Abstract
Genetically encoded probes monitoring H2O2 fluctuations in living organisms are key to decipher redox signaling events. Here we use a new probe, roGFP2-Tpx1.C169S, to monitor pre-toxic fluctuations of peroxides in fission yeast, where the concentrations linked to signaling or to toxicity have been established. This probe is able to detect nanomolar fluctuations of intracellular H2O2 caused by extracellular peroxides; expression of human aquaporin 8 channels H2O2 entry into fission yeast decreasing membrane gradients. The probe also detects H2O2 bursts from mitochondria after addition of electron transport chain inhibitors, the extent of probe oxidation being proportional to the mitochondrial activity. The oxidation of this probe is an indicator of steady-state levels of H2O2 in different genetic backgrounds. Metabolic reprogramming during growth in low-glucose media causes probe reduction due to the activation of antioxidant cascades. We demonstrate how peroxiredoxin-based probes can be used to monitor physiological H2O2 fluctuations.
Similar content being viewed by others
Introduction
Aerobic organisms inevitably produce reactive oxygen species as a side effect of sequential one-electron reduction of oxygen during respiration, among other processes. One of these species, hydrogen peroxide (H2O2), has been traditionally linked to the toxicity associated to aerobic metabolism. However, H2O2 also clearly participates in signaling events, by both regulating different physiological processes and also activating antioxidant cascades (for reviews, see refs. 1,2,3).
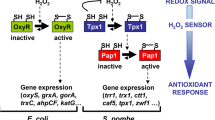
The signaling role of H2O2 is often associated to the direct oxidation of cysteine (Cys) residues via reversible modifications, normally disulfide bridges. Reversibly oxidized thiols are later on reduced by the thioredoxin (Trx) and glutaredoxin (Grx) systems and reduced cofactor4,5. Very few Cys in proteins fulfill the prerequisites for fast and efficient oxidation by moderate fluctuations of peroxides. These special Cys residues are known as thiol switches, and belong to ‘true’ H2O2 protein sensors6. Among the proteins unambiguously responding to pre-toxic doses of H2O2 to induce antioxidant responses are the prokaryotic transcription factor OxyR7 and the Schizosaccharomyces pombe Tpx18,9, which belongs to the family of H2O2 scavengers known as peroxiredoxins (Prxs). OxyR and Prxs display high reactivity to peroxides, with reaction rates in the range of 105–107 M−1 s−110. The gain-of-function of the H2O2-activated sensor, acting either as a transcription factor (in the case of OxyR) or as a redox transducer (in the case of Tpx1), has to be sustained to be capable of inducing an adaptive response. Thus, we have recently proposed that H2O2-oxidized thiols in real protein sensors such as OxyR should also fulfill the requisite of displaying slow reduction rates. On the contrary, in the case of the moonlighting H2O2 peroxidase Tpx1 its reduction rates are very fast; only when the Trx system becomes transiently exhausted Tpx1 switches from a H2O2 scavenger to a signal transducer toward the transcription factor Pap111.
The intracellular levels of H2O2 are tightly controlled by scavenging activities, so that fluctuations over a certain threshold can drive adaptation responses. Measuring those variations has been the focus of attention of many redox biology laboratories for decades. While most measurements are still based on the use of permeable fluorescent dyes which respond with different specificity and sensitivity to H2O2, their use is controversial12. Over a decade ago13, several groups decided to investigate the use of protein-based reporters to measure intracellular redox potentials and, later on, H2O2. Thus, two major families of genetically encoded redox reporters have been developed: the reduction-oxidation-sensitive green fluorescent protein (roGFP)-based proteins13 and HyPer and derivatives14 (for excellent reviews on the use of these genetic reporters, see refs. 15,16).
HyPer was engineered to sense and report intracellular fluctuations of H2O2. It is based on the transcription factor OxyR, which is activated by H2O2 to trigger an antioxidant response. The two Cys residues of OxyR involved in H2O2-dependent disulfide bond formation are located in positions 199 and 2087. In HyPer, the circularly permuted yellow fluorescent protein (cpYFP) is inserted between residues 205 and 206 of OxyR, so that disulfide bond formation generates a conformational change in cpYFP affecting its fluorescent properties. Thus, reduced HyPer has two excitation peaks, with maximal values at 420 and 500 nm, and one emission peak at 516 nm. Upon oxidation with peroxides, the magnitude of the 420 nm peak decreases concomitant with a proportional increase in the magnitude of the 500 nm peak14. These ratiometric changes are specific for H2O2, and other oxidants such as superoxide, oxidized glutathione or other reactive oxygen species are not able to induce them in vitro14.
roGFP has several substitutions on surface-exposed domains, so that disulfide bonds can be formed in response to oxidants and change the fluorescence properties of the protein in an analogous manner to HyPer13. The specificity and sensitivity of roGFP to sense oxidants has been improved during the last years by fusing real H2O2 protein sensors to this fluorescent reporter. Thus, a variant of roGFP has been fused to the Saccharomyces cerevisiae glutathione peroxidase Orp117 or to a Prx lacking its resolving Cys18, yielding the chimeric proteins roGFP2-Orp1 and roGFP2-Tsa2ΔCR. Both, but specially roGFP2-Tsa2ΔCR, have been shown to monitor intracellular H2O2 levels with unprecedented sensitivity in vivo18,19.
Often, characterization of H2O2 fluorescent protein sensors is performed after application of extracellular peroxides, but the exact correlation between extracellular and intracellular peroxide concentrations is not known in most biological systems. In fission yeast, we have recently calculated that the gradient of extracellular-to-intracellular peroxides through fission yeast membranes is around 40:1, but intracellular H2O2 scavenging activities such as Tpx1 can enhance this gradient up to 300:111. In fact, the responsibility to maintain non-toxic peroxide levels relies exclusively on this protein: cells lacking Tpx1 display strong growth defects in the presence of oxygen20, due to low micromolar steady-state levels of H2O2 (specifically, 0.3 µM)11. This suggests that Tpx1 has high specificity and sensitivity for H2O2. With this knowledge in mind, we have expressed in the cytosol of fission yeast a new and ultrasensitive genetically encoded reporter based on this exquisitely sensitive Prx, Tpx1. Similar to its budding yeast ortholog roGFP2-Tsa2ΔCR, the probe is able to sense moderate fluctuations of H2O2 induced by genetic or environmental interventions in fission yeast cells. Its oxidation by extracellular peroxides can be exacerbated by overexpression of human aquaporin 8, which facilitates H2O2 channeling across membranes. The basal level of oxidation of roGFP2-Tpx1.C169S in wild-type cells is around half of its maximum levels, while cells lacking Tpx1 display 70–80% oxidation of the probe, indicating that this extent of oxidation is due to the toxic low micromolar levels. Furthermore, the cytosolic probe is able to sense the leakage of H2O2 from the mitochondria upon addition of the electron transport chain inhibitor antimycin A. Using this Prx-based sensor, we demonstrate that it facilitates quantitative measurements of dynamic and pre-toxic H2O2 fluxes arising from the extracellular milieu or from the mitochondria.
Results
Expression of HyPer and roGFP derivatives in S. pombe
Many genetically encoded reporters have been designed and analyzed in different model systems, which makes it difficult to interpret and compare the results. We decided to create our own probes, based on fission yeast’s Gpx1 and Tpx1 peroxidases, with two main goals: first, to unambiguously compare the sensitivity of previous and new sensors in the same model system; and second, to correlate specific intracellular peroxide concentrations, previously established in fission yeast11, with the oxidation of different cytosolic reporters.
