Abstract
The effect of chemical sympathectomy on cardiovascular parameters and the compensatory role of adrenal hormones, the renin–angiotensin system, and cardiovascular sensitivity to vasoconstrictors were studied in spontaneously hypertensive rats (SHRs) and normotensive Wistar-Kyoto (WKY) rats. Sympathectomy was induced in 20-week-old rats by daily intraperitoneal guanethidine administration (30 mg/kg b.w.) for 2 weeks. Basal blood pressure (BP), heart rate (HR), and restraint stress-induced cardiovascular changes were measured by radiotelemetry. The BP response to catecholamines was determined in rats with implanted catheters. Sympathectomy decreased BP only transiently, and after 14-day guanethidine treatment, BP returned to basal values in both strains. Sympathectomy permanently lowered HR, improved baroreflex sensitivity, and decreased the low-frequency domain of systolic blood pressure variability (a marker of vascular sympathetic activity). Guanethidine also attenuated the BP and HR responses to restraint stress. On the other hand, the BP response to catecholamines was augmented in sympathectomized rats, and this was not due to the de novo synthesis of vascular adrenergic receptors. Sympathectomy caused adrenal enlargement, enhanced the expression of adrenal catecholamine biosynthetic enzymes, and elevated plasma adrenaline levels in both strains, especially in WKY rats. Guanethidine also increased the plasma levels of aldosterone and corticosterone in WKY rats only. In conclusion, sympathectomy produced a transient decrease in BP, a chronic decrease in HR and improvement in baroreflex sensitivity. The effect of sympathectomy on BP was counteracted by increased vascular sensitivity to catecholamines in WKY rats and SHRs and/or by the enhanced secretion of adrenal hormones, which was more pronounced in WKY rats.
Similar content being viewed by others
Introduction
The sympathetic nervous system (SNS) plays an essential role in the regulation of blood pressure (BP) and in the development of human and experimental hypertension [1]. In adult spontaneously hypertensive rats (SHRs), SNS hyperactivity [2], denser vascular sympathetic innervation [3, 4], and numerous other SNS alterations have been described (for a review see refs. [5, 6]). On the other hand, we demonstrated a decrease in the expression of enzymes associated with catecholamine biosynthesis in the sympathetic ganglia and adrenal medulla during hypertension development in SHRs [4], which might compensate for sympathetic hyperactivity in this strain.
The destruction of peripheral sympathetic neurons (chemical sympathectomy) in neonatal SHRs attenuates the development of hypertension, but moderate BP elevation persists in SHRs compared to normotensive Wistar-Kyoto (WKY) rats [7]. This strain difference can be abolished by sympathectomy combined with adrenal demedullation [8] or with α1-adrenergic blockade [9]. The effect of chronic sympathectomy in adult rats with established hypertension is studied less frequently than that in young animals, although the treatment of adult animals offers a better model of the treatment of human essential hypertension. Sympathectomy of 10-week-old SHRs by 6-hydroxydopamine moderately lowers BP and attenuates cardiovascular responses to the noradrenaline-releasing agent tyramine [10]. However, sympathectomy induced by 6-hydroxydopamine in hypertensive animals is incomplete, and the rapid restoration of adrenergic nerve function may occur after the end of the treatment [11, 12]. An effective method of permanent sympathectomy of adult rats is the chronic administration of guanethidine [13, 14]. Guanethidine, which is actively incorporated into peripheral noradrenergic terminals, decreases catecholamine content in the vascular wall and the low-frequency component of systolic blood pressure variability (SBPV; a marker of vascular sympathetic activity) [15], but it does not affect the chromaffin cells of the adrenal medulla or central catecholaminergic cells [13, 16].
The effects of sympathectomy on BP can be counteracted by several compensatory mechanisms (e.g., higher catecholamine release from the adrenal medulla, the increased involvement of the renin–angiotensin system or the enhanced responsiveness of vascular smooth muscle cells to available vasoconstrictors), as reported previously [8, 17, 18]. Therefore, we tested the hypothesis that the BP-lowering effect of chemical sympathectomy in adult rats is counteracted by the above compensatory mechanisms and that the involvement of these compensatory mechanisms may be different in hypertensive SHRs and normotensive WKY rats.
Methods
The methods are described in detail in the Supplementary Information.
Animals and sympathectomy
Chemical sympathectomy was performed in male SHRs and normotensive WKY rats between the ages of 20 and 22 weeks by a daily intraperitoneal administration of guanethidine (30 mg/kg body weight (b.w.)) for 2 weeks [15, 19]; the measurements were performed on the 14th day of guanethidine treatment. Control (CTRL) rats were injected with saline. Animals were kept under standard laboratory conditions (temperature 23 ± 1 °C, 12-h light–dark cycle, Altromin pellet diet, and tap water ad libitum). All experimental procedures were approved by the Ethical Committee of the Institute of Physiology, Czech Academy of Sciences and conformed to the European Convention on Animal Protection and Guidelines on Research Animal Use.
Radiotelemetry
Adult SHRs and WKY rats were implanted with telemetry transmitters (model HD-S10, Data Sciences International, New Brighton) as previously described [15, 20]. After a 10-day recovery period, basal BP and HR were evaluated in freely moving rats, and thereafter, 14-day guanethidine treatment was started. Power spectral analysis of SBPV was used to evaluate the effects of guanethidine on the low-frequency (LF: 0.2–0.75 Hz), and high-frequency (HF: 0.75–4 Hz) components of SBPV as previously described [15]. We also evaluated the effect of restraint stress on cardiovascular parameters. Baroreflex function was determined by the spontaneous sequence technique [21] using Hemolab software (ver. 21.0) programmed by Harald Stauss.
