Abstract
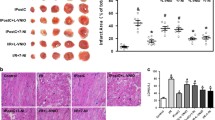
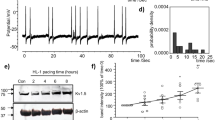
Myocardial ischemia/reperfusion injury worsens in the absence of nitric oxide synthase (NOS). Cilnidipine, a Ca2+ channel blocker, has been reported to activate endothelial NOS (eNOS) and increases nitric oxide (NO) in vascular endothelial cells. We examined whether pretreatment with cilnidipine could attenuate cardiac cell deaths including apoptosis caused by hypoxia/reoxygenation (H/R) injury. HL-1 mouse atrial myocytes as well as H9c2 rat ventricular cells were exposed to H/R, and cell viability was evaluated by an autoanalyzer and flow cytometry; eNOS expression, NO production, and electrophysiological properties were also evaluated by western blotting, colorimetry, and patch clamping, respectively, in the absence and presence of cilnidipine. Cilnidipine enhanced phosphorylation of eNOS and NO production in a concentration-dependent manner, which was abolished by siRNAs against eNOS or an Hsp90 inhibitor, geldanamycin. Pretreatment with cilnidipine attenuated cell deaths including apoptosis during H/R; this effect was reproduced by an NO donor and a xanthine oxidase inhibitor. The NOS inhibitor L-NAME abolished the protective action of cilnidipine. Pretreatment with cilnidipine also attenuated H9c2 cell death during H/R. Additional cilnidipine treatment during H/R did not significantly enhance its protective action. There was no significant difference in the protective effect of cilnidipine under normal and high Ca2+ conditions. Action potential duration (APD) of HL-1 cells was shortened by cilnidipine, with this shortening augmented after H/R. L-NAME attenuated the APD shortening caused by cilnidipine. These findings indicate that cilnidipine enhances NO production, shortens APD in part by L-type Ca2+ channel block, and thereby prevents HL-1 cell deaths during H/R.






Similar content being viewed by others
References
Nabel EG, Braunwald E. A tale of coronary artery disease and myocardial infarction. N Engl J Med. 2012;366:54–63.
Spath NB, Mills NL, Cruden NL. Novel cardioprotective and regenerative therapies in acute myocardial infarction: a review of recent and ongoing clinical trials. Future Cardiol. 2016;12:655–72.
Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, et al. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J. 2017;38:935–41.
Prieto-Moure B, Lloris-Carsí JM, Barrios-Pitarque C, Toledo-Pereyra LH, Lajara-Romance JM, Berda-Antolí M, et al. Pharmacology of Ischemia Reperfusion. Translational research considerations. J Investig Surg. 2016;29:234–49.
Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–36.
Kunst G, Klein AA. Peri-operative anaesthetic myocardial preconditioning and protection - cellular mechanisms and clinical relevance in cardiac anaesthesia. Anaesthesia. 2015;70:467–82.
Schulz R, Kelm M, Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:402–13.
Cohen MV, Yang XM, Downey JM. Nitric oxide is a preconditioning mimetic and cardioprotectant and is the basis of many available infarct-sparing strategies. Cardiovasc Res. 2006;70:231–9.
Bice JS, Jones BR, Chamberlain GR, Baxter GF. Nitric oxide treatments as adjuncts to reperfusion in acute myocardial infarction: a systematic review of experimental and clinical studies. Basic Res Cardiol. 2016;111:23.
Arroyo-Martínez EA, Meaney A, Gutiérrez-Salmeán G, Rivera-Capello JM, GonzálezCoronado V, Alcocer-Chauvet A, et al. Is Local Nitric Oxide Availability Responsible for Myocardial Salvage after Remote Preconditioning? Arq Bras Cardiol. 2016;107:154–62.
Jones SP, Girod WG, Palazzo AJ, Granger DN, Grisham MB, Jourd’Heuil D, et al. Myocardial ischemia-reperfusion injury is exacerbated in absence of endothelial cell nitric oxide synthase. Am J Physiol. 1999;276:H1567–H1573.
Elrod JW, Greer JJ, Bryan NS, Langston W, Szot JF, Gebregzlabher H, et al. Cardiomyocyte-specific overexpression of NO synthase-3 protects against myocardial ischemia-reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:1517–23.