We first expressed HyPer14, roGFP213, and Grx1-roGFP221 in the cytosol of S. pombe cells under the control of the constitutive promoter sty122, using episomal plasmids. The high concentration of the reporters allowed us to monitor probe oxidation directly in exponentially growing cultures, without centrifugation or media transfer. We applied extracellular concentrations of peroxides ranging from 1 to 25 µM (pre-toxic), 50 to 100 µM (signaling toward the antioxidant Pap1 cascade), and 0.2 to 1 mM (toxic, halts growth of wild-type cells), corresponding to intracellular concentrations of 3–80 nM, 0.2–0.3 µM, and 0.7–3 µM, respectively11. As shown in Fig. 1a–c and Supplementary Fig. 1, the three probes HyPer, roGFP2, and Grx1-roGFP2 are oxidized in response to H2O2 treatments; the minimum concentrations of extracellular peroxides triggering probe oxidation are 50, 25, and 25 µM, respectively. HyPer displays a maximum oxidation with extracellular 0.2 mM (Fig. 1a).
Oxidation of HyPer and roGFP derivatives upon extracellular H2O2. Wild-type strain HM123 was transformed with plasmids p605, p406, p379, p429, p407, p407.C169S, and p676, to express HyPer (a), roGFP2 (b), Grx1-roGFP2 (c), roGFP2-Gpx1 (d), roGFP2-Tpx1 (e), roGFP2-Tpx1.C169S (f), and roGFP2- Tsa2ΔCR (g), respectively. The indicated extracellular (EXT.) concentrations of H2O2, classified into ‘Toxic’ (able to temporarily halt growth), ‘Signaling’ (capable to trigger the antioxidant Pap1 cascade) or ‘Pre-toxic’ (unable to activate Pap1), were directly added to MM cultures at an OD600 of 1 in 96-well imaging plates, and growth proceeded at 30 °C with shaking. The corresponding intracellular peroxide concentrations, after applying a 300:1 membrane gradient, are also indicated (INT.). The degree of probe oxidation (amount of probe oxidized per 1) is indicated in the Y axis (OxD roGFP2), except for HyPer (a), for which the ratio 488/405 nm is shown. For each panel, data from three biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 1
We then fused S. pombe Gpx1 and Tpx1 to roGFP2, and determined that the H2O2 thresholds capable of inducing probe oxidation were 25 and 10 µM, respectively (Fig. 1d, e), barely improving the values obtained with the previously published roGFP reporters.
roGFP2-Tpx1.C169S is very sensitive to H2O2 addition
As indicated in the Introduction, we have recently established that Tpx1 is not only exquisitely sensitive to oxidation by peroxides, but also very efficiently recycled by the Trx system11. Thus, we fused Tpx1.C169S to roGFP2. This mutant protein, which lacks the resolving Cys, is capable of interacting with H2O2 but the peroxidatic Cys48-SOH cannot react with the resolving Cys to form a disulfide. Therefore, this mutation eliminates Trx competition and may enhance the oxidation transfer from Cys48-SOH to roGFP2. As shown in Fig. 1f, roGFP2-Tpx1.C169S is able to sense extracellular concentrations as low as 1–5 µM H2O2; this sensitivity is similar to that of the previously characterized reporter roGFP2-Tsa2ΔCR, derived from the S. cerevisiae peroxiredoxin Tsa2, expressed in fission yeast (Fig. 1g). If we consider a gradient of extracellular-to-intracellular peroxides of 300-to-111, an intracellular concentration of as little as 3–16 nM H2O2 can be monitored with both Prx-based probes. On the contrary, HyPer, roGFP, or Grx1-roGFP can only detect concentrations of H2O2 high enough to activate antioxidant cascades, or even halting the growth of cell cultures. As previously described in vitro for roGFP2-Tsa2ΔCR, both Prx-based chimeras are also very sensitive in vivo to other oxidants such as tert-butyl hydroperoxide, menadione (generates in vivo superoxide, which is dismutated to H2O2) and sodium hypochlorite (Supplementary Fig. 2a), but barely or do not respond to high concentrations of a nitric oxide donor, oxidized GSH, or dehydroascorbic acid (Supplementary Fig. 2b). The protein sequence of roGFP2-Tpx1.C169S is provided in Supplementary Fig. 2c. This reporter will be used hereafter to monitor its behavior, often in parallel to the completely different sensor HyPer, in response to physiological, pre-toxic waves of intracellular peroxides.
Effect of AQP8 expression on roGFP2-Tpx1.C169S oxidation
We have demonstrated that the diffusion of H2O2 across fission yeast membranes is limited, and that scavenging mainly by Tpx1 enhances the gradients up to 300:111. We tested whether expression of human AQP8 in S. pombe could decrease those gradients, by monitoring oxidation of cytosolic roGFP2-Tpx1.C169S. Aquaporins (AQPs) are diffusion facilitators for non-charged and partially polar molecules such as water or glycerol. Recent studies have demonstrated that some mammalian AQPs, such as AQP823, also mediate the transport of H2O2 across human cell membranes. As shown in Fig. 2a and Supplementary Fig. 3a, expression of AQP8 in fission yeast facilitates H2O2 entry by a factor of at least five times, and probe oxidation can be detected with as little as 0.2–1 µM extracellular peroxides. Concomitantly, expression of AQP8 in wild-type cells (Fig. 2b) or in strain Δpap1, lacking an antioxidant transcription factor8 (Supplementary Fig. 3b), enhances sensitivity to peroxides on solid plates, without affecting the tolerance to other stressors such as potassium chloride. Importantly, expression of AQP4, which does not channel H2O2 across membranes, does not affect the tolerance of fission yeast to peroxides, and probe oxidation does not differ from that of wild-type cells (Supplementary Fig. 3a, b).
Oxidation of roGFP2-Tpx1.C169S by H2O2 is exacerbated by expression of human AQP8. a Wild-type strain PN513 was co-transformed with plasmid p407.C169S, coding for roGFP2-Tpx1.C169S, and an empty plasmid (left) or plasmid p675 to trigger the constitutive expression of AQP8. H2O2 treatments were performed and oxidation of the reporter estimated as described in Fig. 1. Data from three biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 3. b Expression of AQP8 in fission yeast decreases wild-type tolerance to peroxides. Serial dilutions of MM cultures of strains 972 (WT), AV18 (Δsty1), and 364 carrying pREP.42× (WT + empty plasmid) or p675 (WT + pAQP8) were spotted on MM agar plates and the indicated concentrations of H2O2 or potassium chloride (KCl), and grown for 3–4 days at 30 °C
roGFP2-Tpx1.C169S scavenges H2O2 and affects cell physiology
We have previously shown that cells lacking Tpx1 or expressing the catalytically inactive Tpx1.C48S mutant display severe phenotypes to grow in the presence of oxygen11,20. Nevertheless, Tpx1.C169S can sustain aerobic growth, probably with GSH supplying a thiol group to substitute for Cys16920. To test whether expression of the probe roGFP2-Tpx1.C169S affects cellular H2O2 homeostasis, we transformed strain Δtpx1 with episomal and integrative plasmids containing the chimeric gene roGFP2-tpx1.C169S. As shown in Supplementary Fig. 4, high levels of roGFP2-Tpx1.C169S are able to largely suppress the aerobic growth defects of cells lacking Tpx1. On the contrary, the lower levels accomplished from the integrative plasmid only partially restore aerobic growth.
roGFP2-Tpx1.C169S OxD0, an indicator of H2O2 steady-state levels
Cells lacking Tpx1 display aerobic growth defects due to the absence of the main scavenger of peroxides during aerobic growth20. We aimed at studying whether the new probe would be able to sense the enhanced steady-state levels of peroxides in this strain background. We compared the basal and extracellular H2O2-triggered oxidation ratios of both families of probes, HyPer and roGFP2-Tpx1.C169S. As shown in Fig. 3a and Supplementary Fig. 5, the basal oxidation levels of HyPer expressed in cells lacking Tpx1 were identical to those observed in wild-type cells. Interestingly, HyPer expressed in strain Δtpx1 was now capable of sensing as little as 10–25 µM extracellular H2O2. This reflects the fact that scavenging by Tpx1 is now jeopardized and only a 40-to-1 gradient of extracellular-to-intracellular peroxides applies in this strain background due only to permeability. In wild-type cells, both permeability and H2O2 scavenging generate a 300-to-1 step gradient11.