Cardiovascular response to vasoactive agents
One day prior to BP measurement, catheters were inserted into the left carotid artery and jugular vein of the rats under isoflurane anesthesia. BP and HR were measured in conscious rats kept in small transparent cages (partially restrained) after 24 h of recovery using the PowerLab system (ADInstruments, Bella Vista) between 8 a.m. and 11:30 a.m. [15, 22]. In one group of rats, noncumulative doses of noradrenaline (0.001–10 µg/kg b.w.) or adrenaline (0.001–10 µg/kg b.w.) were administered intravenously. In the second group of rats, single doses of captopril (10 mg/kg b.w.) and pentolinium (5 mg/kg b.w.) were injected intravenously into conscious WKY rats and SHRs [23], followed by noncumulative doses of Ang II (0.1–100 ng/kg b.w.). The extent of sympathectomy was verified by the administration of the catecholamine-releasing agent tyramine (100 µg/kg b.w.) after the end of the dose response experiments.
Blood and tissue sampling
Rats were anesthetized by isoflurane (2.5%), and the blood, the adrenal glands, the femoral arteries, and vascular smooth muscle samples from the thoracic aorta were collected and processed for further use.
Histochemical visualization of monoamines (SPG method)
The histochemical visualization of monoamines in the femoral arteries was performed according to the protocol of de la Torre and Surgeon [24] as described previously [19]. The quantification was performed as described in our previous paper [4].
Catecholamines and corticosteroid measurements
The concentrations of catecholamines (noradrenaline and adrenaline) in the plasma and adrenal homogenates were measured using 3-CAT Research ELISA (LDN, Nordhorn). Plasma normetadrenaline and metadrenaline levels were measured with 2-MET Plasma ELISA Fast Track (LDN). Plasma corticosterone and aldosterone levels were measured using a corticosterone rat/mouse ELISA kit (LDN) and an aldosterone ELISA kit (LDN).
Laser capture microdissection
A total area of 1 mm2 of the adrenal medulla was dissected using the Leica LMD 6000 Laser Microdissection System (Leica Microsystems, Wetzlar) to measure messenger RNA (mRNA) expression.
RNA isolation, reverse transcription, and quantitative real-time PCR
Total RNA from smooth muscle samples was isolated using the RNeasy Fibrous Tissue Mini Kit (Qiagen, Hilden). Laser capture microdissected samples of the adrenal medulla were isolated with the Quick-RNA™ MicroPrep kit (Zymo Research, Irvine). Genomic DNA was removed with RNase-free DNase I (Qiagen). RNA from the vascular smooth muscle cells was transcribed into complementary DNA (cDNA) by the High-Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad). RNA from the adrenal medulla was transcribed using the more sensitive SuperScript® VILO™ cDNA synthesis kit (Thermo Fisher Scientific, Waltham). The gene expression was determined on a LightCycler® 480 System (Roche, Basel,) using HOT FIREPol® Probe qPCR Mix Plus (SolisBioDyne, Tartu) and TaqMan® Gene Expression Assays (Life Technologies). A list of the TaqMan® Gene Expression Assays that were used is available in Supplementary Table 1. The expression data were processed as previously described [4]. Reference genes (Gapdh and Hprt1 in the adrenal medulla and Hmbs and Ywhaz in the aortic smooth muscles) were selected as described in our previous study [25].
Statistics
The data are expressed as the means ± SEM. The normality of distribution was tested by the Shapiro–Wilk test. Statistical significance in the radiotelemetry experiment was determined by two-way repeated measures ANOVA. The BP responses to catecholamines and Ang II were analyzed by three-way ANOVA. Other experiments were analyzed by two-way ANOVA. Bonferroni post-hoc test was used in the case of a significant interaction between factors. The differences were considered to be significant at p < 0.05.
Results
Body weight and adrenal weight
The body weight of SHRs was significantly lower than that of WKY rats before guanethidine treatment (312 ± 3 vs. 343 ± 7 g; p < 0.001). CTRL animals gained a small amount of body weight, while 14-day guanethidine treatment prevented such a change in sympathectomized (SYMPX) SHRs and even caused a body weight loss in WKY rats (CTRL SHRs, 14 ± 3 g; CTRL WKY rats, 6 ± 3 g; SYMPX SHRs, 1 ± 3 g; SYMPX WKY rats, -21 ± 2 g; interaction between strain and treatment: F1,85 = 8.8, p < 0.01). The relative weight of the adrenal glands was significantly greater in CTRL SHRs than in WKY rats. Sympathectomy increased the adrenal weight and abolished this strain difference (CTRL SHRs, 13.8 ± 0.3 mg/100 g; CTRL WKY rats, 11.1 ± 0.2 mg/100 g; SYMPX SHRs, 15.5 ± 0.6 mg/100 g; SYMPX WKY rats, 15.2 ± 0.4 mg/100 g; interaction between strain and treatment: F1,27 = 9.2, p < 0.01).
