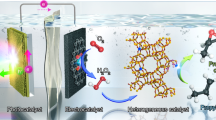
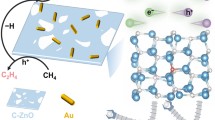
Abstract
The selective oxidative dehydrogenation of ethane (ODHE) is attracting increasing attention as a method for ethylene production. Typically, thermocatalysts operating at high temperatures are needed for C–H activation in ethane. In this study, we describe a low temperature ( < 140 °C) photocatalytic route for ODHE, using O2 as the oxidant. A photocatalyst containing PdZn intermetallic nanoparticles supported on ZnO is prepared, affording an ethylene production rate of 46.4 mmol g–1 h–1 with 92.6% ethylene selectivity under 365 nm irradiation. When we employ a simulated shale gas feed, the photocatalytic ODHE system achieves nearly 20% ethane conversion while maintaining an ethylene selectivity of about 87%. The robust interface between the PdZn intermetallic nanoparticles and ZnO support plays a crucial role in ethane activation through a photo-assisted Mars-van Krevelen mechanism, followed by a rapid lattice oxygen replenishment to complete the reaction cycle. Our findings demonstrate that photocatalytic ODHE is a promising method for alkane-to-alkene conversions under mild conditions.
Similar content being viewed by others
Introduction
Ethylene is one of the most widely used feedstocks in the today’s chemical industry. Currently, high-temperature steam cracking of large hydrocarbons derived from naphtha is the primary method for producing ethylene. The recent discovery of large reserves of shale gas, which contains about 10% ethane (C2H6), has sparked interest in ethane dehydrogenation as an alternative method for producing ethylene1. Researchers are now actively seeking new catalytic materials and technologies capable of efficiently and selectively converting ethane to ethylene.
In recent years, significant progress has been made towards the discovery of efficient catalysts for ethane dehydrogenation, with zeolite-derived and vanadium-based catalysts receiving a lot of attention2,3. Given that the C–H bond energy in C2H6 is exceptionally high (415 kJ mol–1), harsh reaction conditions are typically required to achieve the first hydrogen atom extraction from ethane (the rate-limiting step for direct ethane dehydrogenation)4. Compared with direct ethane dehydrogenation, the oxidative dehydrogenation of ethane (ODHE), which utilizes an oxidant (e.g. O2, CO2, or N2O), is more thermodynamically favorable (for example, C2H6 + 0.5O2 → C2H4 + H2O, ΔGr = –136.2 kJ mol–1) and can deliver both good ethane conversions and acceptable ethylene selectivities5. However, thermochemical ODHE still requires high reaction temperatures ( > 500 °C) to achieve a meaningful ethane conversion, stimulating research towards alternative low-temperature ODHE processes powered by sustainable energy sources6.
Photocatalysis holds great potential for the solar-driven activation of small molecules such as O2, CO2, and CH4 under mild conditions. Metal oxide-based semiconductor photocatalysts, such as ZnO and TiO2, have the ability to generate activated lattice oxygen upon illumination7,8, enabling activation of inert chemical bonds via lattice oxygen-mediated photocatalytic oxidative pathways. For examples, Au-ZnO/TiO27, Au(Pt, Pd, Ag)/ZnO9, and Ag/ZnO10 photocatalysts show activity for the photocatalytic methane coupling, partial oxidation, and combustion, respectively. These catalysts all use photogenerated active oxygen species (O–) derived from TiO2 or ZnO lattice oxygen for C–H bond activation. Moreover, the introduction of metal (metal alloy) nanoparticles with appropriate metal–support interaction boosts interfacial charge transfer and surface active adsorbent conversion, thus speeding up the catalytic reaction kinetics11. Tang et al. reported that AuCu−ZnO photocatalyst achieved methane partial oxidation for methanol and formaldehyde production with efficient charge transfer enhanced by Au and Cu species12. Whilst photocatalytic C–H bond activation in methane conversion has received considerable attention, no research has yet been conducted on photocatalytic ODHE for ethylene production, motivating a detailed investigation.
Herein, we synthesized PdZn intermetallic nanoparticles supported on ZnO (PdZn-ZnO) as a photocatalyst for ODHE with O2. Under 365 nm illumination, a flow photocatalytic system containing PdZn-ZnO delivered a C2H4 production rate of 46.4 mmol g–1 h–1 with 92.6% selectivity. This level of performance was vastly superior to that of photocatalysts containing Pd or other intermetallic nanoparticles on ZnO, whilst also outperforming most high-temperature thermochemical ODHE processes. Our comprehensive characterization studies demonstrate that a robust PdZn-ZnO interface effectively enhances the photogeneration of O– (from ZnO lattice oxygen), which in turn activates the C–H bond in ethane. Further, the PdZn-ZnO metal-support interaction allows fast electron transfer for efficient dioxygen reduction and ZnO lattice oxygen replenishment. The combination of these processes significantly reduced the apparent activation energy for ODHE to only 18.4 kJ mol–1, with PdZn-ZnO delivering an ethane conversion of nearly 20% with about 87% selectivity in simulated shale gas and showing feasibilities in the selective oxidative dehydrogenation of propane and butane. Results identify photocatalysis as a promising strategy for the selective production of ethylene from ethane at low temperatures.
Results and Discussion
Synthesis and characterization of PdZn-ZnO photocatalysts
The X-ray diffraction (XRD) pattern of the Pd-doped Zn5(CO3)2(OH)6 precursor is shown in Supplementary Fig. 1. All peaks can be assigned to monoclinic hydrozincite, Zn5(CO3)2(OH)6 (JCPDS No. 19-1458)13. No peaks were seen for any Pd-containing species, indicating that Pd was highly dispersed in the sample14. Transmission electron microscopy (TEM) revealed Pd-doped Zn5(CO3)2(OH)6 possessed a nanosheet-like structure, with energy dispersive X-ray spectroscopy (EDS) confirming that Zn, O, and Pd were uniformly dispersed in the sample (Supplementary Fig. 2). After annealing at 300 °C in a H2 flow (10 vol.% H2 in Ar) for 4 h, the precursor was transformed into a PdZn-ZnO catalyst. As shown in the XRD pattern (Fig. 1a), the PdZn-ZnO catalyst consists of hexagonal wurtzite ZnO (JCPDS No. 36-1451) and tetragonal intermetallic PdZn nanoparticles (JCPDS No. 06-0620)15,16 After dissolving the ZnO support with dilute hydrochloric acid, the isolated PdZn nanoparticles show a XRD pattern matched well with the predicted PdZn intermetallic nanoparticle (Supplementary Fig. 3). The Pd loading in PdZn-ZnO was determined to be 1.57 wt.%, which was close to the expected nominal value (2.0 wt.%) (Supplementary Table 1). The high-angle annular dark-field scanning TEM (HAADF-STEM) image and EDS element maps confirmed the presence of PdZn intermetallic nanoparticles dispersed on ZnO nanoparticles (Fig. 1b, c). Lattice fringes with spacings of 0.28, 0.25, and 0.22 nm corresponded to ZnO (100), ZnO (101) and PdZn (101) planes, respectively17,18. For comparison, photocatalysts consisting of pristine ZnO and ZnO-supported Pd nanoparticles (Pd-ZnO) were prepared. The structure, morphology, and specific surface area of ZnO and Pd-ZnO photocatalysts were similar to that of PdZn-ZnO (Supplementary Fig. 4 to 6).
a XRD patterns. Inset: crystal structure of PdZn. b HAADF-STEM image. c Energy-dispersive X-ray element maps. d Projection in the [010] direction for tetragonal PdZn. The yellow and blue spheres represent Zn and Pd atoms, respectively. e Aberration-corrected HAADF-STEM image. Inset: schematic of the PdZn intermetallic structure. f Intensity profiles measured from (e). g Aberration-corrected HAADF-STEM image and corresponding atomic-resolution elemental maps.