Basal levels of oxidation, OxD0, of roGFP2-Tpx1.C169S are elevated in cells lacking Tpx1. Strains HM123 (WT) and SG5 (Δtpx1) were transformed with plasmids p605, coding for HyPer (a) and p407.C169S, coding for roGFP2-Tpx1.C169S (b) and H2O2 treatments were performed and oxidation of the reporters estimated as described in Fig. 1. For each panel, data from three biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 5
roGFP2-Tpx1.C169S expressed in Δtpx1 is now able to sense concentrations of peroxides below 1 µM, as shown in Fig. 3b (right panel). But, most importantly, the basal level of oxidation of the probe, OxD0, is also significantly altered, with only 50–60% of the probe being oxidized in a wild-type background (Fig. 3b, left panel) and moving up to 70–80% in strain Δtpx1 (Fig. 3b, right panel). Importantly, the enhanced OxD0 of this probe in Δtpx1 is due to elevated H2O2, since Grx1-roGFP2, designed to sense redox changes in GSH, does not exhibit this increase (Supplementary Fig. 5c). We conclude that basal oxidation levels, OxD0, of roGFP2-Tpx1.C169S can be used to monitor H2O2 steady-state levels in different strain backgrounds.
Effect of genetic mutations on roGFP2-Tpx1.C169S redox state
We have demonstrated that steady-state H2O2 levels, enhanced in Δtpx1, can change basal oxidation of the roGFP2-Tpx1.C169S probe, OxD0 (Fig. 3b). The steady-state oxidation of the probe, however, may also depend on the thiol reducing capacity of the cell. We deleted individual genes coding for components of the Trx and Grx thiol reducing systems. As reported before for other roGFP derivatives18, the Grx system is the major reductant of the roGFP disulfide bond: the basal steady-state levels of oxidized roGFP2-Tpx1.C169S are significantly enhanced in cells lacking Pgr1 (GSH reductase) or Grx1, the main cytosolic glutaredoxin (Fig. 4a and Supplementary Fig. 6a).
Effect of genetic mutations on roGFP2-Tpx1.C169S oxidation. Strains SB36 (Δgrx1) and AD88 (Δpgr1) (a) and strains HM123 (WT), SG61 (Δtrx1), and IC76 (Δtrx1 Δtrx3) (b) were transformed with plasmid p407.C169S and H2O2 treatments were performed and oxidation of the reporter estimated as described in Fig. 1. c Scheme depicting the role of cellular electron donors in roGFP2-Tpx1.C169S probe oxidation and reduction. Blockage of H2O2 detoxification either by lack of Tpx1 recycling or by lack of Ctt1 activity results in accumulation of basal or H2O2-induced oxidized roGFP2.C169S. Recycling of roGFP2.C169S depends on Grx1 at the expense of GSH and Pgr1. d Strains HM123 (WT) and EP160 (Δctt1) were transformed with plasmid p407.C169S and H2O2 treatments were performed and oxidation of the reporter estimated as described in Fig. 1. For each panel, data from three biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 6
Regarding genetic mutations in the cytosolic Trxs, Trx1, and Trx3, they are not expected to exert a major impact on roGFP or on Tpx1.C169S redox state, since the resolving Cys of the peroxidase is missing (see Introduction)20. Nevertheless, these Trxs are required to recycle Tpx124, and therefore extracellularly added H2O2 may not be efficiently scavenged in cells lacking both Trxs. As shown in Fig. 4b, the roGFP2-Tpx1.C169S reporter is not significantly affected under basal conditions in strain Δtrx1. Δtrx1 Δtrx3 cells display an OxD0 of 0.8, confirming that the absence of both Trxs have a larger impact on Tpx1 recycling24. From these experiments, we conclude that the basal oxidation level of roGFP2-Tpx1.C169S can be affected by alterations of the GSH/Grx1 system at the level of probe reduction, and that it responds directly to H2O2 perturbations in the case of Trx mutants (Fig. 4c).
We next monitored whether probe oxidation upon extracellular H2O2 was reversible. We applied different concentrations of H2O2 to wild-type and Δctt1 strains, lacking catalase, and monitored probe oxidation during 7 h (Fig. 4d). In a wild-type background, 2–2.5 h are sufficient for full probe reduction after applying extracellular concentrations of peroxides lower than 100 µM (Fig. 4d, upper panel). These kinetics are very similar in cells lacking Ctt1 (Fig. 4d, lower panel). The oxidized probe in wild-type cells requires longer times (3 and 6 h, respectively) to return to basal OxD values after 0.2 and 1 mM H2O2 stress, which fits with the effect of those toxic concentrations halting culture growth during 1–5 h11,22. Importantly, cell cultures of strain Δctt1 cannot resume growth after 0.2 and 1 mM extracellular stresses, and the roGFP2-Tpx1.C169S probe remains partially (0.2 mM) or fully oxidized (1 mM H2O2) more than 7 h after stress imposition (Fig. 4d, lower panel). In conclusion, the roGFP2-Tpx1.C169S probe is a reversible indicator of intracellular concentrations of peroxides.
roGFP2-Tpx1.C169S can detect mitochondrial H2O2 production
An important source of H2O2 production is the mitochondrial electron transport chain (ETC). We tested whether cytosolic roGFP2-Tpx1.C169S is able to sense H2O2 produced at and diffused out of the mitochondria. We treated cells with antimycin A (ANT), an inhibitor of complex III, which exacerbates accidental reactive oxygen species (ROS) production in the mitochondria due to the accumulation of reduced upstream ETC components25. We first monitored cytosolic HyPer oxidation upon ANT addition in both a wild-type strain and in cells lacking the main H2O2 scavenger, Tpx1. We have represented probe oxidation upon ANT treatments with black lines, and we also used H2O2 treatments as controls (Fig. 5a, b and Supplementary Fig. 7). As shown in Fig. 5a, cytosolic HyPer is unable to sense mitochondrial ROS production upon ANT treatments.
Cytosolic roGFP2-Tpx1.C169S can detect mitochondrial H2O2 production. Strains HM123 (WT) and SG5 (Δtpx1) were transformed with plasmids p605 (a) and p407.C169S (b). The indicated concentrations of H2O2 and antimycin A (ANT) were directly added to MM cultures and oxidation of the reporters was estimated as described in Fig. 1; oxidation of the probe upon ANT treatments is represented with solid (5 µM ANT) or dashed (1 µM ANT) black lines. a Data from two biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 7a. b Data from two (WT) or three (Δtpx1) biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 7b. c Strain Δcoq4 was transformed with plasmid p407.C169S and H2O2 treatments were performed and oxidation of the reporter estimated as described in Fig. 1. Data from three biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 7c
We then treated wild-type cells expressing the roGFP2-Tpx1.C169S reporter with ANT. A small but significant increase of basal oxidation of the probe was observed 30–90 min after ANT exposure (Fig. 5b, top panel, black lines). It is worth mentioning that the growth rates of fission yeast cells grown in MM are not largely affected by ANT-dependent ETC inhibition, indicating that respiration is not the driving force of S. pombe proliferation at high-glucose concentrations26. Oxidation of the roGFP2-Tpx1.C169S probe upon ANT treatment is much more noticeable in Δtpx1 cells, suggesting that H2O2 produced at the mitochondria and dispersed to the cytosol is rapidly scavenged by Tpx1 in wild-type cells (Fig. 5b, bottom panel).