Cardiovascular parameters measured by radiotelemetry
We used radiotelemetry to compare changes in BP, heart rate (HR) and activity over time in adult freely moving SHRs and WKY rats sympathectomized by guanethidine (Table 1 and 2). The mean arterial pressure (MAP), HR, activity, and the LF SBPV in CTRL rats of both strains were higher during the dark phase (active period) than during the light phase (resting period). Untreated SHRs had higher MAP than WKY rats during both the light and dark phases. After 3 days of guanethidine treatment, the MAP was lowered in both strains, and the effect was more pronounced during the dark phase. However, the MAP returned back to the control level after 14 days of guanethidine treatment, and a small persistent decrease in the MAP was found only in WKY rats during the dark phase. SHRs and WKY rats had similar HRs before the onset of guanethidine treatment (Table 1). HR was lowered following 3 days of guanethidine administration during both the light and dark phases in both strains, and the effect persisted until the 14th day of guanethidine treatment. However, during the dark phase, the HR of SYMPX SHRs was higher than that of SYMPX WKY rats. SHRs were more active than WKY rats during the dark phase, and sympathectomy decreased the activity of both strains during the dark phase (Table 1). The LF SBPV (a marker of vascular sympathetic activity) was higher in SHRs than in WKY rats, and the difference was more pronounced during the dark phase (Table 2). The LF SBPV was markedly decreased in both strains after 3 days and 14 days of guanethidine administration, demonstrating the persistent effect of sympathectomy. This finding is consistent with the results obtained from the visualization of monoamines in the sympathetic innervation of the femoral arteries (Fig. 1a). The quantification of the fluorescent signal (Fig. 1b) revealed a strain difference in vascular sympathetic innervation, which was abolished by sympathectomy (interaction between strain and treatment: F1,31 = 9.4, p < 0.01). The fluorescent signal was ~2.5-fold higher in CTRL SHRs than in WKY rats (p < 0.001). Fourteen days of guanethidine treatment reduced the fluorescent signal in both strains (–92% in SYMPX SHRs and –88% in SYMPX WKY rats; p < 0.001 for SHRs and p < 0.01 for WKY rats). The HF SBPV (a marker of cardiac sympathetic activity) was higher in CTRL SHRs, and sympathectomy decreased the HF SBPV in both strains (Table 2).
Histochemical visualization of catecholamines in the femoral arteries (SPG method). Representative images of control (CTRL) and sympathectomized (SYMPX) WKY rats and SHRs (a) and the quantitative evaluation of the fluorescent signal (b). The values are expressed as the mean ± SEM relative to CTRL WKY rats; n = 8–9 rats in each group. The effects of strain and treatment and their interaction were analyzed by two-way ANOVA (the results are shown in the text), and in the case of a significant interaction between the factors, Bonferroni post-hoc test was performed. *p < 0.05 vs. WKY rats; †p < 0.05 vs. CTRL rats
Spontaneous baroreflex function was determined in freely moving SHRs and WKY rats. SHRs had a reduced baroreflex sensitivity compared to that of WKY rats during both the light and dark phases (Table 2). The number of spontaneous baroreflex sequences was substantially decreased by sympathectomy, and baroreflex sensitivity during the dark phase was moderately but significantly increased in SYMPX animals compared to controls.
Since 14-day guanethidine treatment had different effects on BP and LF SBPV in freely moving animals, we examined the responses of the MAP, HR and the LF SBPV to restraint stress, i.e., under the conditions when SNS is activated. We observed a higher MAP response to stress in SHRs than in WKY rats, and this response was attenuated by 14-day guanethidine treatment (CTRL SHRs, 29 ± 2 mmHg; CTRL WKY rats, 14 ± 2 mmHg; SYMPX SHRs, 7 ± 3 mmHg; SYMPX WKY rats, 0 ± 2 mmHg; strain: F1,12 = 24.4, p < 0.001; treatment: F1,12 = 42.4, p < 0.001; interaction: F1,12 = 2.7, NS). The stress-induced HR increase, which was similar in both strains, was attenuated by sympathectomy (CTRL SHRs, 109 ± 12 bpm; CTRL WKY rats, 102 ± 13 bpm; SYMPX SHRs, 23 ± 6 bpm; SYMPX WKY rats, 31 ± 4 bpm; strain: F1,12 = 0.03, NS; treatment: F1,12 = 74.9 p < 0.001; interaction: F1,12 = 0.7, NS). The response of the LF SBPV to stress was markedly higher in CTRL SHRs than in WKY rats, and sympathectomy decreased this parameter in both strains (CTRL SHRs, 19.3 ± 2.2 mmHg2; CTRL WKY rats, 5.9 ± 1.2 mmHg2; SYMPX SHRs, 2.7 ± 0.6 mmHg2; SYMPX WKY rats, 2.2 ± 0.9 mmHg2; interaction between strain and treatment: F1,12 = 18.9, p < 0.001). Thus, after 14 days of guanethidine treatment, sympathectomy-induced cardiovascular changes (decrease in the MAP, HR and the LF SBPV) were more evident under stressful conditions than in freely moving animals.
Cardiovascular response to vasoactive agents
Responses to vasoactive agents were measured in conscious cannulated CTRL and SYMPX SHRs and WKY rats to obtain more detailed information about the effects of sympathectomy on the cardiovascular system. The basal MAP (measured in rats placed in small plastic cages) was higher in SHRs than in WKY rats, and sympathectomy decreased the basal MAP (CTRL SHRs, 157 ± 2 mmHg; CTRL WKY rats, 102 ± 2 mmHg; SYMPX SHR 136 ± 2 mmHg; SYMPX WKY rats, 91 ± 3 mmHg; strain: F1,66 = 233.9, p < 0.001; treatment: F1,66 = 16.7, p < 0.001; interaction: F1,66 = 3.9, p = 0.053). Basal HR was higher in SHRs than in WKY rats, and HR was decreased by sympathectomy (CTRL SHRs, 363 ± 1 bpm; CTRL WKY rats, 323 ± 8 bpm; SYMPX SHRs, 318 ± 6 bpm; SYMPX WKY rats, 282 ± 10 bpm; strain: F1,65 = 44.1, p < 0.001; treatment: F1,65 = 58.8, p < 0.001; interaction: F1,65 = 2.0, NS).
