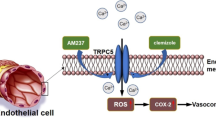
Abstract
Silybin is a flavonolignan extracted from the seeds of Silybum marianum that has been used as a dietary supplement for treating hepatic diseases and components of metabolic syndrome such as diabetes, obesity and hypertension. Transient receptor potential vanilloid 4 (TRPV4) channels are Ca2+-permeable, nonselective cation channels that regulate vascular endothelial function and blood flow. However, the relationship between silybin and TRPV4 channels in small mesenteric arteries remains unknown. In our study, we carried out a molecular docking experiment by using Discovery Studio v3.5 to predict the binding of silybin to TRPV4. Activation of TRPV4 with silybin was detected via intracellular Ca2+ concentration ([Ca2+]i) measurement and patch clamp experiments. The molecular docking results showed that silybin was likely to bind to the ankyrin repeat domain of TPRV4. [Ca2+]i measurements in mesenteric arterial endothelial cells (MAECs) and TRPV4-overexpressing HEK293 (TRPV4–HEK293) cells demonstrated that silybin induced Ca2+ influx by activating TRPV4 channels. The patch clamp experiments indicated that in TRPV4–HEK293 cells, silybin induced TRPV4-mediated cation currents. In addition, in high-salt-induced hypertensive mice, oral administration of silybin decreased systolic blood pressure (SBP) and significantly improved the arterial dilatory response to acetylcholine. Our findings provide the first evidence that silybin could induce mesenteric endothelium-dependent vasodilation and reduce blood pressure in high-salt-induced hypertensive mice via TRPV4 channels, thereby revealing the potential effect of silybin on preventing endothelial dysfunction-related cardiovascular diseases.






Similar content being viewed by others
Change history
20 September 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41440-022-01040-w
References
Bunchorntavakul C, Reddy KR. Review article: herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther. 2013;37:3–17.
Federico A, Dallio M, Loguercio C. Silymarin/Silybin and chronic liver disease: a marriage of many years. Molecules. 2017;22:191.
Loguercio C, Festi D. Silybin and the liver: from basic research to clinical practice. World J Gastroenterol. 2011;17:2288–301.
Janeczko M, Kochanowicz E. Silymarin, a popular dietary supplement shows anti-candida activity. Antibiotics. 2019;8:206.
Li Volti G, Salomone S, Sorrenti V, Mangiameli A, Urso V, Siarkos I, et al. Effect of silibinin on endothelial dysfunction and ADMA levels in obese diabetic mice. Cardiovasc Diabetol. 2011;10:62.
Sciacqua A, Perticone M, Tripepi G, Addesi D, Cassano V, Maio R, et al. Metabolic and vascular effects of silybin in hypertensive patients with high 1-h post-load plasma glucose. Intern Emerg Med. 2019;14:77–84.
Li X, Lin Y, Zhou H, Li Y, Wang A, Wang H, et al. Puerarin protects against endothelial dysfunction and end-organ damage in Ang II-induced hypertension. Clin Exp Hypertens. 2017;39:58–64.
Gonzalez J, Valls N, Brito R, Rodrigo R. Essential hypertension and oxidative stress: new insights. World J Cardiol. 2014;6:353–66.
Konukoglu D, Uzun H. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–40.
Heathcote HR, Lee MD, Zhang X, Saunter CD, Wilson C, McCarron JG. Endothelial TRPV4 channels modulate vascular tone by Ca2+-induced Ca2+ release at inositol 1,4,5-trisphosphate receptors. Br J Pharmacol. 2019;176:3297–317.
Filosa JA, Yao X, Rath G. TRPV4 and the regulation of vascular tone. J Cardiovasc Pharmacol. 2013;61:113–9.
Chen YL, Sonkusare SK. Endothelial TRPV4 channels and vasodilator reactivity. Curr Top Membr. 2020;85:89–117.
O’Neil RG, Heller S. The mechanosensitive nature of TRPV channels. Pflug Arch. 2005;451:193–203.