The (100) crystal plane of PdZn shows an interphase arrangement of Pd and Zn atomic columns and contains of (001) and (010) planes (Fig. 1d). The aberration-corrected HAADF-STEM image of a PdZn nanoparticle (Fig. 1e) in PdZn-ZnO shows highly ordered rectangular arrays with alternating bright and dark columns of atoms. The fast Fourier-transform (FFT) pattern is consistent with the simulation results for a PdZn intermetallic nanoparticle in the [010] direction (Supplementary Fig. 7). The corresponding intensity profile presents a periodic oscillation pattern in two perpendicular directions corresponding to [001] (Fig. 1f). The regular atomic configurations of Pd and Zn atoms in the PdZn nanoparticle were also evident in the corresponding elemental maps (Fig. 1g), further confirming the presence of PdZn intermetallic nanoparticles in PdZn-ZnO.
Photocatalytic ODHE performance evaluation
Photocatalytic ODHE tests were carried out using a home-made continuous flow photoreactor equipped with a 365 nm LED lamp as the light source (see Methods for experimental details). A mixture of C2H6 (5 vol.% in Ar, 18 mL min–1) and O2 (1 vol.% in Ar, 12 mL min–1) with a total flow rate of 30 mL min–1 was introduced as the feed gas. Figure 2a and Supplementary Table 2 show the photocatalytic C2H4 production rate and C2H4 selectivity for PdZn-ZnO and a series of reference samples. PdZn-ZnO exhibited a C2H4 production rate of 46.4 mmol g–1 h–1 with an ethylene selectivity of 92.6%. No liquid carbon-containing products, such as methanol and ethanol, were formed during the photocatalytic reaction (Supplementary Fig. 8). The optimal photocatalyst dosage and nominal Pd loading were determined to be 5.0 mg and 2.0 wt.%, respectively (Supplementary Fig. 9 to 11). The calcination temperature of the precursor was optimized to be 300 °C (Supplementary Fig. 12 and 13). The Pd-ZnO reference delivered a C2H4 production rate of 15.6 mmol g–1 h–1 with 83.4% ethylene selectivity, which is significantly lower than PdZn-ZnO. It should be noted that for pristine ZnO, only CO2 and traces of C2H4 were generated, with no obvious enhancement observed for a physical mixture of ZnO with PdZn nanoparticles (denoted as PdZn-ZnO-mix, Supplementary Fig. 14). We also prepared other intermetallic nanoparticles (AgZn, AuZn, PtZn, CuZn) supported on ZnO (denoted as MZn-ZnO, M = Ag, Au, Pt, and Cu) using methods similar to that used to prepare PdZn-ZnO (Supplementary Fig. 15). However, CO2 was the major product for all these photocatalysts. In addition, catalysts containing PdZn nanoparticles on other metal oxide supports, including TiO2, In2O3, CeO2, and Al2O3 were prepared (Supplementary Fig. 16). PdZn-TiO2 prefer to over-oxidize C2H6 to CO2 with a low C2H4 selectivity of 47.6%. PdZn-In2O3, PdZn-CeO2, and PdZn-Al2O3 showed negligible activity under the same reaction conditions. Clearly, the ZnO support actively participated in photocatalytic ODHE over PdZn-ZnO. Above results indicated that PdZn intermetallic nanoparticles, ZnO, and the interfacial contact between PdZn and ZnO were indispensable to the outstanding photocatalytic performance.
a ODHE tests over PdZn-ZnO and comparison photocatalysts. Reaction condition: 5.0 mg photocatalyst, C2H6 (5 vol.% in Ar, 18 mL min–1) + O2 (1 vol.% in Ar, 12 mL min–1), 365 nm LED, 600 mW cm–2. b Photocatalytic activity at a total flow rate of 30 mL min–1 with different C2H6/O2 feed gas ratios. c Photocatalytic stability test for 12 h of continuous irradiation. d Performance comparison with reported thermocatalysts. e Photocatalytic activity at 136 °C under dark-light switch conditions. f Arrhenius plots measured in the dark and light conditions. Light intensity = 55.9 mW cm–2. g Wavelength-dependent AQE and diffuse reflectance spectra of ZnO and PdZn-ZnO. Error bars represent standard deviations obtained from three independent measurements.
Figure 2b shows that the C2H6/O2 ratio had a strong influence on the activity and selectivity of PdZn-ZnO for ethane-to-ethylene conversion. The C2H4 selectivity decreased from nearly 100% to 67.7% as the C2H6 (5 vol.% in Ar)/O2 (1 vol.% in Ar) feed gas ratio was changed from 30/0 to 6/24 (total flow rate = 30 mL min–1). The production rate of C2H4 followed a volcano-type relationship, with the highest rate achieved at a feed gas ratio of 18/12. The effect of the total flow rate was then investigated at the C2H6/O2 ratio of 18/12 (Supplementary Fig. 17). The C2H4 production rate increased gradually from 5 mL min–1 to 30 mL min–1, reaching a maximum of 46.4 mmol g–1 h–1. The selectivity to C2H4 remained above 92% under these flow conditions. For a batch reactor (flow rate = 0), the best performance was realized after about 180 s of irradiation (15.0% C2H6 conversion, 6579.3 μmol g–1 C2H4 production, and 85% ethylene selectivity, Supplementary Fig. 18), with the short time to reach the optimum being due to depletion of O2 in the batch reactor. At higher reactant concentrations, the ethylene production rate exhibited a substantial increase, reaching up to 225.9 mmol g–1 h–1. Notably, the ethylene selectivity showed a gradual decline from 92.6% to 61.9% as the concentration of reactants increased (Supplementary Fig. 19). This suggests the importance of finding a balance between the rate of product formation and selectivity. During a 12 h of continuous photocatalytic stability test under the optimized reaction condition, the production rate of C2H4 over PdZn-ZnO fluctuated around 45 mmol g–1 h–1 with no obvious deactivation and a stable ethylene selectivity of around 92% (Fig. 2c). The spent catalyst showed almost no structural changes as confirmed by XRD (Supplementary Fig. 20). The nearly 100% carbon balance, supported by thermogravimetric and Raman spectroscopy analyses, confirms the absence of carbon deposition (Supplementary Fig. 21-23).