The ganglionic blocker pentolinium decreased the MAP in all experimental groups (CTRL SHRs, –47 ± 3 mmHg; CTRL WKY rats, –38 ± 1 mmHg; SYMPX SHRs, –7 ± 4 mmHg; SYMPX WKY rats, –15 ± 4 mmHg; interaction between strain and treatment: F1,26 = 8.1, p < 0.01). The effect of pentolinium on the MAP was more pronounced in CTRL SHRs than in WKY rats (p < 0.05), and this strain difference was abolished by sympathectomy. Pentolinium decreased the HR more in CTRL WKY rats than in SHRs, and guanethidine treatment altered the HR response to pentolinium in both strains (CTRL SHRs, –16 ± 5 bpm; CTRL WKY rats, –26 ± 6 bpm; SYMPX SHRs, 26 ± 6 bpm; SYMPX WKY rats, –10 ± 10 bpm; strain: F1,25 = 11.2, p < 0.01; treatment: F1,25 = 17.7, p < 0.001; interaction: F1,25 = 3.4, NS). The catecholamine-releasing agent tyramine increased the MAP similarly in SHRs and WKY rats, and the effect was almost completely abolished by sympathectomy (CTRL SHRs, 48 ± 4 mmHg; CTRL WKY rats, 49 ± 5 mmHg; SYMPX SHRs, 4 ± 1 mmHg; SYMPX WKY rats, 6 ± 2 mmHg; strain: F1,65 = 0.005, NS; treatment: F1,65 = 215.8, p < 0.001; interaction: F1,65 = 1.7, NS). The effect of tyramine on HR was similar to its effect on the MAP (CTRL SHRs, 93 ± 13 bpm; CTRL WKY rats, 94 ± 15 bpm; SYMPX SHRs, –3 ± 3 bpm; SYMPX WKY rats, 2 ± 2 bpm; strain: F1,65 = 0.2, NS; treatment: F1,65 = 94.7, p < 0.001; interaction: F1,65 = 1.1, NS). The experiments with pentolinium and tyramine confirmed the persistent effect of sympathectomy after 14 days of guanethidine administration.
The acute administration of the angiotensin-converting enzyme inhibitor captopril decreased the MAP more in SHRs than in WKY rats, and sympathectomy did not change the MAP response to captopril (CTRL SHRs, –15 ± 4 mmHg; CTRL WKY rats, –4 ± 1 mmHg; SYMPX SHRs, –18 ± 3 mmHg; SYMPX WKY rats, –10 ± 2 mmHg; strain: F1,36 = 8.6, p < 0.01; treatment: F1,36 = 2.3, NS; interaction: F1,36 = 0.2, NS). Captopril administration caused an increase in HR in WKY rats but not in SHRs with or without sympathectomy (CTRL SHRs, 2 ± 7 bpm; CTRL WKY rats, 29 ± 7 bpm; SYMPX SHRs, 7 ± 5 bpm; SYMPX WKY rats, 32 ± 8 bpm; strain: F1,36 = 16.0, p < 0.01; treatment: F1,36 = 0.4, NS; interaction: F1,36 = 0.001, NS).
BP changes upon the administration of increasing doses of noradrenaline, adrenaline, and angiotensin II were analyzed by three-way ANOVA with the following factors: strain (SHRs vs. WKY rats), treatment (CTRL rats vs. SYMPX rats), and the dose of the particular vasoconstrictor. Regarding the MAP response to noradrenaline administration, the effect of strain vs. treatment interaction on the MAP response depended on the noradrenaline dose (Fig. 2a; strain vs. treatment interaction: p < 0.001 at 0.01 µg/kg dose, NS at 0.1 µg/kg dose, p < 0.001 at 1 µg/kg dose). There was no strain difference in the increase in the MAP after 0.01 µg/kg noradrenaline administration, and sympathectomy augmented the MAP response in both strains (p < 0.001 for WKY rats, p < 0.01 for SHRs). Upon the administration of noradrenaline at a dose of 0.1 µg/kg, the increase in the MAP was more pronounced in SHRs than that in WKY rats (p < 0.001), and sympathectomy augmented the MAP response in both strains (p < 0.001). Upon the administration of noradrenaline at a dose of 1 µg/kg, the MAP responses in CTRL SHRs and WKY rats were similar, sympathectomy augmented the responses in both strains (p < 0.001), and SYMPX SHRs responded with a greater increase in the MAP than that in SYMPX WKY rats (p < 0.001). When the MAP response to adrenaline administration was analyzed by three-way ANOVA, there was no significant interaction between strain, treatment, and dose. The MAP response to adrenaline was augmented in SHRs regardless of the adrenaline dose or guanethidine treatment (Fig. 2b). The effect of sympathectomy on the MAP response depended on the adrenaline dose. The MAP response upon the administration of 0.01 µg/kg adrenaline was similar in CTRL and SYMPX rats, but the MAP responses were augmented in SYMPX rats upon the administration of 0.1 and 1 µg/kg adrenaline (p < 0.001 for both doses). When the MAP response to Ang II administration was analyzed by three-way ANOVA, there was no significant interaction between strain, treatment, and dose (Fig. 2c). The effect of both strain and treatment on the MAP response to Ang II depended on the dose. The MAP responses were more pronounced in SHRs than in WKY rats upon the administration of 5, 10, 20, 50, and 100 ng/kg Ang II (p < 0.01). Sympathectomy augmented the MAP responses upon the administration of 5, 10, and 20 µg/kg Ang II (p < 0.01).
Mean arterial pressure (MAP) response to the intravenous administration of vasoconstrictors. Noncumulative doses of noradrenaline (a), adrenaline (b), or angiotensin II (c) were administered to conscious control (CTRL) and sympathectomized (SYMPX) WKY rats and SHRs. The values are expressed as the mean ± SEM; n = 6–8 rats for noradrenaline and adrenaline, n = 9–11 rats for angiotensin II. The effects of strain, treatment, and dose and their interactions were analyzed by three-way ANOVA (the results are listed in Supplementary Table 2), and in the case of significant interaction of the factors, Bonferroni post-hoc test was performed
Gene expression in vascular smooth muscle cells and in the adrenal medulla
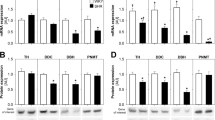
The most abundantly expressed adrenergic receptors subtype in aortic smooth muscles was Adra1d followed by Adra1b (Table 3). The mRNA expression of Adra1a, Adra2a, Adra2b, Adra2c, Adrb1, and Adrb2 was too low for reliable quantification by quantitative real-time PCR. The mRNA expression levels of Adra1d and Adra1b were lower in SHRs than in WKY rats and were not affected by sympathectomy. The G-proteins that participate in α1-adrenergic contraction are the Gq and G11 subtypes, which are encoded by the Gnaq and Gna11 genes. The mRNA expression of Gnaq was lower in the vascular smooth muscles of SHRs than of WKY rats while the expression of Gna11 was similar between the groups. There was no effect of sympathectomy on the mRNA expression of Gnaq or Gna11.