Consistent with the chemical inhibition of the ETC with ANT, genetic blockage also triggers H2O2 leakage out of the membrane. Thus, strains carrying individual gene deletions of ETC components display growth defects on solid MM plates, which can be alleviated by the addition of antioxidants or oxygen depletion. We proposed that elevated steady-state levels of H2O2 due to electron leakage compromises the growth of these cells27. As shown now with the roGFP2-Tpx1.C169S probe, the steady-state levels of H2O2 in Δcoq4 cells, lacking a protein required for ubiquinone biosynthesis27, drive 70–80% oxidation of the probe. This confirms the massive (and constant) H2O2 leakage from the mitochondria to the cytosol in this strain background, probably at the level of complex I28 (Fig. 5c).
Effect of glucose depletion on roGFP2-Tpx1.C169S oxidation
The balance between respiration and fermentation largely depends on nutrient availability. In most cell types, including tumors and yeast, growth in nutrient-rich media induces fermentation and inhibits respiration (Crabtree effect29,30). Glucose-rich conditions inhibit respiratory metabolism in all cell types to different extents. Since ROS production is linked to mitochondrial metabolism, we decided to determine the effect of low glucose in roGFP2-Tpx1.C169S basal oxidation, OxD0. Thus, we compared the OxD0 in standard MM (MM-Glu), which contains 2% glucose as the only carbon source, with that in MM-Gly (1.85% glycerol and 0.15% glucose). To our surprise, OxD0 is very low in MM-Gly (Fig. 6a and Supplementary Fig. 8), suggesting that the levels of intracellular H2O2 are lower in cells grown in respiratory-prone conditions.
Effect of low-glucose media on roGFP2-Tpx1.C169S oxidation. a Strain HM123 transformed with p407.C169S was grown in MM-Glu or MM-Gly (respiratory-prone conditions), and H2O2 treatments were performed and oxidation of the reporter estimated as described in Fig. 1. Data from three biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 8a. b Scheme depicting the effect of glucose deprivation in the activation and inactivation of the Sty1 and Pka1 kinases, respectively. Activation of the Sty1 pathway triggers the transcription of Atf1-dependent antioxidant genes such as ctt1 or tpx1. Inactivation of the Pka1 kinase results in the de-repression of gluconeogenic genes and upregulation of mitochondrial oxygen consumption. The Pyp1 phosphatase is a negative regulator of the Sty1 kinase. c Strains HM123 (WT), EP16 (Δsty1), EP193 (Δatf1), and ED1150 (Δcgs1) were transformed with plasmid p407.C169S and basal OxD0 levels determined as described in Fig. 1. Data from three biological replicates with error bars (S.D.) and individual data points overlaid (black dots) are shown. d Strains MC22 (Δpka1), MC24 (Δpka1 Δsty1), and AZ64 (Δpyp1) were transformed with plasmid p407.C169S and H2O2 treatments were performed and oxidation of the reporter estimated as described in Fig. 1. Data from three biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 8b
All cell types respond directly to glucose availability by activating signaling cascades. In S. pombe, the Pka1- and the Sty1-dependent gene expression programs are triggered upon glucose depletion, and are drivers of this glucose-dependent catabolite repression (Fig. 6b). The Pka1 kinase is specifically inactivated upon low-glucose conditions, and the downstream Rst2 transcription factor upregulates, among others, the gluconeogenic fbp1 gene and respiratory-related genes. Deletion of pka1 causes de-repression of fbp131, and high respiratory rates, as determined with oxygen consumption32, even in the presence of elevated glucose levels. The Sty1 kinase and the Atf1 transcription factor are activated not only by glucose deprivation but also by many other environmental signals, and trigger a massive anti-stress gene program, which includes antioxidant activities33.
Final steady-state ROS levels depend on the equilibrium between their synthesis and scavenging. We tested whether activation of the Sty1-Atf1 antioxidant cascade by glucose depletion could cause a decrease in basal H2O2 levels explaining the low OxD0 of roGFP2-Tpx1.C169S in cells grown in respiratory-prone medium. We determined the OxD0 of strains lacking components of the glucose-sensing Pka1 and Sty1 pathways, grown in standard MM (MM-Glu) or in MM-Gly. Unlike wild-type cells, strains lacking Atf1 display very similar levels of OxD0 in both media (Fig. 6c), while cells lacking Cgs1, an inhibitor of Pka1 kinase, behave as wild-type cells. Furthermore, cells lacking Pka1 display constitutive activation of the Sty1-Atf1 cascade through an unknown mechanism32. Concomitantly, the basal level of oxidation of the probe in MM-Glu is significantly lower in cells lacking Pka1 (Fig. 6d, left panel OxD0: 0.26) than in wild-type cells (Fig. 6a, left panel; OxD0: 0.55), in a Sty1-dependent manner (Fig. 6d, center panel, OxD0 in Δpka1 Δsty1 cells: 0.6). These results suggest that upregulation of the Sty1-Atf1 antioxidant response causes the low levels of intracellular peroxides of strain Δpka1. Indeed, constitutive activation of only the MAP kinase Sty1 pathway, by deletion of its main phosphatase Pyp1, is sufficient to trigger low levels of oxidation of the roGFP2-Tpx1.C169S reporter in cells grown in MM-Glu (Fig. 6d, right panel, OxD0 in Δpyp1 cells: 0.3).
Monitoring mitochondrial activity with roGFP2-Tpx1.C169S
As explained above, fission yeast grown in low-glucose concentrations shifts toward increased respiration, even though catabolite repression is not as dramatic as in other microbes27,34. It has been recently reported that glucose-dependent repression is more effective in standard YE medium than in MM, since oxygen consumption and ANT-dependent growth inhibition are lower in YE27,35.
We searched for YE-based growth media altering mitochondrial activity to different extents. We determined oxygen consumption rates of wild-type cultures grown in standard YE rich media, containing 3% glucose, YE-0.08% glucose (this concentration of glucose in MM triggers a shift of fermentation-to-respiration in fission yeast, as nicely reported by Yanagida and colleagues26), and YE-3% glycerol (this media has been recently described to elicit an extreme carbon starvation response at the transcriptional level36). As shown in Fig. 7a, oxygen consumption at the same cell density is significantly lower in YE-3% glucose cultures, being maximal in YE-3% glycerol and intermediate in YE-0.08% glucose cell cultures. Furthermore, the presence of ANT largely compromises the growth of fission yeast in YE-0.08% glucose and YE-3% glycerol, but not in YE-3% glucose (Supplementary Fig. 9a).
