advocates perspective that was incorporated into each section and wrote the patients perspective section. C.H. and N.J. prepared the tables involving clinical trials. D.G. extensively edited the review. All authors contributed to editing the final version review.
Corresponding author
Ethics declarations
Competing interests
V.S. has received research grants to JHU from Abbvie, Biocept, Pfizer, Novartis, and Puma Biotechnology and is a member of the Data Safety Monitoring Board at Immunomedics, Inc. K.R. has received travel expenses from Brainlab, Accuray, and Elekta, research funding from Accuracy and Elekta, honorariums from Elekta and NCCN, and is a member of the Data Safety Monitoring Board at Biomimtix. C.H. has received funding from Roche/Genentech (honoraria, consulting/advisory), Pfizer (consulting/advisory), Johns Hopkins Hospital (consulting/advisory), NIH/NCI Cancer Moonshot (consulting/advisory), and Eli Lilly (travel, accommodations, expenses). D.G., L.K., and N.J. have no conflicts to disclose.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joe, N.S., Hodgdon, C., Kraemer, L. et al. A common goal to CARE: Cancer Advocates, Researchers, and Clinicians Explore current treatments and clinical trials for breast cancer brain metastases. npj Breast Cancer 7, 121 (2021). https://doi.org/10.1038/s41523-021-00326-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41523-021-00326-5
- Springer Nature Limited