As shown in Fig. 2d and Supplementary Table 3, the photocatalytic ODHE performance of PdZn-ZnO was superior to representative thermocatalysts reported to date when considering the three main reaction parameters: temperature, C2H4 production rate, and selectivity19,20,21,22,23,24,25,26,27,28,29,30. Moreover, the as-developed PdZn-ZnO photocatalytic system also demonstrated exceptional performance for the oxidative dehydrogenation of propane (a propene production rate of 54.0 mmol g–1 h–1 with 89.4% propene selectivity) and butane (a butene production rate of 59.1 mmol g–1 h–1 with 95.9% butene selectivity) (Supplementary Fig. 24). Additionally, by replacing the ethane feed gas with a simulated shale gas (45 vol.% CH4, 5 vol.% C2H6, balanced with Ar), a C2H4 production rate of about 60 mmol g–1 h–1 was achieved, with about 87% ethylene selectivity at nearly 20% ethane conversion (Supplementary Fig. 25).
It is noteworthy that the surface temperature of the PdZn-ZnO photocatalyst increased under light irradiation due to the photothermal effects, and reached 136 °C at 600 mW cm–2. (Supplementary Fig. 26a). As depicted in Fig. 2e, no significant product formation was observed by electrically heating the reactor containing PdZn-ZnO to 136 °C in the dark. However, on exposing the photocatalyst to light irradiation, the C2H4 production rate was greatly enhanced at the same temperature, and showed a linear relationship with the light intensity (Supplementary Fig. 26b). The results indicate that the ethane oxidative dehydrogenation process involved a photocatalytic mechanism. Arrhenius plots based on ethane reaction rates (Rethane) at different temperatures are shown in Fig. 2g. The apparent activation energy for ODHE over PdZn-ZnO under light conditions (18.4 kJ mol–1) was significantly lower than in the dark (66.9 kJ mol–1), implying that photons changed the reaction pathway for ODHE and significantly reduced the reaction energy barrier. Rethane values decreased under light conditions at temperatures greater than 180 °C, which is explained by shortened lifetime of photogenerated charge carriers and increased oxygen consumption due to ethane overoxidation at such high temperatures (Supplementary Fig. 27 and 28)31. The wavelength-dependent apparent quantum efficiency (AQE) for photocatalytic ODHE over PdZn-ZnO was found to be 7.2%, 5.5%, 2.8%, and 0.3% under monochromatic irradiation at 350, 365, 380, and 400 nm, respectively (Fig. 2f). This trend closely matched the absorbance spectrum of ZnO, indicating that photo-excitation of ZnO was a key process in ethylene production.
Mechanistic investigations
PdZn-ZnO and two reference samples, ZnO and Pd-ZnO, were selected for further mechanistic investigations. Firstly, temperature-programmed desorption experiments for C2H6 and O2 (C2H6-TPD and O2-TPD) were conducted, with the results shown in Fig. 3a. The data show that C2H6 (m/z = 30) desorbed at 190.7, 233.8 and 254.6 °C on ZnO, Pd-ZnO, and PdZn-ZnO, respectively. Clearly, PdZn-ZnO shows a higher desorption temperature than the other samples, indicating a strong chemical adsorption towards C2H6. Furthermore, PdZn-ZnO demonstrates the highest ethane adsorption strength when compared to PdZn-TiO2, PdZn-In2O3, PdZn-CeO2, and PdZn-Al2O3 (Supplementary Fig. 29). This heightened ethane adsorption capability could be a key factor contributing to the significantly improved photocatalytic ODHE performance observed in PdZn-ZnO compared to its counterparts. In the O2-TPD profiles, O2 (m/z = 32) desorption signals were observed between 400 and 500 °C for all three samples, which can be ascribed to the desorption of chemisorbed oxygen species. The O2 adsorption capacity of pristine ZnO was modest, and largely attributable to O2 adsorption/dissociation on oxygen defects produced by sample pretreatment in a hydrogen atmosphere32. Once Pd or PdZn nanoparticles were introduced, the amount of adsorbed oxygen increased significantly, indicating that Pd and PdZn were the dominant active sites for O2 adsorption and activation33.
a C2H6-TPD and O2-TPD spectra for ZnO, Pd-ZnO and PdZn-ZnO. b In situ EPR spectra for ZnO exposed to Ar in the dark and under 365 nm irradiation, and exposed to C2H6 under 365 nm irradiation. c Evolution of reactants and products during ODHE monitored by online MS. d Time-resolved online MS data for C2H6 dehydrogenation over ZnO, Pd-ZnO and PdZn-ZnO. e In situ FT-IR spectra collected from PdZn-ZnO in a feed gas (3 vol.% C2H6 + 0.4 vol.% O2, balanced with Ar) at different irradiation times.
The efficient separation and transport of photogenerated charge carriers are critical for photocatalytic reactions34. The photoluminescence (PL) intensity under 365 nm excitation was notably decreased for Pd-ZnO and PdZn-ZnO compared with pristine ZnO, indicating improved separation of photo-excited electron-hole pairs (Supplementary Fig. 30). To investigate the carrier dynamics further, time-resolved transient absorption (TA) spectra were then recorded under 365 nm excitation and probed at 520 nm (Supplementary Fig. 31). The results showed that PdZn-ZnO had the longest lifetime of photogenerated charge carriers (187.3 ps), compared to Pd-ZnO (132.9 ps) and ZnO (118.6 ps), confirming its superior carrier separation efficiency. Next, in situ electron paramagnetic resonance (EPR) experiments were conducted to investigate the electron transfer and ethane activation processes. As shown in Fig. 3b and Supplementary Fig. 32, under dark conditions exposed to Ar atmosphere, both ZnO and PdZn-ZnO showed a symmetric EPR peak with a g value of 2.003, which could be attributed to unpaired electrons trapped at oxygen vacancies (Ov) in ZnO35. Under light irradiation, a symmetric EPR peak at g = 1.959 appeared for ZnO due to trapped photogenerated electrons (i.e. Zn+ species)36. However, this trapped electron signal was weak for PdZn-ZnO, suggesting that the excited electrons in ZnO were transferred to PdZn nanoparticles, which acted as electron acceptors37. Additionally, anisotropic EPR signals, namely gx = 2.023, gy = 2.019, and gz = 2.003, also appeared under irradiation, which can be assigned to O– species created by the ZnO lattice oxygen (formally O2–) trapping photogenerated holes38. After switching the contacting atmosphere from Ar to C2H6 under light conditions, the intensity of the EPR signal related to O– species decreased significantly for both ZnO and PdZn-ZnO, inferring the reaction of surface O– with C2H6 molecules. The results provide strong evidence for enhanced interfacial electron transfer from ZnO to PdZn in PdZn-ZnO, whilst also establishing the crucial role of photogenerated surface O– derived from lattice oxygen in C2H6 activation.