Tominaga M, Ohya Y, Tsukashima A, Kobayashi K, Takata Y, Koga T, et al. Ambulatory blood pressure monitoring in patients with essential hypertension treated with a new calcium antagonist, cilnidipine. Cardiovasc Drugs Ther. 1997;11:43–48.
Kobayashi N, Mori Y, Mita S, Nakano S, Kobayashi T, Tsubokou Y, et al. Effects of cilnidipine on nitric oxide and endothelin-1 expression and extracellular signal-regulated kinase in hypertensive rats. Eur J Pharm. 2001;422:149–57.
Bahrudin U, Morisaki H, Morisaki T, Ninomiya H, Higaki K, Nanba E, et al. Ubiquitin-proteasome system impairment caused by a missense cardiac myosin-binding protein C mutation and associated with cardiac dysfunction in hypertrophic cardiomyopathy. J Mol Biol. 2008;384:896–907.
Tanno S, Yamamoto K, Kurata Y, Adachi M, Inoue Y, Otani N, et al. Protective effects of topiroxostat on an ischemia-reperfusion model of rat hearts. Circ J. 2018;82:1101–11.
Bahrudin U, Morikawa K, Takeuchi A, Kurata Y, Miake J, Mizuta E, et al. Impairment of ubiquitin-proteasome system by E334K cMyBPC modifies channel proteins, leading to electrophysiological dysfunction. J Mol Biol. 2011;413:857–78.
Courtemanche M, Ramirez RJ, Nattel S, Am J. Physiol. Ionic mechanisms underlying human atrial action potential properties: insights from a mathematical model. Am J Physiol. 1998;275:H301–H321. https://doi.org/10.1152/ajpheart.1998.275.1.H301
Brutsaert DL. Cardiac endothelial-myocardial signaling: Its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev. 2003;83:59–115.
Boveris A, Costa LE, Poderoso JJ, Carreras MC, Cadenas E. Regulation of mitochondrial respiration by oxygen and nitric oxide. Ann N Y Acad Sci. 2000;899:121–35.
Ding Y, Vaziri ND. Calcium channel blockade enhances nitric oxide synthase expression by cultured endothelial cells. Hypertension. 1998;32:718–23.
Ishii M. Pharmacokinetics study of FRC-8653 (Cilnidipine). Jpn Pharm Ther. 1993;21:43–52.
Jiang J, Cyr D, Babbitt RW, Sessa WC, Patterson C. Chaperone-dependent regulation of endothelial nitric-oxide synthase intracellular trafficking by the cochaperone/ubiquitin ligase CHIP. J Biol Chem. 2003;278:49332–41.
Nedvetsky PI, Sessa WC, Schmidt HH. There’s NO binding like NOS binding: protein-protein interactions in NO/cGMP signaling. Proc Natl Acad Sci USA. 2002;99:16510–2.
Smolenski PA, Muller H, Kronich P, Kugler P, Walter U, Schnitzer JE, et al. Calcium-dependent membrane association sensitizes soluble guanylyl cyclase to nitric oxide. Nat Cell Biol. 2002;4:307–11.
Lee YM, Cheng PY, Chen SY, Chung MT, Sheu JR. Wogonin suppresses arrhythmias, inflammatory responses, and apoptosis induced by myocardial ischemia/reperfusion in rats. J Cardiovasc Pharm. 2011;58:133–42.
Li J, Zhang H, Zhang C. Role of inflammation in the regulation of coronary blood flow in ischemia and reperfusion: Mechanisms and therapeutic implications. J Mol Cell Cardiol. 2012;52:865–72.
Gottlieb RA. Cell death pathways in acute ischemia/reperfusion injury. J Cardiovasc Pharm Ther. 2011;16:233–8.
Koenitzer JR, Freeman BA. Redox signaling in inflammation: Interactions of endogenous electrophiles and mitochondria in cardiovascular disease. Ann N Y Acad Sci. 2010;1203:45–52.
Szewczyk A, Jarmuszkiewicz W, Koziel A, Sobieraj I, Nobik W, Lukasiak A, et al. Mitochondrial mechanisms of endothelial dysfunction. Pharm Rep. 2015;67:704–10.
Zhang P, Lu Y, Yu D, Zhang D, Hu W. Trap1 provides protection against myocardial ischemia-reperfusion injury by ameliorating mitochondrial dysfunction. Cell Physiol Biochem. 2015;36:2072–82.