SHRs exhibited lower mRNA expression of enzymes involved in catecholamine biosynthesis (Th, Ddc, Dbh, and Pnmt) in the adrenal medulla than that exhibited by WKY rats (Table 3). There were no strain differences in the mRNA expression of genes involved in catecholamine reuptake or degradation (Net, Comt, Maoa, and Maob). Sympathectomy increased the mRNA expression of Th, Dbh, Pnmt, and Maob in the adrenal medulla.
Catecholamine content in the adrenal gland
There was a lower noradrenaline content in the adrenal glands of CTRL SHRs than of WKY rats, and sympathectomy increased the noradrenaline content in the adrenal glands of WKY rats (CTRL SHRs, 2.4 ± 0.3 µg; CTRL WKY rats, 4.8 ± 0.3 µg; SYMPX SHRs, 2.6 ± 0.2 µg; SYMPX WKY rats, 7.3 ± 0.8 µg per adrenal gland; interaction between strain and treatment: F1,27 = 4.5, p < 0.05). SHRs also exhibited a lower adrenal content of adrenaline than that of WKY rats, and sympathectomy increased the adrenaline content in the adrenal glands irrespective of strain (CTRL SHRs, 12.8 ± 0.4 µg; CTRL WKY rats, 14.0 ± 0.5 µg; SYMPX SHRs, 13.4 ± 0.4 µg; SYMPX WKY rats, 16.1 ± 0.6 µg per adrenal gland; strain: F1,27 = 13.1, p < 0.001; treatment: F1,27 = 6.3, p < 0.05; interaction: F1,27 = 1.8, NS).
Plasma catecholamines and corticosteroids
There was no strain difference in the plasma levels of noradrenaline or normetadrenaline in CTRL rats. Sympathectomy decreased the plasma levels of noradrenaline and normetadrenaline in both strains (Fig. 3a, b). CTRL SHRs exhibited similar plasma adrenaline levels but higher plasma metadrenaline levels compared to those exhibited by CTRL WKY rats (Fig. 3c, d; p < 0.05). Sympathectomy increased the plasma levels of adrenaline and metadrenaline, and this effect was more pronounced in WKY rats (fourfold increase in SYMPX WKY rats, p < 0.001 vs. 1.5-fold increase in SYMPX SHRs, p < 0.001 for WKY rats, and p < 0.05 for SHRs). The evaluation of plasma corticosteroid levels revealed that CTRL SHRs exhibited plasma levels of corticosterone similar to those of WKY rats (Fig. 3e) and plasma aldosterone levels lower than those of WKY rats (Fig. 3f, p < 0.01). Sympathectomy increased the plasma levels of both corticosterone and aldosterone in WKY rats (p < 0.05 for corticosterone and p < 0.001 for aldosterone) but not in SHRs.
Plasma concentrations of adrenal hormones and their metabolites. Noradrenaline (a), adrenaline (b), normetadrenaline (c), metadrenaline (d), corticosterone (e), and aldosterone (f) levels in control (CTRL) and sympathectomized (SYMPX) WKY rats and SHRs. The values are expressed as the mean ± SEM; n = 8 rats for each group. The effects of strain and treatment and their interaction were analyzed by two-way ANOVA (the results are listed in Supplementary Table 3), and in the case of a significant interaction between the factors, Bonferroni post-hoc test was performed. *p < 0.05 vs. WKY rats; †p < 0.05 vs. CTRL rats
Discussion
In the present study, the effects of guanethidine-induced sympathectomy on the cardiovascular system and adrenal glands of adult hypertensive SHRs and normotensive WKY rats were evaluated. We demonstrated that chronic guanethidine treatment in SHRs and WKY rats decreased the MAP, HR, LF SBPV, and HF SBPV (markers of sympathetic vascular and cardiac activity, respectively [26]). However, the MAP decrease was only temporary. During the light phase, the MAP returned to basal levels in both strains after 14-day guanethidine treatment despite the persistent effect of sympathectomy (as demonstrated by several measurements, i.e., decreased LF SBPV, reduced MAP response to pentolinium and tyramine, and low catecholamine content in the vascular wall). Moreover, stress-induced MAP and HR increases were attenuated by sympathectomy in both strains. MAP recovery in sympathectomized animals may be explained by compensatory mechanisms, e.g., the augmented BP response to catecholamines and/or increased plasma adrenaline levels. Plasma adrenaline levels increased more in sympathectomized WKY rats than in SHRs, and this increase was accompanied by higher plasma levels of other adrenal hormones, such as corticosterone and aldosterone, in WKY rats only.