Oxygen isotope labeling tracing experiments were utilized to explore the key role of lattice oxygen in photocatalytic ODHE. Figure 3c shows the results of the 18O isotope experiments, with online mass spectrometry (MS) used to detect 18O-containing products of ODHE (see Methods for more details). Firstly, a gas mixture containing C2H6 and 18O2 was fed to the reactor in the dark to remove residual gas impurities in the flow chamber. Upon irradiation of PdZn-ZnO, strong signals due to H216O and C16O18O were observed confirming that surface lattice oxygen (16O) on ZnO was an active site for water production and ethane conversion during photocatalytic ODHE. This result was further corroborated by the 18O-tracer experiments (Supplementary Fig. 33), in which 18O had been pre-incorporated in the ZnO lattice of PdZn-ZnO. After 10 min of illumination, the light was turned-off and the atmosphere switched to a gas mixture of C2H6 and 16O2. The detection of 18O in the oxygen-containing gas products (H218O, C16O18O) confirmed that 18O from the 18O2 feed replenished Ov during the first irradiation period and could participate in the following ODHE reaction. This lattice oxygen replenishment process was also confirmed by the light-induced oxygen isotope exchange effect between 18O2 and PdZn-ZnO (Supplementary Fig. 34).
Time-resolved online MS spectra for C2H6 dehydrogenation over PdZn-ZnO, Pd-ZnO, and ZnO were then collected (Fig. 3d). An ethane gas flow was first introduced into the reactor, with a H2O signal being detected for all photocatalysts under light irradiation as a result of lattice oxygen consumption and Ov generation on ZnO during the ODHE reaction. The light was then turned off and the atmosphere switched to O2 for a period of time. When the light was again turned on, the signal for O2 in the gas phase decayed, indicating the consumption of O2 to fill Ov sites. PdZn-ZnO showed the highest water generation and dioxygen consumption signals under illumination, indicating a significantly promoted lattice oxygen-mediated photocatalytic ODHE through a photo-enhanced Mars-van Krevelen (M-K) pathway. That is, the photogenerated surface O– from lattice oxygen on ZnO extracts hydrogen atoms from ethane, resulting in the formation of water molecules and Ov. Then, O2 adsorbed on PdZn is activated by photo-excited electrons, with the O2 molecule dissociating to fill Ov and complete the reaction cycle.
In situ Fourier transform infrared (FT-IR) spectra were collected to gain additional information about the reaction intermediates and reaction mechanism (see Methods for experiment details). As shown in Fig. 3e, a series of absorption peaks appeared over PdZn-ZnO in a feed gas (3 vol.% C2H6 + 0.4 vol.% O2, balanced with Ar) under light conditions, with the intensities of the peaks gradually increasing with irradiation time. A Zn–OH bending vibration at 1417 cm–1 was detected39, indicating the presence of surface-adsorbed hydroxyl groups (*OH) generated by hydrogen atom extraction from ethane on surface lattice oxygen (i.e. photo-generated O– sites) of ZnO40. The absorption peaks at 1445/1620 cm–1, together with the absorption band around 2950 cm–1, could be assigned to C = C and C–H stretching vibrations of adsorbed C2 intermediates (*C2H4+n, n = 0, 1, 2)41,42. The peaks at 1569 cm–1 and 1340 cm–1 were assigned to adsorbed carboxyl species (HCOO*) species associated with ethane overoxidation products, which were also evidences by gaseous CO2 peaks at 2334 cm–1 and 2362 cm–1 43. The FT-IR results demonstrate the formation of surface-adsorbed *OH, *C2H4+n, and HCOO* during ODHE over PdZn-ZnO. These are the expected intermediates for lattice oxygen-mediated photocatalytic ODHE (along with some minor intermediates and products associated with the over-oxidation of ethane).
To gain a better understanding of the electron transfer processes and reaction transition states (TS) occurring at the interface between the metal nanoparticle (PdZn or Pd) and the ZnO support, density functional theory (DFT) calculations were performed. Two models were built and optimized to simulate the structure of PdZn-ZnO and Pd-ZnO. A Pd15Zn15 cluster supported on ZnO(101) (denoted as Pd15Zn15-ZnO) was used to represent PdZn-ZnO, whilst a Pd30 cluster supported on ZnO(101) (denoted as Pd30-ZnO) was used to represent Pd-ZnO, as shown in Fig. 4a, d. A charge density difference comparison between the two models revealed that charge redistribution is localized in a limited region near the interface between metal nanoparticles and ZnO supports (Fig. 4b, e). Bader charge analysis predicted increased charge accumulation ( + 8.32 qe) on the Pd15Zn15 cluster (Fig. 4c) compared to the Pd30 cluster ( + 5.82 qe, Fig. 4f), indicating that electron transfer from PdZn to ZnO was enhanced compared to electron transfer from Pd to ZnO. The higher binding energy of the Pd 3d signal in PdZn-ZnO than in Pd-ZnO also signifies a more pronounced electron deficiency state associated with interfacial electron transfer (Supplementary Fig. 35). DFT calculations also showed that the strong interfacial electron delocalization and transfer in Pd15Zn15-ZnO resulted in a more negative Pd d-band center position (–1.68 eV) compared with Pd30-ZnO (–1.54 eV) (Fig. 4g). The more negative d-band center in the case of supported PdZn contributes to a downshifted antibonding state for oxygen adsorbates, leading to an increased d orbital occupancy and weakened adsorption energy of activated oxygen adsorbates44,45. This was expected to be beneficial for subsequent lattice oxygen replenishment processes on adjacent ZnO during ODHE.
a and d Model structures for Pd15Zn15-ZnO (a) and Pd30-ZnO (d). b and e Charge density difference plots for Pd15Zn15-ZnO (b) and Pd30-ZnO (e). The Pd, Zn, and O atoms are shown in purple, gray, and red. The yellow and cyan surfaces correspond to regions of charge gain (accumulation) and loss (depletion). The isovalue of the isosurfaces is 3.0 × e Å–3. (c and f) Bader charge distributions for Pd15Zn15-ZnO (c) and Pd30-ZnO (f). The red and blue spheres indicate the extent of charge depletion (positive) and accumulation (negative). g Density of states of Pd atoms in Pd15Zn15-ZnO and Pd30-ZnO. The horizontal dashed lines indicate the calculated d-band center. E-EF represents the energy relative to the Fermi energy level. h Calculated potential energy diagrams for ODHE to C2H4 on Pd15Zn15-ZnO and Pd30-ZnO.
The reaction kinetics of dioxygen activation and ethane dehydrogenation to ethylene over Pd15Zn15-ZnO and Pd30-ZnO were then simulated, and the energy profiles are shown in Fig. 4h. Additional information on the models and energy values are summarized in Supplementary Fig. 36-37 and Supplementary Table 4-5. The adsorption energy of O2 on Pd15Zn15-ZnO was found to be significantly higher (–2.35 eV) than that on Pd30-ZnO (–1.54 eV). The adsorbed oxygen (*O2) then dissociates to two oxygen atoms (*O2 → 2*O, TS1), with activation barriers (Ea) of 0.68 eV and 1.08 eV on Pd15Zn15-ZnO and Pd30-ZnO, respectively. These results are consistent with the rapid oxygen consumption over PdZn-ZnO observed on the time-resolved online MS (Fig. 3d).