Pike MM, Luo CS, Clark MD, Kirk KA, Kitakaze M, Madden MC, et al. Nmr measurements of na+ and cellular energy in ischemic rat heart: Role of na(+)-h+ exchange. Am J Physiol. 1993;265:2017–26.
Smith MA, Schnellmann RG. Calpains, mitochondria, and apoptosis. Cardiovasc Res. 2012;96:32–37.
Zhang JY, Wu F, Gu XM, Jin ZX, Kong LH, Zhang Y, et al. The blockade of transmembrane cl(-) flux mitigates i/r-induced heart injury via the inhibition of calpain activity. Cell Physiol Biochem. 2015;35:2121–34.
Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ros-induced ros release: an update and review. Biochim Biophys Acta. 2006;1757:509–17.
Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004;15:1983–92.
Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–90.
Thandroyen FT, McCarthy J, Burton KP, Opie LH. Ryanodine and caffeine prevent ventricular arrhythmias during acute myocardial ischemia and reperfusion in rat heart. Circ Res. 1988;62:306–14.
Zhao CY, Greenstein JL, Winslow RL. Mechanisms of the cyclic nucleotide cross-talk signaling network in cardiac L-type calcium channel regulation. J Mol Cell Cardiol. 2017;106:29–44.
Takahara A, Nakamura Y, Wagatsuma H, Aritomi S, Nakayama A, Satoh Y, et al. Long-term blockade of L/N-type Ca(2+) channels by cilnidipine ameliorates repolarization abnormality of the canine hypertrophied heart. Br J Pharm. 2009;158:1366–74.
Hayashi T, Yamaguchi T, Sakakibara Y, Taguchi K, Maeda M, Kuzuya M, et al. eNOS-dependent antisenscence effect of a calcium channel blocker in human endothelial cells. PLoS ONE. 2014;9:e88391.
Matsubara M, Yao K, Hasegawa K. Benidipine, a dihydropyridine-calcium channel blocker, inhibits lysophosphatidylcholine-induced endothelial injury via stimulation of nitric oxide release. Pharm Res. 2006;53:35–43.
Fan L, Yang Q, Xiao XQ, Grove KL, Huang Y, Chen ZW, et al. Dual actions of cilnidipine in human internal thoracic artery: inhibition of calcium channels and enhancement of endothelial nitric oxide synthase. J Thorac Cardiovasc Surg. 2011;141:1063–9.
Batova S, DeWever J, Godfraind T, Balligand JL, Dessy C, Feron O. The calcium channel blocker amlodipine promotes the unclamping of eNOS from caveolin in endothelial cells. Cardiovasc Res. 2006;71:478–85.
Acknowledgements
EA Pharma. Co. (Tokyo, Japan) and Fuji yakuhin Co. (Saitama, Japan) kindly provided cilnidipine and topiroxostat, respectively. Daiichi Sankyo Co. kindly provided azelnidipine.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
IH reported receiving lecturer’s fees from Mochida Pharmaceutical Company, Sanwa Kagaku Kenkyusho Co. Ltd, PFeizer Co. Ltd. and Fuji Yakuhin Co. Ltd., and research grants from EA Pharma. Co. Teijin Pharma, Fuji Yakuhin Co. Ltd. and Sanwa Kagaku Kenkyusho Co. Ltd.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Minato, H., Hisatome, I., Kurata, Y. et al. Pretreatment with cilnidipine attenuates hypoxia/reoxygenation injury in HL-1 cardiomyocytes through enhanced NO production and action potential shortening. Hypertens Res 43, 380–388 (2020). https://doi.org/10.1038/s41440-019-0391-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0391-7
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Effects of Neonatal Administration of Non-Opiate Analogues of Leu-Enkephalin to Heart Tissue Homeostasis of Prepubertal Albino Rats Exposed to Hypoxia
Bulletin of Experimental Biology and Medicine (2022)
-
Current Updates on Potential Role of Flavonoids in Hypoxia/Reoxygenation Cardiac Injury Model
Cardiovascular Toxicology (2021)
-
Does cilnidipine, a dual L- and N-type Ca2+ blocker, shows promise in drug repositioning approaches?
Hypertension Research (2020)