roGFP2-Tpx1.C169S oxidation by ANT is proportional to mitochondrial activity. a Oxygen consumption of S. pombe cells grown in different growth media. Wild-type strain 972 was grown on YE media with 3% glycerol (YE-3% Gly), 0.08% glucose (YE-0.08% Glu), or 3% glucose (YE-3% Glu), and when cultures reached an OD600 of 0.5 oxygen consumption was recorded (see Methods). Data from three biological replicates with error bars (S.D.) and individual data points overlaid (black dots) are shown. b, c Strains HM123 (WT) (b) and SG5 (Δtpx1) (c) transformed with plasmid p407.C169S were grown in YE media containing 3% glycerol (YE-3% Gly—green circles), 0.08% glucose (YE-0.08% Glu—red diamonds), or 3% glucose (YE-3% Glu—yellow triangles). At an OD600 of 1, cells were transferred to filtered MM, and probe oxidation upon ANT treatment (5 µM) was monitored for the times indicated. Ten minutes after addition of 1 mM H2O2 was used to determine 100% probe oxidation in each strain and condition, while 0% was the starting OxD0 for each strain and condition. Data from three biological replicates are shown, with error bars (S.D.) displayed in Supplementary Fig. 9bc. d roGFP2-Tpx1.C169S oxidation upon ANT treatment at 45 min from data of panels (b) and (c) is displayed. Data from three biological replicates with error bars (S.D.) and individual data points overlaid (black dots) are shown
We grew wild-type and Δtpx1 cells expressing roGFP2-Tpx1.C169S in the three media, and applied the ETC inhibitor ANT to determine whether enhanced mitochondrial activity could cause increased levels of H2O2 and concomitant probe oxidation. As shown before for MM-based media, the OxD0 varied depending on the media (data not shown). To test the effect of ANT on probe oxidation in the three media, we monitored the percentage of oxidation from each starting OxD0 levels. As shown in Fig. 7b–d and Supplementary Fig. 9bc, in both genetic backgrounds the effect of ANT treatment has a significantly larger effect on roGFP2-Tpx1.C169S oxidation when cells are grown in YE-3% glycerol than in standard YE-3% glucose, with an intermediate oxidation in YE-0.08% glucose. We conclude that roGFP2-Tpx1.C169S oxidation after addition of ETC inhibitors can be used as readout of mitochondrial activity.
Discussion
Redox biology is in need of reliable methods to measure in vivo fluctuations of H2O2. It may be quite easy to quantify large increases, which are normally linked to transient or permanent toxicity. Here, we demonstrate that the Prx-based sensor roGFP2-Tpx1.C169S can monitor differences in steady-state peroxide concentrations of strains carrying defects in the production or scavenging of H2O2. We recently described the gradients established across fission yeast membranes upon extracellular additions of peroxides11. Taking advantage of this knowledge, we can establish that our probe is able to detect intracellular H2O2 fluctuations in the low nanomolar (~10 nM) range, which is below the level where antioxidant redox signaling and toxicity occurs—at least 200–300 nM intracellular is required to trigger activation of the pre-toxic Pap1 signaling cascade11.
We have demonstrated the specificity and sensitivity of roGFP2-Tpx1.C169S to detect subtle fluctuations of intracellular peroxides by modifying extracellular-to-intracellular peroxide gradients through overexpression of AQP8 in fission yeast. Two fluorescent H2O2 reporters, HyPer and a permeable dye, PY1-ME, had been used in mammalian cells to demonstrate that AQPs facilitate H2O2 entry after the addition of 20–50 µM H2O2 to the growing media37,38. Using our reporter roGFP2-Tpx1.C169S, we monitor cytosolic peroxide fluctuations upon 0.1–0.5 µM extracellular peroxides.
The extent of probe oxidation, OxD0, can be used as a readout of steady-state levels of H2O2. Thus, roGFP2-Tpx1.C169S senses basal oxidative stress in cells lacking Tpx1, so that the percentage of basal roGFP2-Tpx1.C169S oxidation in Δtpx1 strain is 20% higher than that of wild-type cells (Fig. 3b). Simple calculations can correlate OxD0 to intracellular steady-state levels of peroxides. In wild-type cells, 25–50 µM extracellular peroxides, which according to the 300:1 gradients corresponds to 0.08–0.16 µM intracellular H2O2, are able to oxidize the probe up to 75–85% (Fig. 3b, left panel). This degree of probe oxidation is observed under basal conditions in cells lacking Tpx1; we have recently reported that the toxic levels of peroxides observed in strain Δtpx1 are in the order of 0.3 µM11.
By using this probe, we have demonstrated the role of moderate and/or severe H2O2 fluctuations in cell biology. For instance, we have confirmed that Tpx1 is the main peroxide scavenger at low concentrations of H2O2, while the role of catalase as a peroxide scavenger starts to be relevant at concentrations of applied peroxides ≥ 0.1 mM (Fig. 4d). We have also shown that reduction of roGFP2-Tpx1.C169S depends on the Grx/GSH system (Fig. 4a), and that probe reduction is sufficiently slow as to allow sensitive and sustained oxidation upon very mild peroxide concentrations. However, probe reduction timely reflects H2O2 scavenging in living cells: while upon 1 mM H2O2 extracellular stress wild-type cells can resume growth after ~5 h and roGFP2-Tpx1.C169S becomes reduced at the same time, cells lacking Ctt1 cannot resume growth when exposed to this level of oxidative stress and the probe remains oxidized for as long as 7 h (Fig. 4d).
Genetic alterations of the Trx or GSH/Grx systems can influence the redox state of roGFP2-Tpx1.C169S at the level of oxidation (in a Tpx1-dependen manner) or reduction, respectively (see Fig. 4c). This suggests that interventions known to affect not only H2O2 homeostasis but also the reduced-to-oxidized ratios of redoxins or GSH may affect roGFP2-Tpx1.C169S redox state.
During the course of our study, we have attempted to demonstrate mitochondrial ROS production during respiratory-prone conditions by depleting glucose from the media. As explained in the Results section, glucose-mediated catabolite repression is not dramatic in S. pombe, as it is in E. coli or S. cerevisiae. Thus, we have used MM-based (Fig. 6) and YE-based (Fig. 7) media with different concentrations of carbon sources to accomplish varying degrees of mitochondrial metabolism, and monitor their impact on roGFP2-Tpx1.C169S oxidation. We initially (Fig. 6) compared probe oxidation in cells grown in standard, high-glucose MM (MM-Glu) or in respiratory-prone MM-Gly. To our surprise, respiratory-prone media, known to induce mitochondrial activity, did not cause roGFP2-Tpx1.C169S oxidation but rather probe reduction (Fig. 6), suggesting that intracellular H2O2 levels are lower in cells grown in this media. Glucose depletion triggers, among others, the Sty1-Atf1 cascade and its antioxidant gene expression program35. In cells lacking the transcription factor Atf1, where the antioxidant program cannot be induced upon glucose deprivation, the OxD0 in MM-Gly is not lower than in MM-Glu (Fig. 6c). On the contrary, cells lacking the Sty1 phosphatase Pyp1, or the kinase Pka1, where the antioxidant gene expression program is constitutively engaged even with high glucose, the OxD0 of our probe in MM-Glu is very low, similar to that of a wild-type cells grown in MM-Gly (Fig. 6d). We conclude that glucose depletion causes the upregulation of the antioxidant gene expression program, and as a consequence, steady-state levels of peroxides are dampened, since scavenging prevails over mitochondrial H2O2 production.
An alternative strategy to promote mitochondrial H2O2 leakage different from lowering glucose from the media is the use of ETC inhibitors. Thus, we have demonstrated that addition of ANT, a complex III inhibitor which favors H2O2 release from the mitochondria, can cause cytosolic roGFP2-Tpx1.C169S oxidation. Furthermore, the extent of probe oxidation upon ANT addition can be used as readout of changes in mitochondrial activity due to glucose availability (Fig. 7). Thus, we have first measured oxygen consumption in three types of YE-based media to characterize their mitochondrial activities (Fig. 7a). The extent of probe oxidation upon inhibition by ANT is directly proportional to the mitochondrial activity in each media (Fig. 7b–d). However, this strategy does not allow the measurement of physiological mitochondrial H2O2 production unless the ETC inhibitor is added. Expression of the probe in the mitochondrial matrix or inter-membrane space may be required to monitor H2O2 release upon massive ETC usage during mitochondrial respiration in the absence of ANT or other inhibitors, and experiments are underway.
