Abstract
Cystic fibrosis (CF) is a life-limiting disease caused by defective or deficient cystic fibrosis transmembrane conductance regulator (CFTR) activity. The recent advent of the FDA-approved CFTR modulator drug ivacaftor, alone or in combination with lumacaftor or tezacaftor, has enabled treatment of the majority of patients suffering from CF. Even before the identification of the CFTR gene, gene therapy was put forward as a viable treatment option for this genetic condition. However, initial enthusiasm has been hampered as CFTR gene delivery to the lungs has proven to be more challenging than expected. This review covers the contemporary clinical and scientific knowledge base for small molecule CFTR modulator drug therapy, gene delivery vectors and CRISPR/Cas9 gene editing and highlights the prospect of these technologies for future treatment options.


Similar content being viewed by others
References
Griesenbach U, Pytel KM, Alton EW. Cystic fibrosis gene therapy in the UK and elsewhere. Hum Gene Ther. 2015;26:266–75.
Kleizen B, Braakman I, de Jonge HR. Regulated trafficking of the CFTR chloride channel. Eur J Cell Biol. 2000;79:544–56.
Sheppard DN, Welsh MJ. Structure and function of the CFTR chloride channel. Physiol Rev. 1999;79(1 Suppl):S23–45.
Schneider EK, Huang JX, Carbone V, Baker M, Azad MA, Cooper MA, et al. Drug-drug plasma protein binding interactions of ivacaftor. J Mol Recognit. 2015;28:339–48.
Tizzano EF, Buchwald M. CFTR expression and organ damage in cystic fibrosis. Ann Interna Med. 1995;123:305–8.
O’Sullivan BP, Flume P. The clinical approach to lung disease in patients with cystic fibrosis. Semin Respir Crit Care Med. 2009;30:505–13.
Solomon M. Cystic fibrosis-update on diagnosis and treatment from cystic fibrosis etiology, diagnosisn and treatments. In: Leatte PN, editors Treatments for cystic fibrosis; 2009.
Schneider EK, Reyes-Ortega F, Li J, Velkov T. Can cystic fibrosis patients finally catch a breath with lumacaftor/ivacaftor? Clin Pharmacol Ther. 2017;101:130–41.
Gill DR, Hyde SC. Delivery of genes into the CF airway. Thorax. 2014;69:962–4.
Conway SP, Pond MN, Hamnett T, Watson A. Compliance with treatment in adult patients with cystic fibrosis. Thorax. 1996;51:29–33.
Worldwide CF https://www.cfww.org (2018).
Dean M, Santis G. Heterogeneity in the severity of cystic fibrosis and the role of CFTR gene mutations. Hum Genet. 1994;93:364–8.
Goodman BE, Percy WH. CFTR in cystic fibrosis and cholera: from membrane transport to clinical practice. Adv Physiol Educ. 2005;29:75–82.
Griesenbach U, Alton EW. Moving forward: cystic fibrosis gene therapy. Hum Mol Genet. 2013;22(R1):R52–8.
Griesenbach U, Alton EW. Current status and future directions of gene and cell therapy for cystic fibrosis. BioDrugs. 2011;25:77–88.
Griesenbach U, Geddes DM, Alton EW. Advances in cystic fibrosis gene therapy. Curr Opin Pulm Med. 2004;10:542–6.
Davis PB, Yasothan U, Kirkpatrick P. Ivacaftor. Nat Rev. Drug Discov. 2012;11:349–50.
Sala MA, Jain M. Tezacaftor for the treatment of cystic fibrosis. Expert Rev Respir Med. 2018:12;725–32.
Aiuti A, Roncarolo MG, Naldini L. Gene therapy for ADA-SCID, the first marketing approval of an ex vivo gene therapy in Europe: paving the road for the next generation of advanced therapy medicinal products. EMBO Mol Med. 2017;9:737–40.
Sheridan C. Gene therapy finds its niche. Nat Biotechnol. 2011;29:121–8.
Aiuti A. Advances in gene therapy for ADA-deficient SCID. Curr Opin Mol Ther. 2002;4:515–22.