Our study confirmed the greater role of the SNS in SHRs than in WKY rats, as documented by higher LF SBPV and HF SBPV, a more pronounced MAP decrease after treatment with the ganglionic blocker pentolinium and a higher fluorescent signal of catecholamines in the femoral arteries in SHRs, which is in accordance with previous reports [4, 15, 23, 27, 28]. Sympathectomy reduced the MAP, HR, and the LF SBPV and HF SBPV in both SHRs and WKY rats after 3 days of guanethidine treatment. HR and the LF and HF SBPV were still reduced after 14 days of guanethidine treatment, but the MAP returned back to the control level after this period. Accordingly, it was reported that chronic guanethidine treatment of adult Sprague-Dawley rats either decreased [29] or did not significantly change [13] the resting BP. However, a decrease in the MAP after 14 days of guanethidine administration was observed under the conditions of restraint stress, as we previously demonstrated [15]; thus, the method of BP measurement might influence the results of the experiment. MAP recovery in freely moving animals cannot be ascribed to an insufficient degree of sympathectomy in either rat strain. The LF SBPV was still decreased after 14 days of guanethidine treatment. In both SHRs and WKY rats, guanethidine treatment decreased the catecholamine fluorescent signal to <15% of that in nonsympathectomized controls. Moreover, the MAP responses to pentolinium and tyramine were also attenuated by sympathectomy.
However, after the disruption of sympathetic innervation to the vasculature, vascular tone can be maintained by the increased postjunctional sensitivity of vascular smooth muscle cells to various vasoconstrictors [18]. In our experiment, sympathectomy increased MAP sensitivity to noradrenaline (up to 14-fold) and adrenaline (up to tenfold) in both SHRs and WKY rats. Accordingly, the increased BP response to the α1-adrenergic agonist phenylephrine has been demonstrated in adult sympathectomized Wistar [30] and WKY rats [31]. To determine whether the sympathectomy-induced augmentation of the MAP response is generalized or specific for catecholamines, the MAP response to Ang II was also measured. Sympathectomy increased the sensitivity of MAP to Ang II (up to twofold) in both SHRs and WKY rats, but this change was less pronounced than in the case of catecholamines. A smaller impact of sympathectomy on cardiovascular sensitivity to Ang II than to catecholamines was also described by Rizzoni et al. [31]. Kamikihara et al. [30] reported that increased vascular sensitivity to phenylephrine in animals sympathectomized by reserpine is accompanied by increased mRNA expression of Adra1d in the rat tail artery. In the present study, we found no effect of guanethidine treatment on the mRNA expression of adrenergic receptors and G-protein subtypes that mediate adrenergic vasoconstriction in aortic smooth muscles. The contradictory results might be caused by the use of different sympatholytic agents or the examination of various vascular beds. Nevertheless, the increased sensitivity of the cardiovascular system to catecholamines can be achieved by mechanisms other than the de novo synthesis of adrenergic receptors, e.g., by their increased affinity for noradrenaline [32]. We also cannot exclude the possibility of vascular remodeling, which was described previously in the denervated rat caudal artery [33].
Spontaneous baroreflex function was evaluated in freely moving SHRs and WKY rats. We demonstrated that SHRs had lower baroreflex sensitivity than that of WKY rats and that sympathectomy improved baroreflex sensitivity in both strains. This finding is in accordance with reports that impaired baroreflex sensitivity is associated with sympathetic hyperactivity in SHRs as well as in human essential hypertension [10, 34], and baroreflex sensitivity can be augmented by sympathectomy [10, 35]. However, strain differences in baroreflex sensitivity persisted in sympathectomized SHRs and WKY rats. Impaired baroreflex function in SHRs is consistent with the augmented MAP decrease, but the attenuated HR increase to the acute administration of the angiotensin-converting enzyme inhibitor captopril in SHRs when compared to WKY rats. On the other hand, different HR responses to the ganglionic blocker pentolinium in SHRs and WKY rats is probably not associated with baroreflex function (as pentolinium impairs the baroreflex response), but rather with a higher intrinsic rate of atrial pacemaker in SHRs [36].
In our study, guanethidine treatment decreased the plasma levels of noradrenaline and normetadrenaline by 50% in both SHRs and WKY rats, which is in line with the data obtained in neonatally sympathectomized SHRs [37]. Sympathectomy also increased the plasma adrenaline and metadrenaline levels in both strains, with the effect being three times stronger in WKY rats. Plasma noradrenaline predominantly originates from peripheral sympathetic nerve endings, whereas the adrenal medulla is considered to be the source of <10% of plasma noradrenaline under normal conditions. However, the adrenal contribution to plasma noradrenaline levels can increase up to 30–45% in stressed rats [38], suggesting that the adrenal gland can partially compensate for the lack of noradrenaline. Adrenal hypertrophy, the increase in the adrenal levels of noradrenaline and adrenaline and a higher activity of adrenal Th have already been described in sympathectomized Sprague-Dawley rats [39, 40]. We also observed adrenal hypertrophy in guanethidine-treated rats, and this effect was more pronounced in WKY rats than in SHRs. Adrenal growth was accompanied by an increase in adrenal adrenaline content in both strains and increased adrenal noradrenaline content in WKY rats. We previously reported lower mRNA expression of catecholamine biosynthetic enzymes in the adrenal glands of young and adult SHRs when compared to WKY rats [4]. Sympathectomy of adult rats increased the mRNA expression of the Th, Dbh, and Pnmt genes similarly in the adrenal medulla of both SHRs and WKY rats, and the strain difference persisted.
Finally, the plasma levels of corticosterone and aldosterone were increased twofold by sympathectomy in WKY rats but not in SHRs. Aldosterone secretion is regulated by Ang II; thus, a greater contribution of the renin–angiotensin system to BP maintenance in sympathectomized animals might explain the increased plasma aldosterone levels. It has been shown that the sympathetic–renal interaction contributes to hypertension development in SHRs [41], and renal denervation lowers BP in this strain [42]. Guanethidine decreases plasma renin activity while increasing Ang II receptors in the adrenal gland [39]. However, in our study, sympathectomy did not change the acute MAP response to angiotensin-converting enzyme inhibitor in either strain, and further experiments are necessary to determine the contribution of the kidney and renin–angiotensin system to BP maintenance in sympathectomized animals. On the other hand, both corticosterone and aldosterone are released under stressful conditions [43]. It is possible that sympathectomy is a more stressful intervention for WKY rats than for SHRs since body weight loss and adrenal growth were more pronounced in sympathectomized WKY rats than in sympathectomized SHRs.