Pd15Zn15-ZnO was found to have an Ea of 0.32 eV for *O-assisted C–H bond scission in C2H6 (*O + *C2H6 → *C2H5 + *OH, TS2), which was 0.54 eV lower than that of Pd30-ZnO, indicating that ethane activation was kinetically favored over PdZn-ZnO. Subsequently, the β-H of *C2H5 is extracted to form *C2H4 and *H on ZnO (TS3). Then, *OH and *H combine to form H2O (TS4) via two possible pathways, with the oxygen in H2O coming from either surface lattice oxygen (M-K pathway) or dissociated dioxygen (Langmuir-Hinshelwood, L-H pathway). The barriers of these two pathways over Pd15Zn15-ZnO were calculated to be 0.71 eV and 0.92 eV, respectively (Supplementary Fig. 38 and 39), suggesting that the M-K pathway is kinetically favored. The M-K pathway leaves an Ov on ZnO and an additional *O on PdZn, respectively, followed by a ZnO lattice oxygen replenishment process between the two species (TS5) with an Ea of 0.81 eV. Pd30-ZnO exhibits larger Ea for both TS4 and TS5 compared with Pd15Zn15-ZnO (Supplementary Fig. 40 and 41). We compared the energy profiles of the first two reaction steps (TS1 and TS2 as potential rate-determining steps) with models constructed for other Pd-based intermetallic nanoparticles on ZnO (Pd15In15-ZnO, Pd15Cu15-ZnO and Pd15Ni15-ZnO). Results showed that Pd15Zn15-ZnO exhibited the lowest Ea for both oxygen activation and hydrogen extraction from ethane, validating its outstanding photocatalytic ODHE performance (Supplementary Fig. 42 and 43).
Based on the results presented, we propose a probable reaction mechanism for the selective photocatalytic ODHE over PdZn-ZnO (Fig. 5). Initially, photoexcited electron-hole pairs are generated in ZnO under UV (365 nm) irradiation. The photogenerated electrons in ZnO are efficiently transferred to PdZn nanoparticles, activating O2 to adsorbed oxygen atoms. Meanwhile, the photogenerated holes reaching the surface lattice oxygen of ZnO to form O–, which activates C–H bond scission in C2H6 to form *C2H5 and *OH. Subsequently, β-H cleavage in *C2H5 leads to the formation of *C2H4 and a water molecule, both of which desorb from the photocatalysts (leaving an Ov on the ZnO surface). Finally, the Ov is filled by the adsorbed oxygen atom spilling over from the PdZn sites.
In summary, we report the first example of selective photocatalytic ODHE with O2 for ethylene production. A PdZn-ZnO photocatalyst, containing PdZn intermetallic nanoparticles, was prepared via thermal hydrogen reduction of a Pd-doped Zn5(CO3)2(OH)6 precursor. The strong electron transfer interactions at the PdZn-ZnO interface promotes the photogeneration of surface O– active sites on ZnO to activate the C–H bonds in ethane, leading to the formation of ethylene and water. Photo-excited electron transfer from ZnO to PdZn facilitated dioxygen dissociation, allowing fast replenishment of ZnO lattice oxygen consumed in the Mars-van Krevelen mechanism. Photocatalytic ODHE on PdZn-ZnO had an apparent activation energy of 18.4 kJ mol–1 under irradiation conditions, much lower than that in the dark (66.9 kJ mol–1). The PdZn-ZnO photocatalyst shows an ethylene production rate of 46.4 mmol g–1 h–1 with 92.6% selectivity at low temperatures, exhibiting high alkene production rates and selectivity for photocatalytic dehydrogenation of propane and butane, as well as ethane in simulated shale gas. This work offers a proof-of-concept investigation, demonstrating that low-temperature photocatalytic ODHE is a promising new approach for ethylene production from ethane-containing feedstocks. Future work should be directed towards more detailed investigation of reaction mechanisms and also reactor designs for process scale-up.
Methods
Chemicals
Zn(NO3)2·6H2O, Na2CO3 and Cu(NO3)2·3H2O were obtained from Beijing Innochem Science & Technology Co., Ltd. Na2PdCl4, H2PtCl6, AgNO3, HAuCl4 were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. C2H6 (5 vol.% in Ar), O2 (1 vol.% in Ar) and a mixed reactant gas (3 vol.% C2H6, 1 vol.% O2, balanced with Ar) were purchased from Beijing Beiyang United Gas Co., Ltd. 18O isotopically labeled oxygen (1 vol.% 18O2 in Ar) was obtained from Shanghai Maotoo Specialty Gases Co., Ltd. All materials were used without further purification. Deionized water was used in the synthesis of all catalysts.
Preparation of catalysts
-
(a)
PdZn-ZnO. Pd-doped Zn5(CO3)2(OH)6 precursor (Pd-Zn5(CO3)2(OH)6) was synthesized according a commonly used coprecipitation method. Typically, for the preparation of the PdZn-ZnO photocatalyst, 2.97 g Zn(NO3)2·6H2O and a certain amount of Na2PdCl4 were dissolved in deionized water. Then, a Na2CO3 aqueous solution was added dropwise to the metal salt solution under continuous stirring. Next, the Pd-Zn5(CO3)2(OH)6 product was collected by centrifugation, washed several times with deionized water and then finally vacuum freeze-dried for 24 h. Next, the Pd-Zn5(CO3)2(OH)6 precursor was heated at 5 °C min–1 to 300 °C under a H2/Ar (10/90) flow (300 mL min–1), then held at 300 oC for 4 h.
-
(b)
MZn-ZnO. The synthesis methods for AuZn-ZnO, AgZn-ZnO, PtZn-ZnO, CuZn-ZnO photocatalysts (with the mass percentage of Au, Ag, Pt or Cu 2 wt.% relative to ZnO), were similar to those described for the PdZn-ZnO photocatalyst with different metals salts being used as required.
-
(c)
ZnO and Pd-ZnO. Pristine ZnO photocatalyst was prepared by heating Zn5(CO3)2(OH)6 at 300 °C in a H2/Ar (10/90) flow (300 mL min–1) for 4 h. The Pd-ZnO photocatalysts was synthesized using a NaBH4 reduction method46 with the above synthesized ZnO as raw material, followed by heating in a nitrogen gas stream at 300 °C for 4 h.
-
(d)
PdZn-ZnO-mix. For the synthesis of PdZn-ZnO-mix sample, 1.0 g of PdZn-ZnO was immersed in 30 mL of an aqueous 1 mol L–1 HCl solution for 10 min to dissolve the ZnO support, then collected by centrifugation and washed with water. The obtained PdZn nanoparticles was dispersed in 100 mL of deionized water together with 980 mg of ZnO. The PdZn and ZnO dispersion was sonicated for 30 min, followed by freeze-dried for 24 h.