Candotti F, Shaw KL, Muul L, Carbonaro D, Sokolic R, Choi C, et al. Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans. Blood. 2012;120:3635–46.
Cooney AL, McCray PB, Jr, Sinn PL. Cystic fibrosis gene therapy: looking back, looking forward. Genes. 2018;9 pii: E538. https://doi.org/10.3390/genes9110538.
Stern M, Ulrich K, Geddes DM, Alton EW. Poly (D, L-lactide-co-glycolide)/DNA microspheres to facilitate prolonged transgene expression in airway epithelium in vitro, ex vivo and in vivo. Gene Ther. 2003;10:1282–8.
Schuster BS, Kim AJ, Kays JC, Kanzawa MM, Guggino WB, Boyle MP, et al. Overcoming the cystic fibrosis sputum barrier to leading adeno-associated virus gene therapy vectors. Mol Ther. 2014;22:1484–93.
Xia E, Munegowda MA, Cao H, Hu J. Lung gene therapy-How to capture illumination from the light already present in the tunnel. Genes Dis. 2014;1:40–52.
Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D, et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol. 2000;18:970–3.
Mitomo K, Griesenbach U, Inoue M, Somerton L, Meng C, Akiba E, et al. Toward gene therapy for cystic fibrosis using a lentivirus pseudotyped with Sendai virus envelopes. Mol Ther. 2010;18:1173–82.
Joseph PM, O’Sullivan BP, Lapey A, Dorkin H, Oren J, Balfour R, et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. I. Methods, safety, and clinical implications. Hum Gene Ther. 2001;12:1369–82.
Perricone MA, Morris JE, Pavelka K, Plog MS, O’Sullivan BP, Joseph PM, et al. Aerosol and lobar administration of a recombinant adenovirus to individuals with cystic fibrosis. II. Transfection efficiency in airway epithelium. Hum Gene Ther. 2001;12:1383–94.
Harvey BG, Leopold PL, Hackett NR, Grasso TM, Williams PM, Tucker AL, et al. Airway epithelial CFTR mRNA expression in cystic fibrosis patients after repetitive administration of a recombinant adenovirus. J Clin Investig. 1999;104:1245–55.
Bellon G, Michel-Calemard L, Thouvenot D, Jagneaux V, Poitevin F, Malcus C, et al. Aerosol administration of a recombinant adenovirus expressing CFTR to cystic fibrosis patients: a phase I clinical trial. Hum Gene Ther. 1997;8:15–25.
Crystal RG, McElvaney NG, Rosenfeld MA, Chu CS, Mastrangeli A, Hay JG, et al. Administration of an adenovirus containing the human CFTR cDNA to the respiratory tract of individuals with cystic fibrosis. Nat Genet. 1994;8:42–51.
Hay JG, McElvaney NG, Herena J, Crystal RG. Modification of nasal epithelial potential differences of individuals with cystic fibrosis consequent to local administration of a normal CFTR cDNA adenovirus gene transfer vector. Hum Gene Ther. 1995;6:1487–96.
Knowles MR, Hohneker KW, Zhou Z, Olsen JC, Noah TL, Hu PC, et al. A controlled study of adenoviral-vector-mediated gene transfer in the nasal epithelium of patients with cystic fibrosis. New Engl J Med. 1995;333:823–31.
Walters RW, Grunst T, Bergelson JM, Finberg RW, Welsh MJ, Zabner J. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J Biol Chem. 1999;274:10219–26.
Vidovic D, Carlon MS, da Cunha MF, Dekkers JF, Hollenhorst MI, Bijvelds MJ, et al. rAAV-CFTRDeltaR rescues the cystic fibrosis phenotype in human intestinal organoids and cystic fibrosis mice. Am J Respir Crit Care Med. 2016;193:288–98.
Vidovic D, Gijsbers R, Quiles-Jimenez A, Dooley J, Van den Haute C, Van, et al. Noninvasive imaging reveals stable transgene expression in mouse airways after delivery of a nonintegrating recombinant adeno-associated viral vector. Hum Gene Ther. 2016;27:60–71.