In conclusion, we have exploited the use of fission yeast Tpx1.C169S as an exquisitely sensitive H2O2 sensor and signal transducer to roGFP. We have used the fission yeast model system to demonstrate the behavior of roGFP2-Tpx1.C169S, and provide numbers to redox biology events, such as mitochondrial H2O2 production, activation of signaling cascades and toxicity linked to enhanced H2O2 levels. We have also described that low-glucose-driven respiratory-prone conditions, known to stimulate mitochondrial activity, also trigger antioxidant signaling cascades, with a final decrease in cytosolic H2O2 levels. This indicates that steady-state levels of peroxides have to be experimentally determined to confirm or dismiss oxidative stress in a giving cellular context, since measuring only H2O2 production or scavenging may not be sufficient to anticipate which of both prevails. A remarkable example of complexity regarding glucose metabolism and H2O2 levels is tumor progression. Thus, cancer cells had been proposed to enhance not only glycolysis and glucose uptake, as proposed by Warburg in the 50s39, but also mitochondrial metabolism, being respiration the main energy source in tumor cells (for excellent reviews on cancer metabolic reprogramming, see refs. 40,41,42). Importantly, both mitochondrial H2O2 production and scavenging are enhanced in tumors compared with the surrounding normal cells43. Therefore, without in vivo measurements it is unpredictable to anticipate the steady-state levels of peroxides in cancer cells. Now, we expect our probe to be expressed and tested in other model systems, including human tumor cell lines, to find out whether the same intracellular H2O2 thresholds define the boundaries between aerobic metabolism, activation of antioxidant cascades, and toxicity linked to oxidative stress.
Methods
Fission yeast growth media and genetic manipulations
For most of the experiments, cells were grown in filtered minimal medium (MM or MM-Glu, carrying 2% glucose) at 30 °C44, supplemented with uracil, adenine, leucine, and/or cysteine in auxotrophic strains. We also used, when indicated, MM-Gly, containing as carbon source 1.85% glycerol and 0.15% glucose. Yeast extract (YE) rich medium or autoclaved MM could not be used for direct fluorescence measurements in the plate reader, since they display strong interference with intracellular fluorescence. When indicated, cells were grown in rich medium (YE) with various concentrations and sources of carbon supplies: 3% glucose, 0.08% glucose, or 3% glycerol.
Generation of plasmids and strains
All the plasmids described here express fluorescent reporters under the control of the constitutive sty1 promoter22, and most of them are episomal, with an average of 7–8 plasmid copies per cell45. According to previous quantifications of other proteins expressed from the same promoter11, the intracellular protein concentration arising from these constructs is in the order of 2–10 µM. HyPer and Grx1-roGFP2 were PCR-amplified from the original pHyPer-Cyto (Evrogen) and pLPCX cyto Grx1-roGFP221 plasmids, and cloned after a HA-coding sequence, yielding plasmids p605 and p379; these plasmids allowed the expression of HA-HyPer and HA-Grx1-linker (L)-roGFP2, respectively. We also PCR-amplified from pLPCX cyto Grx1-roGFP2 the DNA sequences coding for roGFP2 open reading frame and for the intermediate linker (L), coding for a 30 amino acids-long glycine-rich domain, and cloned them in tandem yielding plasmid p406, coding for roGFP2-L. This plasmid was used as a backbone to clone three PCR-amplified open reading frames from S. pombe, yielding plasmids p429, p407, and p407.C169S, allowing the expression of roGFP2-L-Gpx1, roGFP2-L-Tpx1, and roGFP2-L-Tpx1.C169S, respectively. p406 was also used to clone a synthetic open reading frame (supplied by IDT Technologies) coding for Tsa2ΔCR, yielding plasmid p676 coding for roGFP2-Tsa2ΔCR. We also generated an integrative plasmid for the expression of roGFP-Tpx1.C169S under the control of the constitutive sty1 promoter, named p504′.C169S. To express human AQPs in fission yeast, we ordered synthetic genes coding for AQP8 and AQP4 flanked by an endoplasmic reticulum driving coding sequence at 5′, and Myc and FLAG tag coding sequence at 3′ (IDT Technologies), and cloned them in the ura4 episomal vector pREP.42x plasmid46, with the nmt promoter substituted with the sty1 promoter, yielding p675 (AQP8) and p682 (AQP4). The absence of mutations in each of these plasmids was confirmed by DNA sequencing. The genotypes of the strains used in this study are shown in Supplementary Table 1. Some of these strains were constructed for this study as follows. Strain SG63 was derived from the Δpap1 strain of a S. pombe haploid deletion collection (Bioneer), by replacing the kanMX6 with natMX6 cassette. Strain AD88 was constructed by crossing AD8411 (h− pgr1::natMX6) with JA212 (h+ leu1-32; our laboratory stock) and selecting clones in MM plates supplemented with leucine. Strain SG60 was obtained by replacing kanMX6 cassette from strain MJ1547 (h+ trx1::kanMX6) with natMX6 cassette. Strains SG61 and EP16 were obtained by insertion of an ura4 cassette in the trx1 and sty1 loci, respectively, of strain PN513 (h− ura4-D18 leu1-32; our laboratory stock). Strain SG260 was obtained by mating MJ1547 (h+ trx1::kanMX6) with SG25748 (h− trx3::natMX6). Strain EP160 was obtained by crossing CN51349 (h− ctt1::ura4 ade6-M216 leu1-32 ura4-D18) with JA368 (h+ ura4-D18 leu1-32; our laboratory stock) and selecting clones in MM supplemented with leucine but lacking uracile. Strain AZ81 was obtained by crossing AZ61 (h+ pka1::kanMX6 sty1::ura4, obtained by insertion of an ura4 cassette in the sty1 locus of a pka1::kanMX6 strain from Bioneer collection) with JA365 (h− ura4-D18; our laboratory stock) and selecting clones in MM plates. The Δpyp1 strain of the Bioneer S. pombe haploid deletion collection was crossed with 972 to yield strain AZ64 after selection in plates supplemented with leucine. Strains HM123 (wild-type), SG5 (Δtpx1), Δcoq4, AD88 (Δpgr1), SG61 (Δtrx1), IC76 (Δtrx1 Δtrx3), EP160 (Δctt1), MC22 (Δpka1), and MC24 (Δpka1 Δsty1) were transformed with the episomal plasmids described above following standard genetic techniques50, and transformants were selected in MM plates lacking leucine; only strain SB36 (Δgrx1), without leucine auxotrophy, was crossed with HM123 carrying p407.C169S, and plasmid acquisition was selected by fluorescence. The integrative plasmid p504′.C169S was inserted at the leu1-32 loci of strain SG5 (Δtpx1), yielding strain MC167.C169S.
Growth of strains expressing HyPer or roGFP derivatives
For wild-type backgrounds, standard MM-based early stationary phase pre-cultures were diluted in filtered MM (either MM-Glu or MM-Gly), or in YE-based media (see above) to reach an OD600 of 1 after 4–5 duplications. In the case of some strain backgrounds displaying defects to grow under aerobic conditions, such as Δtpx1, Δpgr1, and Δcoq4, pre-cultures were grown under anaerobic conditions, using capped-bottles filled to the top with MM medium at 30 °C without shaking; these anaerobic pre-cultures were then diluted in normal aerobic MM-containing flasks (Δtpx1) or anaerobic capped-bottles (Δpgr1 and Δcoq4), till cultures reached an OD600 of 1 after 3–4 duplications. For strains Δtrx1 and Δtrx1 Δtrx3, auxotrophic for cysteine, MM aerobic cultures contained 38 µg/ml cysteine. The fluorescence of 190 µl of these cultures, at an OD600 of 1, was directly monitored in 96-well plates in a Fluorstar OMEGA (BMG Labtech) as described below. In the case of strain MC98.C169S, expressing low levels of roGFP2-Tpx1.C169S from an integrative plasmid, MM cultures at an OD600 of 1 obtained as above where gently centrifuged at 1000 × g for 3 min at room temperature, and 75% of the supernatant was withdrawn; the remaining buffer was used to resuspend the cell pellets reaching an OD600 of 4; this concentrated cultures were monitored in the fluorescence plate reader. In the case of strains growing in YE-based media, cell cultures were centrifuged and resuspended in the same volume of standard MM to allow fluorescence readings.