Characterizations
The structure and crystallinity of the photocatalysts were examined by X-ray diffraction (XRD, Bruker AXSD8 Advance, Germany). The diffractometer was equipped with a Cu Kα radiation source (λ = 1.5405 Å) operating at 40 kV. Morphologies and structure of the photocatalysts were studied using TEM (JEM, 2100 F, Japan). The aberration-corrected HAADF-STEM images and corresponding high-resolution EDS analyses were performed using a JEOL JEM-ARM300F atomic resolution electron microscope with a double spherical aberration corrector. Nitrogen adsorption/desorption isotherms were collected at 77 K on a Quadrasorb SI MP apparatus. Specific surface areas were calculated via the BET method. Diffuse reflectance spectra were recorded on a Cary 7000 (Agilent) spectrometer equipped with an integrating sphere attachment. The actual metal contents in the photocatalysts were determined by inductively coupled plasma-optical emission spectroscopy (ICP-OES, Varian 710).
C2H6-TPD profiles were obtained using an AutoChem II 2920 (Micromeritics Instrument Corporation). Photocatalysts (200.0 mg) were pretreated under a He atmosphere for 60 min at 150 °C, using a heating rate of 10 °C min–1. After cooling to 50 °C, photocatalysts were kept under a pure C2H6 atmosphere for 60 min to achieve adsorption saturation. Next, the samples were kept under a He flow (50 mL min–1) for 60 min to remove any weakly physically adsorbed ethane. Finally, the C2H6-TPD tests were carried out by heating the photocatalysts from 50 °C to 800 °C at a heating rate of 10 °C min–1 under a He carrier gas, with a mass spectrometer used for C2H6 detection. The test method for O2-TPD experiments was similar to C2H6-TPD tests, except that C2H6 is replaced by O2.
In situ EPR spectra were collected on a Bruker E500 spectrometer. Before the EPR tests, the catalyst was pre-treated at 150 °C for 2 h under an Ar flow to remove any surface adsorbed species. 50.0 mg of photocatalyst was loaded into a quartz tube and evacuated. Then, Ar or C2H6 gas was introduced into the EPR tube. EPR spectra were recorded in the dark and light conditions at 110 K.
Time-resolved online MS experiments were performed in a homemade fixed-bed stainless steel flow reactor (volume = 56.0 mL) with a quartz window on the top for irradiation of photocatalysts. The reactor was coupled to a mass spectrometer (SPIMS 2000, Hexin mass spectrum). Before each test, 20.0 mg of photocatalyst was uniformly dispersed on a glass fiber membrane and the modified membrane was then pretreated in an Ar gas flow at 200 °C for 2 h to remove any impurities from the membrane.
In situ Fourier transform infrared spectroscopy (FT-IR) data were collected on a Bruker Vertex 70 V FT-IR spectrometer equipped with a narrowband HgCdTe detector and a transmission reaction chamber (Harrick) connected to an evacuation line (∼ 10–7 mbar). 5.0 mg of PdZn-ZnO photocatalyst was pressed into a self-supported pellet (7.0 mm in diameter) and placed in the transmission chamber. In a typical in situ FT-IR experiment, the photocatalyst pellet was first purged with Ar (100 mL min–1) for 30 min to remove impurities adsorbed on the surface. Then, reactant gas (3 vol.% C2H6, 0.4 vol.% O2, balanced with Ar, 30 mL min–1) was introduced into the chamber. After 30 min of reactant adsorption, a background FT-IR spectrum was then recorded. Further FT-IR spectra were then collected every minute in the reaction atmosphere under illumination from a 150 mW continuous diode laser (375 nm), which served as the light source. Each spectrum was recorded by averaging 200 scans collected at a scanning velocity of 40 kHz and a resolution of 4 cm–1.
Photocatalytic activity measurements
Photocatalytic ODHE tests were conducted in a custom-built fixed-bed stainless steel flow reactor (volume = 56.0 mL) with a quartz window on the top for light irradiation (Supplementary Fig. 44). Typically, photocatalyst (5.0 mg) was uniformly spread on a glass fiber membrane (Whatman, GE Healthcare Life Sciences, catalog number 1823-047) and then placed in the reactor (parallel to the quartz window). Then, a reaction gas comprising C2H6 (5 vol.% in Ar, 18 mL min–1) and O2 (1 vol.% in Ar, 12 mL min–1) with a total flow rate of 30 mL min–1 was introduced to the reactor. After purging for 30 min to remove the air in the reactor. a 365 nm LED lamp (100 W 365 nm LED, Beijing PerfectLight Technology Co., Ltd., PLS-LED100C) was applied as light source to drive the reaction. Gas samples in the outlet were analyzed using a gas chromatograph (Shimadzu GC-2014, Shimadzu Co., Japan) equipped with three channels. The first channel analyzed hydrocarbons in an HP PLOT Al2O3 column with He as a carrier gas and a flame ionization detector (FID). The second channel analyzed CO2, N2, Ar, O2, CH4, and CO with a combination of micropacket Haysep Q, H-N, and Molsieve 13× columns using He as the carrier gas and a thermal conductivity detector (TCD). The third channel analyzes H2 using a micropacket HayeSep Q and Molsieve 5 Å column with N2 as a carrier gas and a TCD detector. For the batch-reactor test, a batch type reactor (volume = 56.0 mL) was used. After evacuation of the reaction system, reaction gas (3 vol.% C2H6, 0.4 vol.% O2, balanced with Ar) was introduced into the reactor until the pressure reached 0.3 MPa, after which the photocatalyst was exposed to the 365 nm LED light source.
The C2H4 selectivity is calculated according to the following equation.
where \(n({{{{{{\rm{C}}}}}}}_{2}{{{{{{\rm{H}}}}}}}_{4})\), \(n({{{{{\rm{C}}}}}}{{{{{{\rm{H}}}}}}}_{4})\) and \(n({{{{{\rm{CO}}}}}}_{2})\) represent the moles of C2H4, CH4 and CO2 at the outlet, respectively. The conversion rate is calculated based on products according to the following equation.
where \({n}_{0}({{{{{{\rm{C}}}}}}}_{2}{{{{{{\rm{H}}}}}}}_{6})\) represents the moles of C2H6 at the inlet. The carbon balance is calculated according to the following equation.
where \({n}_{1}\left({{{{{{\rm{C}}}}}}}_{2}{{{{{{\rm{H}}}}}}}_{6}\right)\) represent the moles of C2H6 at the outlet, respectively. The AQE was calculated according to the following equation.
where n(electrons) and n(photons) represent the number of reacted electrons and the number of incident photons, respectively. According to the chemical equation (2C2H6 + O2 → 2C2H4 + 2H2O, 2C2H6 + 7O2 → 4CO2 + 6H2O), n(electrons) = 2n(C2H4) + 7n(CO2), where n(C2H4) and n(CO2), represent the moles of produced C2H4 and CO2, respectively. n(photons) = IAt/E, where I, A, t, and E represent light intensity (W cm–2), irradiation area (12.57 cm–2), irradiation time (s) and photon energy (J), respectively. E = hc/λ, where h, c, and λ represent Planck’s constant, light speed, and monochromatic light wavelength, respectively. The light intensity I at different wavelengths (λ = 350, 365, 380, 400, 450, 500, 600, and 700 nm) was measured to be 51.3, 52.6, 50.2, 49.8, 50.4, 50.5, 51.3, and 49.8 mW cm–2 by xenon lamp (300 W Xe lamp, Beijing Perfectlight Technology Co. Ltd, PLS-SXE300D) source and band pass filter (Supplementary Fig. 45). Since the surface temperature of the catalyst was around room temperature under monochromatic light irradiation, the contribution of heat induced by illumination during AQE tests was not considered.