Cao S, Anishkin A, Zinkevich NS, Nishijima Y, Korishettar A, Wang Z, et al. Transient receptor potential vanilloid 4 (TRPV4) activation by arachidonic acid requires protein kinase A-mediated phosphorylation. J Biol Chem. 2018;293:5307–22.
Liu L, Guo M, Lv X, Wang Z, Yang J, Li Y, et al. Role of transient receptor potential vanilloid 4 in vascular function. Front Mol Biosci. 2021;8:677661.
Zhou T, Wang Z, Guo M, Zhang K, Geng L, Mao A, et al. Puerarin induces mouse mesenteric vasodilation and ameliorates hypertension involving endothelial TRPV4 channels. Food Funct. 2020;11:10137–48.
Shao J, Han J, Zhu Y, Mao A, Wang Z, Zhang K, et al. Curcumin induces endothelium-dependent relaxation by activating endothelial TRPV4 channels. J Cardiovasc Transl Res. 2019;12:600–7.
Zhang X, Mao A, Xiao W, Zhang P, Han X, Zhou T, et al. Morin induces endothelium-dependent relaxation by activating TRPV4 channels in rat mesenteric arteries. Eur J Pharmacol. 2019;859:172561.
Meduru H, Wang YT, Tsai JJ, Chen YC. Finding a potential dipeptidyl peptidase-4 (DPP-4) inhibitor for type-2 diabetes treatment based on molecular docking, pharmacophore generation, and molecular dynamics simulation. Int J Mol Sci. 2016;17:920.
Tang H, Wang H, Lin Q, Fan F, Zhang F, Peng X, et al. Loss of IP3 receptor-mediated Ca(2+) release in mouse B cells results in abnormal B cell development and function. J Immunol. 2017;199:570–80.
Zhang P, Sun C, Li H, Tang C, Kan H, Yang Z, et al. TRPV4 (Transient Receptor Potential Vanilloid 4) mediates endothelium-dependent contractions in the aortas of hypertensive mice. Hypertension. 2018;71:134–42.
Khaldan A, Bouamrane S, En-Nahli F, El-Mernissi R, El Khatabi K, Hmamouchi R, et al. Prediction of potential inhibitors of SARS-CoV-2 using 3D-QSAR, molecular docking modeling and ADMET properties. Heliyon 2021;7:e06603.
Takahashi N, Hamada-Nakahara S, Itoh Y, Takemura K, Shimada A, Ueda Y, et al. TRPV4 channel activity is modulated by direct interaction of the ankyrin domain to PI(4,5)P(2). Nat Commun. 2014;5:4994.
Inada H, Procko E, Sotomayor M, Gaudet R. Structural and biochemical consequences of disease-causing mutations in the ankyrin repeat domain of the human TRPV4 channel. Biochemistry. 2012;51:6195–206.
Kren V. Chirality matters: biological activity of optically pure silybin and its congeners. Int J Mol Sci. 2021;22:7885.
Xie Y, Zhang D, Zhang J, Yuan J. Metabolism, transport and drug-drug interactions of silymarin. Molecules. 2019;24:3693.
Aghazadeh S, Amini R, Yazdanparast R, Ghaffari SH. Anti-apoptotic and anti-inflammatory effects of Silybum marianum in treatment of experimental steatohepatitis. Exp Toxicol Pathol. 2011;63:569–74.
Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol. 2013;5:109–13.
Marino Z, Crespo G, D’Amato M, Brambilla N, Giacovelli G, Rovati L, et al. Intravenous silibinin monotherapy shows significant antiviral activity in HCV-infected patients in the peri-transplantation period. J Hepatol. 2013;58:415–20.
Abenavoli L, Izzo AA, Milic N, Cicala C, Santini A, Capasso R. Milk thistle (Silybum marianum): A concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytother Res. 2018;32:2202–13.
Pignatelli P, Carnevale R, Menichelli D. Silybin and metabolic disorders. Intern Emerg Med. 2019;14:1–3.