In vivo measurement of HyPer and roGFP-derivatives oxidation
HyPer has two excitation maxima at 420 and 500 nm and one emission peak at 516 nm14. Similarly, roGFP2 exhibits two excitation maxima at 400 nm and 475–490 nm when fluorescence emission is monitored at 510 nm. Either HyPer or roGFP2 oxidation was monitored using a fluorescence plate reader FLUOstar OMEGA (BMG Labtech). In both cases, we used excitation filters of 400-10 and 485BP12, combined with emission filter EM520. For each strain to be analyzed, we transferred 190 µl of the MM culture at an OD600 of 1 (strains carrying episomal plasmids) or 4 (strains with integrative plasmids) to as many wells of a 96-well imaging plate (Krystal Microplate™ 215003, Porvair Sciences) as experimental treatments were to be tested. Recording at the two excitation wavelengths was initiated, with four readings every 1–1.5 min, with continuous shaking at 30 °C. After these four cycles, 10 µl of MM containing or not the different treatments were added to the wells to accomplish the final concentrations of reagents indicated in the figures, and recording rapidly proceeded. To one well, the oxidant N,N,N′,N′-tetramethylazodicarboxamide (diamide) was added to a final concentration of 5 mM, and to another well the reductant dithiothreitol (DTT) was added to a final concentration of 50 mM. These samples served as fully oxidized and fully reduced sensor controls for roGFP derivatives (see below); for some strains (Δpgr1, Δcoq4, and Δtrx1 Δtrx3 strains) or growth conditions (MM-Gly and low glucose YE cultures), treatment with 1 mM H2O2 were used as fully oxidized sensor references instead of diamide. S. pombe cells displayed basal levels of fluorescence at 510 nm after excitation at either 400 or 485 nm, and this basal fluorescence changed with diamide and DTT treatments; therefore, for each strain expressing HyPer or roGFP derivatives, we grew cultures of the same genetic background lacking the plasmids, and performed the same treatments on the 96-well plates as to their genetic counterparts expressing the chimeras; then, after recording, for each specific treatment we subtracted the fluorescence values of the empty strain to those of the strain expressing the reporter. In the case of YE-based cultures, in which we had to change the culture media for fluorescence recording, we did not subtract the fluorescence values of the empty strains. For HyPer, diamide and DTT only had modest effects in oxidation and reduction of the probe, and could not be used as controls of maximal oxidation/reduction; therefore, we only represent the data as ratio 488/405 nm. For roGFP derivatives, we determined the degree of sensor oxidation (OxD) with the following Eq. (1) as described51:
where I represents the fluorescence intensity at 510 nm after excitation at either 405 nm or 488 nm of the sample treated with H2O2 at a given time and concentration, the control values intensity after diamide or DTT treatments were the fluorescence intensity values after 5–10 min of these control treatments. When indicated (Fig. 7, YE-based cultures) the OxD values of the cultures were expressed in a 0-to-100% scale, 0 being the starting OxD for each strain in the specific culture media and 100% being the maximum level of oxidation upon 1 mM H2O2 10 min after stress imposition. All experiments were performed in biological triplicates (or duplicates, when indicated) using cells obtained from three independent cell cultures. For each new strain to be tested, HM123 carrying plasmid p407.C169S was always added and analyzed in the same 96-well plate, to use it as an internal control of basal probe oxidation.
Oxygen sensitivity assay on solid plates
For survival on solid plates, S. pombe strains were grown, diluted and spotted on MM plates and plates were incubated at 30 °C for 3–4 days. To grow cells in solid media in an anaerobic environment, we incubated the plates at 30 °C in a tightly sealed plastic bag containing a water-activated Anaerocult A sachet (Merck, Darmstadt, Germany)20, or in a nitrogen-filled anaerobic chamber (Forma Anaerobic System, Thermo Electron Corp.).
Measurement of oxygen consumption
Oxygen consumption of 2 × 107 cells in 1 ml was performed as described before27. Briefly, cells were grown in YE media containing different concentrations of carbon sources till they reached an OD600 of 0.5. Cells were harvested, and resuspended in 1 ml of standard MM to a final OD600 of 1. The measurements were made using a Hansatech Oxygraph (Hansatech), with readings being recorded during 10 min. Each one of the measurements was performed from biological triplicates.
Growth curves
Cells from cultures of wild-type strain 972 in standard YE at an OD600 of 1 were harvested and resuspended in YE with different carbon sources to an OD600 of 0.1. Cell growth was monitored using an assay based on automatic measurements of optical densities, as previously described52. Briefly, for each resuspended culture we placed 100-µl samples, with or without ANT (5 µM), into 96-well non-coated polystyrene microplates with an adhesive plate seal. Each experimental condition was measured in duplicate (technical replicates). We used Power Wave microplate scanning spectrophotometer (Bio-Tek) to obtain the growth curves. Incubation temperature was kept at 30 °C, the microplates were subjected to continuous shaking and the readings were done every 10 min during a 48 h period. The OD600 was automatically recorded using Gen5 software. Each one of the growth curves was performed from biological triplicates.
Statistics
For in vivo fluorescence probe oxidation quantification, OxD, one independent culture of the strain of interest was grown for each replicate. In all figure panels, values of mean of n = 3 are indicated, with the mean ± s.d displayed in supplementary figures. Only in some specific figure panels, results from biological duplicates are shown. The exact n value is described in each figure legend. In the case of spot assays, 3-to-4 independent experiments with very similar results were performed.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Garcia-Santamarina, S., Boronat, S. & Hidalgo, E. Reversible cysteine oxidation in hydrogen peroxide sensing and signal transduction. Biochemistry 53, 2560–2580 (2014).
Stocker, S., Van Laer, K., Mijuskovic, A. & Dick, T. P. The conundrum of hydrogen peroxide signaling and the emerging role of peroxiredoxins as redox relay hubs. Antioxid Redox Signal 28, 558–573 (2017).
Antunes, F. & Brito, P. M. Quantitative biology of hydrogen peroxide signaling. Redox Biol. 13, 1–7 (2017).
Lu, J. & Holmgren, A. The thioredoxin superfamily in oxidative protein folding. Antioxid. Redox Signal 21, 457–470 (2014).
Herrero, E., Ros, J., Belli, G. & Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim Biophys. Acta 1780, 1217–1235 (2008).
Winterbourn, C. C. Are free radicals involved in thiol-based redox signaling? Free Radic. Biol. Med. 80, 164–170 (2015).
Zheng, M., Aslund, F. & Storz, G. Activation of the OxyR transcription factor by reversible disulfide bond formation. Science 279, 1718–1721 (1998).
Vivancos, A. P. et al. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc. Natl Acad. Sci. USA 102, 8875–8880 (2005).
Bozonet, S. M. et al. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. J. Biol. Chem. 280, 23319–23327 (2005).
Winterbourn, C. C. & Hampton, M. B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 45, 549–561 (2008).
Domenech, A., Ayte, J., Antunes, F. & Hidalgo, E. Using in vivo oxidation status of one- and two-component redox relays to determine H2O2 levels linked to signaling and toxicity. BMC Biol. 16, 61 (2018).
Winterbourn, C. C. The challenges of using fluorescent probes to detect and quantify specific reactive oxygen species in living cells. Biochim Biophys. Acta 1840, 730–738 (2014).
Hanson, G. T. et al. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 279, 13044–13053 (2004).
Belousov, V. V. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 3, 281–286 (2006).
Lukyanov, K. A. & Belousov, V. V. Genetically encoded fluorescent redox sensors. Biochim Biophys. Acta 1840, 745–756 (2014).