Computational details
First-principles spin-polarized calculations were performed using the Vienna ab initio Simulation Program (VASP)47,48. The generalized gradient approximation (GGA) in the Perdew-Burke-Ernzerhof (PBE) form and a cutoff energy of 700 eV for planewave basis set were adopted49. A 3 × 3 × 1 Monkhorst-Pack50 k grid was used for sampling the Brillouin zones in the structure calculations. Ion-electron interactions were described by the projector augmented wave (PAW) method51. The convergence criteria for structure optimization was a maximum force less than 0.02 eV Å–1 on each atom with an energy change less than 1 × 10–5 eV. The DFT-D3 semiempirical correction was described via Grimme’s scheme method52,53.
Since the main exposed facets of ZnO were (101), a ZnO(101) model was used for the calculations. The ZnO(101) surface was modelled by a 3 × 3 unit cell based on an initial structure with lattice parameters a = 18.4 Å, b = 19.5 Å, c = 25.4 Å, α = β = 90°, γ = 105°. Pd15Zn15 cluster with fifteen Pd atoms and fifteen Zn atom was used to simulate a PdZn intermetallic nanoparticle, whilst Pd30 cluster with thirty Pd atoms was used to simulate a Pd nanoparticle. The above Pd15Zn15 and Pd30 clusters were placed on ZnO(101) surface to form models Pd15Zn15-ZnO(101) and Pd30-ZnO(101), respectively. Models similar to Pd15Zn15-ZnO(101) were used to build Pd15Cu15-ZnO(101), Pd15Ni15-ZnO(101), and Pd15In15-ZnO(101).
To calculate the kinetic energy barrier of chemical reactions, the climbing image nudged elastic band (CI-NEB) method was used to search for the TS54,55, with convergence criteria a force below 0.05 eV Å−1. Ea was calculated using Ea = ETS – EIS, where ETS and EIS represents the energy of TS and the initial state.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request. Received: ((will be filled in by the editorial staff)) Accepted: ((will be filled in by the editorial staff)) Published online: ((will be filled in by the editorial staff)) Source data are provided with this paper.
References
Li, X., Pei, C. & Gong, J. Shale gas revolution: catalytic conversion of C1–C3 light alkanes to value-added chemicals. Chem 7, 1755–1801 (2021).
Melzer, D. et al. Design and synthesis of highly active MoVTeNb–oxides for ethane oxidative dehydrogenation. Nat. Commun. 10, 4012 (2019).
Yang, Z. et al. Coking–resistant iron catalyst in ethane dehydrogenation achieved through siliceous zeolite modulation. J. Am. Chem. Soc. 142, 16429–16436 (2020).
Xie, Z., Wang, X., Chen, X., Liu, P. & Chen, J. G. General descriptors for CO2–assisted selective C–H/C–C bond scission in ethane. J. Am. Chem. Soc. 144, 4186–4195 (2022).
Venegas, J. M., McDermott, W. P. & Hermans, I. Serendipity in catalysis research: boron–based materials for alkane oxidative dehydrogenation. Acc. Chem. Res. 51, 2556–2564 (2018).
Cavani, F., Ballarini, N. & Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 127, 113–131 (2007).
Song, S. et al. A selective Au–ZnO/TiO2 hybrid photocatalyst for oxidative coupling of methane to ethane with dioxygen. Nat. Catal. 4, 1032–1042 (2021).
Zhang, W. et al. High-performance photocatalytic nonoxidative conversion of methane to ethane and hydrogen by heteroatoms-engineered TiO2. Nat. Commun. 13, 2806 (2022).
Song, H. et al. Direct and selective photocatalytic oxidation of CH4 to oxygenates with O2 on cocatalysts/ZnO at room temperature in water. J. Am. Chem. Soc. 141, 20507–20515 (2019).
Chen, X. et al. Photocatalytic oxidation of methane over silver decorated zinc oxide nanocatalysts. Nat. Commun. 7, 12273 (2016).
Parastaev, A. et al. Boosting CO2 hydrogenation via size–dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 3, 526–533 (2020).
Luo, L. et al. Binary Au–Cu reaction sites decorated ZnO for selective methane oxidation to C1 oxygenates with nearly 100% selectivity at room temperature. J. Am. Chem. Soc. 144, 740–750 (2022).
Xiong, P. et al. Zn–ion transporting, in situ formed robust solid electrolyte interphase for stable zinc metal anodes over a wide temperature range. ACS Energy Lett. 8, 1613–1625 (2023).
Zhang, F. et al. Catalytic activity of Cu/ZnO catalysts mediated by MgO promoter in hydrogenation of methyl acetate to ethanol. J. Energy Chem. 61, 203–209 (2021).
Ye, C. et al. PdZn intermetallic compound stabilized on ZnO/nitrogen–decorated carbon hollow spheres for catalytic semihydrogenation of alkynols. Nano Res 15, 3090–3098 (2022).
Hu, M. et al. MOF–confined sub–2 nm atomically ordered intermetallic PdZn nanoparticles as high–performance catalysts for selective hydrogenation of acetylene. Adv. Mater. 30, 1801878 (2018).
Qiu, Y. et al. Construction of Pd–Zn dual sites to enhance the performance for ethanol electro–oxidation reaction. Nat. Commun. 12, 5273 (2021).
Zabilskiy, M. et al. Mechanistic study of carbon dioxide hydrogenation over Pd/ZnO–based catalysts: the role of palladium–zinc alloy in selective methanol synthesis. Angew. Chem. Int. Ed. 60, 17053–17059 (2021).
Huang, R. et al. Direct insight into ethane oxidative dehydrogenation over boron nitrides. ChemCatChem 9, 3293–3297 (2017).
Sarkar, B. et al. MoO3 nanoclusters decorated on TiO2 nanorods for oxidative dehydrogenation of ethane to ethylene. Appl. Catal., B 217, 637–649 (2017).
Luongo, G. et al. Highly selective oxidative dehydrogenation of ethane to ethylene via chemical looping with oxygen uncoupling through structural engineering of the oxygen carrier. Adv. Energy Mater. 12, 2200405 (2022).