In conclusion, sympathectomy of adult hypertensive SHRs and normotensive WKY rats by guanethidine administration decreased HR and improved baroreflex sensitivity in both strains. Basal BP was lowered by sympathectomy only temporarily, but the effect of guanethidine was revealed under stressful conditions. BP recovery might be explained by compensatory mechanisms, such as the more than tenfold increase in BP sensitivity to catecholamines and the increased plasma levels of adrenaline. The involvement of the renin–angiotensin system in BP maintenance in sympathectomized rats also cannot be excluded.
SHRs are characterized by sympathetic hyperactivity and are often considered a model of human essential hypertension. Our data indicate that sympathectomy in adult animals with established hypertension transiently lowers BP. Nevertheless, the long-term reduction of sympathetic vascular tone (decreased LF SBPV) is associated with HR reduction, an attenuated BP response to stress and an enhancement of baroreflex sensitivity. These improvements might decrease overall cardiovascular risk represented by increased HR and low baroreflex sensitivity in hypertensive patients.
References
Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–46.
Judy WV, Farrell SK. Arterial baroreceptor reflex control of sympathetic nerve activity in the spontaneously hypertensive rat. Hypertension. 1979;1:605–14.
Cassis LA, Stitzel RE, Head RJ. Hypernoradrenergic innervation of the caudal artery of the spontaneously hypertensive rat: an influence upon neuroeffector mechanisms. J Pharm Exp Ther. 1985;234:792–803.
Vavřínová A, Behuliak M, Bencze M, Vaněčková I, Zicha J. Which sympathoadrenal abnormalities of adult spontaneously hypertensive rats can be traced to a prehypertensive stage? Hypertens Res. 2019;42:949–59.
Head RJ. Hypernoradrenergic innervation: its relationship to functional and hyperplastic changes in the vasculature of the spontaneously hypertensive rat. Blood Vessels. 1989;26:1–20.
de Champlain J. Pre- and postsynaptic adrenergic dysfunctions in hypertension. J Hypertens Suppl. 1990;8(Suppl7):S77–85.
Lee RM, Triggle CR, Cheung DW, Coughlin MD. Structural and functional consequence of neonatal sympathectomy on the blood vessels of spontaneously hypertensive rats. Hypertension. 1987;10:328–38.
Lee RM, Borkowski KR, Leenen FH, Tsoporis J, Coughlin M. Combined effect of neonatal sympathectomy and adrenal demedullation on blood pressure and vascular changes in spontaneously hypertensive rats. Circ Res. 1991;69:714–21.
Korner P, Bobik A, Oddie C, Friberg P. Sympathoadrenal system is critical for structural changes in genetic hypertension. Hypertension. 1993;22:243–52.
Ferrari AU, Daffonchio A, Franzelli C, Mancia G. Potentiation of the baroreceptor-heart rate reflex by sympathectomy in conscious rats. Hypertension. 1991;18:230–5.
Finch L, Leach GD. The contribution of the sympathetic nervous system to the development and maintenance of experimental hypertension in the rat. Br J Pharm. 1970;39:317–24.
Yamori Y, Yamabe H, De Jong W, Lovenberg W, Sjoerdsma A. Effect of tissue norepinephrine depletion by 6-hydroxydopamine on blood pressure in spontaneously hypertensive rats. Eur J Pharm. 1972;17:135–40.
Johnson EM Jr., O’Brien F. Evaluation of the permanent sympathectomy produced by the administration of guanethidine to adult rats. J Pharm Exp Ther. 1976;196:53–61.
Franco-Colín M, Villanueva I, Piñón M, Racotta R. The effects of sympathectomy and dexamethasone in rats ingesting sucrose. Int J Biol Sci. 2006;2:17–22.
Behuliak M, Bencze M, Polgárová K, Kuneš J, Vaněčková I, Zicha J. Hemodynamic response to gabapentin in conscious spontaneously hypertensive rats. Hypertension. 2018;72:676–85.
Johnson EM Jr., Manning PT. Guanethidine-induced destruction of sympathetic neurons. Int Rev Neurobiol. 1984;25:1–37.
Lo M, Julien C, Barres C, Medeiros I, Allevard AM, Vincent M, et al. Blood pressure maintenance in hypertensive sympathectomized rats. II. Renin-angiotensin system and vasopressin. Am J Physiol. 1991;261(4 Pt 2):R1052–6.
Fleming WW. Postjunctional supersensitivity: a cellular homeo-static mechanism. Trends Pharm Sci. 1981;2:152–4.
Bencze M, Behuliak M, Vavřínová A, Zicha J. Altered contractile responses of arteries from spontaneously hypertensive rat: the role of endogenous mediators and membrane depolarization. Life Sci. 2016;166:46–53.
Vaněčková I, Vokurková M, Rauchová H, Dobešová Z, Pecháňová O, Kuneš J, et al. Chronic antioxidant therapy lowers blood pressure in adult but not in young Dahl salt hypertensive rats: the role of sympathetic nervous system. Acta Physiol (Oxf). 2013;208:340–9.
Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hypertens Suppl. 1985;3(Suppl3):S79–81.
Kunes J, Dobesová Z, Zicha J. Altered balance of main vasopressor and vasodepressor systems in rats with genetic hypertension and hypertriglyceridaemia. Clin Sci (Lond). 2002;102:269–77.
Zicha J, Dobešová Z, Behuliak M, Pintérová M, Kuneš J, Vaněčková I. Nifedipine-sensitive blood pressure component in hypertensive models characterized by high activity of either sympathetic nervous system or renin-angiotensin system. Physiol Res. 2014;63:13–26.
de la Torre JC, Surgeon JW. Histochemical fluorescence of tissue and brain monoamines: results in 18 min using the sucrose-phosphate-glyoxylic acid (SPG) method. Neuroscience. 1976;1:451–3.