Meyer, A. J. & Dick, T. P. Fluorescent protein-based redox probes. Antioxid. Redox Signal 13, 621–650 (2010).
Gutscher, M. et al. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 284, 31532–31540 (2009).
Morgan, B. et al. Real-time monitoring of basal H2O2 levels with peroxiredoxin-based probes. Nat. Chem. Biol. 12, 437–443 (2016).
Fujikawa, Y. et al. Mouse redox histology using genetically encoded probes. Sci. Signal 9, rs1 (2016).
Jara, M. et al. The peroxiredoxin Tpx1 is essential as a H2O2 scavenger during aerobic growth in fission yeast. Mol. Biol. Cell 18, 2288–2295 (2007).
Gutscher, M. et al. Real-time imaging of the intracellular glutathione redox potential. Nat. Methods 5, 553–559 (2008).
Paulo, E. et al. A genetic approach to study H2O2 scavenging in fission yeast–distinct roles of peroxiredoxin and catalase. Mol. Microbiol. 92, 246–257 (2014).
Bienert, G. P. & Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim Biophys. Acta 1840, 1596–1604 (2014).
Calvo, I. A. et al. Dissection of a redox relay: H2O2-dependent activation of the transcription factor Pap1 through the peroxidatic Tpx1-thioredoxin cycle. Cell Rep. 5, 1413–1424 (2013).
Gonzalez-Flecha, B. & Demple, B. Metabolic sources of hydrogen peroxide in aerobically growing Escherichia coli. J. Biol. Chem. 270, 13681–13687 (1995).
Takeda, K., Starzynski, C., Mori, A. & Yanagida, M. The critical glucose concentration for respiration-independent proliferation of fission yeast, Schizosaccharomyces pombe. Mitochondrion 22, 91–95 (2015).
Zuin, A. et al. Mitochondrial dysfunction increases oxidative stress and decreases chronological life span in fission yeast. PLoS ONE 3, e2842 (2008).
Gomes, F., Tahara, E. B., Busso, C., Kowaltowski, A. J. & Barros, M. H. nde1 deletion improves mitochondrial DNA maintenance in Saccharomyces cerevisiae coenzyme Q mutants. Biochem J. 449, 595–603 (2013).
Crabtree, H. G. Observations on the carbohydrate metabolism of tumours. Biochem. J. 23, 536–545 (1929).
Diaz-Ruiz, R., Rigoulet, M. & Devin, A. The Warburg and Crabtree effects: on the origin of cancer cell energy metabolism and of yeast glucose repression. Biochim Biophys. Acta 1807, 568–576 (2011).
Hoffman, C. S. & Winston, F. Glucose repression of transcription of the Schizosaccharomyces pombe fbp1 gene occurs by a cAMP signaling pathway. Genes Dev. 5, 561–571 (1991).
Zuin, A. et al. Lifespan extension by calorie restriction relies on the Sty1 MAP kinase stress pathway. Embo J. 29, 981–991 (2010).
Chen, D. et al. Global transcriptional responses of fission yeast to environmental stress. Mol. Biol. Cell 14, 214–229 (2003).
Heslot, H., Goffeau, A. & Louis, C. Respiratory metabolism of a “petite negative” yeast Schizosaccharomyces pombe 972h. J. Bacteriol. 104, 473–481 (1970).
Malecki, M. et al. Functional and regulatory profiling of energy metabolism in fission yeast. Genome Biol. 17, 240 (2016).
Vassiliadis, D., Wong, K. H., Andrianopoulos, A. & Monahan, B. J. A genome-wide analysis of carbon catabolite repression in Schizosaccharomyces pombe. BMC Genomics 20, 251 (2019).
Bertolotti, M. et al. Tyrosine kinase signal modulation: a matter of H2O2 membrane permeability? Antioxid. Redox Signal 19, 1447–1451 (2013).
Miller, E. W., Dickinson, B. C. & Chang, C. J. Aquaporin-3 mediates hydrogen peroxide uptake to regulate downstream intracellular signaling. Proc. Natl Acad. Sci. USA 107, 15681–15686 (2010).
Warburg, O. On the origin of cancer cells. Science 123, 309–314 (1956).
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev. Cell Dev. Biol. 27, 441–464 (2011).
Pavlova, N. N. & Thompson, C. B. The emerging hallmarks of cancer metabolism. Cell Metab. 23, 27–47 (2016).
DeBerardinis, R. J. & Chandel, N. S. Fundamentals of cancer metabolism. Sci. Adv. 2, e1600200 (2016).
Chandel, N. S. & Tuveson, D. A. The promise and perils of antioxidants for cancer patients. N. Engl. J. Med 371, 177–178 (2014).
Alfa, C., Fantes, P., Hyams, J., McLeod, M. & Warbrick, E. Experiments with Fission Yeast: A Laboratory Course Manual. (Cold Spring Harbor, N.Y, 1993).
Brun, C., Dubey, D. D. & Huberman, J. A. pDblet, a stable autonomously replicating shuttle vector for Schizosaccharomyces pombe. Gene 164, 173–177 (1995).
Maundrell, K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene 123, 127–130 (1993).
Garcia-Santamarina, S. et al. Is oxidized thioredoxin a major trigger for cysteine oxidation? Clues from a redox proteomics approach. Antioxid. Redox Signal 18, 1549–1556 (2013).
Garcia-Santamarina, S., Boronat, S., Ayte, J. & Hidalgo, E. Methionine sulphoxide reductases revisited: free methionine as a primary target of H2O2 stress in auxotrophic fission yeast. Mol. Microbiol. 90, 1113–1124 (2013).
Nakagawa, C. W., Yamada, K. & Mutoh, N. Two distinct upstream regions are involved in expression of the catalase gene in Schizosaccharomyces pombe in response to oxidative stress. J. Biochem. 123, 1048–1054 (1998).
Moreno, S., Klar, A. & Nurse, P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 194, 795–823 (1991).
Morgan, B., Sobotta, M. C. & Dick, T. P. Measuring E(GSH) and H2O2 with roGFP2-based redox probes. Free Radic. Biol. Med. 51, 1943–1951 (2011).
Calvo, I. A. et al. Genome-wide screen of genes required for caffeine tolerance in fission yeast. PLoS ONE 4, e6619 (2009).
Acknowledgements
We acknowledge Rosa Bretón and Santiago Lamas for providing plasmid pLPCX Grx1-roGFP2, and Guy Lenaers for strain Δcgs1. This work is supported by the Ministerio de Ciencia, Innovación y Universidades (Spain), PLAN E and FEDER (BFU2015-68350-P and PGC2018-093920-B-I00 to E.H.) and by Unidad de Excelencia María de Maeztu (MDM-2014-0370). The Oxidative Stress and Cell Cycle group is also supported by Generalitat de Catalunya (Spain) (2017-SGR-539). E.H. is recipient of an ICREA Academia Award (Generalitat de Catalunya, Spain).
Author information
Authors and Affiliations
Contributions
M.C., L.C., E.B. and M.M.-B. performed most experiments. M.C., L.C., S.B., I.M.-F., R.S., J.A. and E.H. analyzed the data. E.H. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Communications thanks Bruce Morgan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carmona, M., de Cubas, L., Bautista, E. et al. Monitoring cytosolic H2O2 fluctuations arising from altered plasma membrane gradients or from mitochondrial activity. Nat Commun 10, 4526 (2019). https://doi.org/10.1038/s41467-019-12475-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-019-12475-0
- Springer Nature Limited
This article is cited by
-
Impact of silver nanoparticles on secondary metabolite composition and toxicity in anise (Pimpinella anisum L.) callus culture
BMC Plant Biology (2024)
-
Mitochondrial H2O2 release does not directly cause damage to chromosomal DNA
Nature Communications (2024)