Solsona, B., Concepción, P., Hernández, S., Demicol, B. & Nieto, J. M. L. Oxidative dehydrogenation of ethane over NiO–CeO2 mixed oxides catalysts. Catal. Today 180, 51–58 (2012).
Dang, D., Chen, X., Yan, B., Li, Y. & Cheng, Y. Catalytic performance of phase–pure M1 MoVNbTeOx/CeO2 composite for oxidative dehydrogenation of ethane. J. Catal. 365, 238–248 (2018).
Ykrelef, A. et al. Mixed oxide TiSiO prepared by non–hydrolytic Xerogel method as a diluter of nickel oxide for the oxidative dehydrogenation of ethane. Catal. Today 299, 93–101 (2018).
Zhang, Z. et al. Low-temperature, highly selective, highly stable Nb2O5–NiO/Ni–foam catalyst for the oxidative dehydrogenation of ethane. Catal. Sci. Technol. 8, 4383–4389 (2018).
Lu, J. et al. Coking–and sintering–resistant palladium catalysts achieved through atomic layer deposition. Science 335, 1205–1208 (2012).
Kong, L. et al. Oxidative dehydrogenation of ethane to ethylene over Mo–incorporated mesoporous SBA–16 catalysts: The effect of MoOx dispersion. Appl. Catal. A–Gen. 510, 84–97 (2016).
Kong, L. et al. Template–induced mesoporous Ni–Al oxide catalysts with tuned physico–chemical properties for the oxidative dehydrogenation of ethane. Chem. Eng. J. 452, 139247 (2023).
Cao, X. et al. Boron and nitrogen co–doped porous carbon nanospheres for oxidative dehydrogenation of ethane to ethylene. Carbon 197, 120–128 (2022).
Yan, P., Chen, Y. & Cheng, Y. Industrially potential MoVNbTeOx@FoamSiC structured catalyst for oxidative dehydrogenation of ethane. Chem. Eng. J. 427, 131813 (2022).
Shi, R. et al. Structural disorder in higher-temperature phases increases charge carrier lifetimes in metal halide perovskites. J. Am. Chem. Soc. 144, 19137–19149 (2022).
Yang, Y. et al. Oxygen–vacancy–induced O2 activation and electron–hole migration enhance photothermal catalytic toluene oxidation. Cell Rep. Phys. Sci. 3, 101011 (2022).
Rieboldt, F., Bechstein, R., Besenbacher, F. & Wendt, S. Vicinal rutile TiO2 surfaces and their interactions with O2. J. Phys. Chem. C. 118, 3620–3628 (2014).
Xu, C. et al. Nanostructured materials for photocatalysis. Chem. Soc. Rev. 48, 3868–3902 (2019).
Kang, L. et al. Photo–thermo catalytic oxidation over a TiO2–WO3–supported platinum catalyst. Angew. Chem. Int. Ed. 59, 12909–12916 (2020).
Zhu, S. et al. Efficient photooxidation of methane to liquid oxygenates over ZnO Nanosheets at atmospheric pressure and near room temperature. Nano Lett. 21, 4122–4128 (2021).
Shoji, S. et al. Photocatalytic uphill conversion of natural gas beyond the limitation of thermal reaction systems. Nat. Catal. 3, 148–153 (2020).
Wang, P. et al. Selective photocatalytic oxidative coupling of methane via regulating methyl intermediates over metal/ZnO nanoparticles. Angew. Chem. Int. Ed. 62, e202304301 (2023).
Wang, W. et al. Photocatalytic C–C coupling from carbon dioxide reduction on copper oxide with mixed–valence copper(I)/copper(II.).J. Am. Chem. Soc 143, 2984–2993(2021).
Bian, Y., Kim, M., Li, T., Asthagiri, A. & Weaver, J. F. Facile dehydrogenation of ethane on the IrO2(110) surface. J. Am. Chem. Soc. 140, 2665–2672 (2018).
Zhang, R. et al. Photocatalytic oxidative dehydrogenation of ethane using CO2 as a soft oxidant over Pd/TiO2 catalysts to C2H4 and syngas. ACS Catal. 8, 9280–9286 (2018).
Ye, L., Duan, X. & Xie, K. Electrochemical oxidative dehydrogenation of ethane to ethylene in a solid oxide electrolyzer. Angew. Chem. Int. Ed. 60, 21746–21750 (2021).
He, Y. et al. In situ identification of reaction intermediates and mechanistic understandings of methane oxidation over hematite: a combined experimental and theoretical study. J. Am. Chem. Soc. 142, 17119–17130 (2020).
Luo, M. et al. PdMo bimetallene for oxygen reduction catalysis. Nature 574, 81–85 (2019).
Lee, B.-H. et al. Supramolecular tuning of supported metal phthalocyanine catalysts for hydrogen peroxide electrosynthesis. Nat. Catal. 6, 234–243 (2023).
Wang, P. et al. Efficient photocatalytic aerobic oxidation of bisphenol A via gas–liquid–solid triphase interfaces. Mater. Today Energy 23, 100908 (2022).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total–energy calculations using a plane–wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane–wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin–zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Blöchl, P. E. Projector augmented–wave method. Phys. Rev. B 50, 17953–17979 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT–D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Harl, J., Schimka, L. & Kresse, G. Assessing the quality of the random phase approximation for lattice constants and atomization energies of solids. Phys. Rev. B 81, 115126 (2010).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G. & Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Acknowledgements
The authors are grateful for financial support from the National Key R&D Program of China (2023YFA1507201), the National Natural Science Foundation of China (51825205, 52120105002, 22272190, 22088102), the Beijing Natural Science Foundation (2222035), the CAS Project for Young Scientists in Basic Research (YSBR-004), the DNL Cooperation Fund, CAS (DNL202016), and the Youth Innovation Promotion Association of the CAS. GINW acknowledges funding support from the Royal Society Te Apārangi (for a James Cook Research Fellowship), the MacDiarmid Institute for Advanced Materials and Nanotechnology, and the Energy Education Trust of New Zealand. The authors would like to thank Yueru Wu from Shiyanjia Lab (www.shiyanjia.com) for assistance with the TPD experiments.
Author information
Authors and Affiliations
Contributions
R.S. and T.Z. supervised the project. P.W. conceived the idea for the project and performed catalyst synthesis, characterization and photocatalytic tests. X.Z. participated in data processing for DFT calculations. J.Z. and R.S. assisted in data analysis. P.W. wrote the manuscript. R.S., G.I.N.W., J.T. and T.Z. revised the manuscript. All authors discussed the results and commented on the manuscript at all stages.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Liang Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, P., Zhang, X., Shi, R. et al. Photocatalytic ethylene production by oxidative dehydrogenation of ethane with dioxygen on ZnO-supported PdZn intermetallic nanoparticles. Nat Commun 15, 789 (2024). https://doi.org/10.1038/s41467-024-45031-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-45031-6
- Springer Nature Limited