Hajiaghamohammadi AA, Ziaee A, Oveisi S, Masroor H. Effects of metformin, pioglitazone, and silymarin treatment on non-alcoholic Fatty liver disease: a randomized controlled pilot study. Hepat Mon. 2012;12:e6099.
Yao J, Zhi M, Gao X, Hu P, Li C, Yang X. Effect and the probable mechanisms of silibinin in regulating insulin resistance in the liver of rats with non-alcoholic fatty liver. Braz J Med Biol Res. 2013;46:270–7.
Wah Kheong C, Nik Mustapha NR, Mahadeva S. A randomized trial of silymarin for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2017;15:1940–9 e1948.
Grace MS, Bonvini SJ, Belvisi MG, McIntyre P. Modulation of the TRPV4 ion channel as a therapeutic target for disease. Pharmacol Ther. 2017;177:9–22.
Zhang T, Kawaguchi N, Yoshihara K, Hayama E, Furutani Y, Kawaguchi K, et al. Silibinin efficacy in a rat model of pulmonary arterial hypertension using monocrotaline and chronic hypoxia. Respir Res. 2019;20:79.
Perticone F, Sciacqua A, Maio R, Perticone M, Maas R, Boger RH, et al. Asymmetric dimethylarginine, L-arginine, and endothelial dysfunction in essential hypertension. J Am Coll Cardiol. 2005;46:518–23.
Yao J, Zhi M, Minhu C. Effect of silybin on high-fat-induced fatty liver in rats. Braz J Med Biol Res. 2011;44:652–9.
Sun YH, Zhao J, Jin HT, Cao Y, Ming T, Zhang LL, et al. Vasorelaxant effects of the extracts and some flavonoids from the buds of Coreopsis tinctoria. Pharm Biol. 2013;51:1158–64.
Zhang BN, Hou YL, Liu BJ, Liu QM, Qiao GF. The Rhododendron dauricum L. Flavonoids Exert Vasodilation and Myocardial Preservation. Iran J Pharm Res. 2010;9:303–11.
Pan Z, Feng T, Shan L, Cai B, Chu W, Niu H, et al. Scutellarin-induced endothelium-independent relaxation in rat aorta. Phytother Res. 2008;22:1428–33.
Ham J, Lim W, Bazer FW, Song G. Silibinin stimluates apoptosis by inducing generation of ROS and ER stress in human choriocarcinoma cells. J Cell Physiol. 2018;233:1638–49.
Kim SH, Kim KY, Yu SN, Seo YK, Chun SS, Yu HS, et al. Silibinin induces mitochondrial NOX4-mediated endoplasmic reticulum stress response and its subsequent apoptosis. BMC Cancer. 2016;16:452.
Ham J, Kim J, Bazer FW, Lim W, Song G. Silibinin-induced endoplasmic reticulum stress and mitochondrial dysfunction suppress growth of endometriotic lesions. J Cell Physiol. 2019;234:4327–41.
Acknowledgements
This work was fully supported by the National Natural Science Foundation of China [81622007, 81960662, 81800430], the Chang Jiang Scholars Program [Q2015106], Fundamental Research Funds for the Central Universities [JUSRP51704A], the National First-Class Discipline Program of Food Science and Technology [JUFSTR20180101], Postdoctoral Research Funding Program of Jiangsu Province [2020Z427], Wuxi Health Commission [1286010241210430], Wuxi Health Commission grant no. M202044 and Foundation of Wuxi Science and Technology Bureau grant no. CSE31N1702.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In the version of this article initially published, the acknowledgements section omitted Wuxi Health Commission grant no. M202044 and Foundation of Wuxi Science and Technology Bureau grant no. CSE31N1702.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wen, X., Peng, Y., Zheng, B. et al. Silybin induces endothelium-dependent vasodilation via TRPV4 channels in mouse mesenteric arteries. Hypertens Res 45, 1954–1963 (2022). https://doi.org/10.1038/s41440-022-01000-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-022-01000-4
- Springer Nature Singapore Pte Ltd.