Vavřínová A, Behuliak M, Zicha J. The importance of the selection of appropriate reference genes for gene expression profiling in adrenal medulla or sympathetic ganglia of spontaneously hypertensive rat. Physiol Res. 2016;65:401–11.
Yoshimoto T, Eguchi K, Sakurai H, Ohmichi Y, Hashimoto T, Ohmichi M, et al. Frequency components of systolic blood pressure variability reflect vasomotor and cardiac sympathetic functions in conscious rats. J Physiol Sci. 2011;61:373–83.
Chiu EK, McNeill JR. Role of autonomic function in the antihypertensive effect of vasopressin withdrawal in spontaneous hypertension. Am J Hypertens. 1992;5:187–92.
Head RJ, Cassis LA, Robinson RL, Westfall DP, Stitzel RE. Altered catecholamine contents in vascular and nonvascular tissues in genetically hypertensive rats. Blood Vessels. 1985;22:196–204.
Benarroch EE, Schmelzer JD, Ward KK, Nelson DK, Low PA. Noradrenergic and neuropeptide Y mechanisms in guanethidine-sympathectomized rats. Am J Physiol. 1990;259(2 Pt 2):R371–5.
Kamikihara SY, Mueller A, Lima V, Akinaga J, Nojimoto FD, Castilho A, et al. alpha1-Adrenoceptors in proximal segments of tail arteries from control and reserpinised rats. Naunyn Schmiedebergs Arch Pharm. 2007;376:117–26.
Rizzoni D, Perlini S, Mircoli L, Porteri E, Franzelli C, Castellano M, et al. Enhanced vascular reactivity in the sympathectomized rat: studies in vivo and in small isolated resistance arteries. J Hypertens. 2000;18:1041–9.
Colucci WS, Gimbrone MA Jr., McLaughlin MK, Halpern W, Alexander RW. Increased vascular catecholamine sensitivity and alpha-adrenergic receptor affinity in female and estrogen-treated male rats. Circ Res. 1982;50:805–11.
Malheiros-Lima MR, Pires W, Fonseca IAT, Joviano-Santos JV, Ferreira AJ, Coimbra CC, et al. Physical exercise-induced cardiovascular and thermoregulatory adjustments are impaired in rats subjected to cutaneous artery denervation. Front Physiol. 2018;9:74
Head GA. Baroreflexes and cardiovascular regulation in hypertension. J Cardiovasc Pharm. 1995;26(Suppl2):S7–16.
Mircoli L, Fedele L, Benetti M, Bolla GB, Radaelli A, Perlini S, et al. Preservation of the baroreceptor heart rate reflex by chemical sympathectomy in experimental heart failure. Circulation. 2002;106:866–72.
Dyke AC, Angus JA, Korner PI. A functional study of the development of the cardiac sympathetic neuroeffector junction in the SHR. J Hypertens. 1989;7:345–53.
Tipton CM, Sturek MS, Oppliger RA, Matthes RD, Overton JM, Edwards JG. Responses of SHR to combinations of chemical sympathectomy, adrenal demedullation, and training. Am J Physiol. 1984;247(1 Pt 2):H109–18.
Goldstein DS, McCarty R, Polinsky RJ, Kopin IJ. Relationship between plasma norepinephrine and sympathetic neural activity. Hypertension. 1983;5:552–9.
Qiu J, Nelson SH, Speth RC, Wang DH. Regulation of adrenal angiotensin receptor subtypes: a possible mechanism for sympathectomy-induced adrenal hypertrophy. J Hypertens. 1999;17:933–40.
Kvetnansky R, Weise VK, Thoa NB, Kopin IJ. Effects of chronic guanethidine treatment and adrenal medullectomy on plasma levels of catecholamines and corticosterone in forcibly immobilized rats. J Pharm Exp Ther. 1979;209:287–91.
Grisk O, Rose HJ, Lorenz G, Rettig R. Sympathetic-renal interaction in chronic arterial pressure control. Am J Physiol Regul Integr Comp Physiol. 2002;283:R441–50.
Skrzypecki J, Gawlak M, Huc T, Szulczyk P, Ufnal M. Renal denervation decreases blood pressure and renal tyrosine hydroxylase but does not augment the effect of hypotensive drugs. Clin Exp Hypertens. 2017;39:290–4.
Kubzansky LD, Adler GK. Aldosterone: a forgotten mediator of the relationship between psychological stress and heart disease. Neurosci Biobehav Rev. 2010;34:80–6.
Acknowledgements
The work documented in this paper was made possible by research grants from the Czech Science Foundation (GACR 16-10349Y), the Charles University Grant Agency (GAUK 1071416) and the Ministry of Health of the Czech Republic (15-25396 A).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vavřínová, A., Behuliak, M., Bencze, M. et al. Sympathectomy-induced blood pressure reduction in adult normotensive and hypertensive rats is counteracted by enhanced cardiovascular sensitivity to vasoconstrictors. Hypertens Res 42, 1872–1882 (2019). https://doi.org/10.1038/s41440-019-0319-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-019-0319-2
- Springer Nature Singapore Pte Ltd.
Keywords
This article is cited by
-
Both central sympathoexcitation and peripheral angiotensin II-dependent vasoconstriction contribute to hypertension development in immature heterozygous Ren-2 transgenic rats
Hypertension Research (2022)
-
Development of the hypersecretory phenotype in the population of adrenal chromaffin cells from prehypertensive SHRs
Pflügers Archiv - European Journal of Physiology (2021)
-
Effects of Low-Dose L-Thyroxine on Stress Resistance in Animals with Experimental Deficit of Sympathetic Neural Influences
Neuroscience and Behavioral Physiology (2021)