Wagner JA, Reynolds T, Moran ML, Moss RB, Wine JJ, Flotte TR, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–3.
Johnson LG, Olsen JC, Naldini L, Boucher RC. Pseudotyped human lentiviral vector-mediated gene transfer to airway epithelia in vivo. Gene Ther. 2000;7:568–74.
Burney TJ, Davies JC. Gene therapy for the treatment of cystic fibrosis. Appl Clin Genet. 2012;5:29–36.
Fischer A, Hacein-Bey-Abina S, Cavazzana-Calvo M. Gene therapy of primary T cell immunodeficiencies. Gene. 2013;525:170–3.
Hacein-Bey Abina S, Gaspar HB, Blondeau J, Caccavelli L, Charrier S, Buckland K, et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. J Am Med Assoc. 2015;313:1550–63.
Griesenbach U, Inoue M, Meng C, Farley R, Chan M, Newman NK, et al. Assessment of F/HN-pseudotyped lentivirus as a clinically relevant vector for lung gene therapy. Am J Respir Crit Care Med. 2012;186:846–56.
Lee TW, Matthews DA, Blair GE. Novel molecular approaches to cystic fibrosis gene therapy. Biochem J. 2005;387(Pt 1):1–15.
Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26:549–51.
Griesenbach U, Ferrari S, Geddes DM, Alton EW. Gene therapy progress and prospects: cystic fibrosis. Gene Ther. 2002;9:1344–50.
Maclachlan TK, Lukason M, Collins M, Munger R, Isenberger E, Rogers C, et al. Preclinical safety evaluation of AAV2-sFLT01- a gene therapy for age-related macular degeneration. Mol Ther. 2011;19:326–34.
Ruiz FE, Clancy JP, Perricone MA, Bebok Z, Hong JS, Cheng SH, et al. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum Gene Ther. 2001;12:751–61.
Alton EW, Boyd AC, Porteous DJ, Davies G, Davies JC, Griesenbach U, et al. A phase I/IIa safety and efficacy study of nebulized liposome-mediated gene therapy for cystic fibrosis supports a multidose trial. Am J Respir Crit Care Med. 2015;192:1389–92.
Alton E, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, et al. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. 2015;3:684–91.
Marangi M, Pistritto G. Innovative therapeutic strategies for cystic fibrosis: moving forward to CRISPR technique. Front Pharmacol. 2018;9:396.
Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–23.
Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell. 2013;154:1370–9.
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–8.
Fan Z, Perisse IV, Cotton CU, Regouski M, Meng Q, Domb C, et al. A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI insight. 2018;3 pii: 123529.
Crane AM, Kramer P, Bui JH, Chung WJ, Li XS, Gonzalez-Garay ML, et al. Targeted correction and restored function of the CFTR gene in cystic fibrosis induced pluripotent stem cells. Stem Cell Rep. 2015;4:569–77.
Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72.
Martiniano SL, Sagel SD, Zemanick ET. Cystic fibrosis: a model system for precision medicine. Curr Opin Pediatr. 2016;28:312–7.
Davies JC, Cunningham S, Harris WT, Lapey A, Regelmann WE, Sawicki GS, et al. Safety, pharmacokinetics, and pharmacodynamics of ivacaftor in patients aged 2-5 years with cystic fibrosis and a CFTR gating mutation (KIWI): an open-label, single-arm study. Lancet Respir Med. 2016;4:107–15.
Wainwright CE, Elborn JS, Ramsey BW, Marigowda G, Huang X, Cipolli M, et al. Lumacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del CFTR. N Engl J Med. 2015;373:220–31.
Fajac I, De Boeck K. New horizons for cystic fibrosis treatment. Pharmacol Ther. 2017;170:205–11.
Taylor-Cousar JL, Munck A, McKone EF, van der Ent CK, Moeller A, Simard C, et al. Tezacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 2017;377:2013–23.
Donaldson SH, Pilewski JM, Griese M, Cooke J, Viswanathan L, Tullis E, et al. Tezacaftor/Ivacaftor in subjects with cystic fibrosis and F508del/F508del-CFTR or F508del/G551D-CFTR. Am J Rrespir Crit Care Med. 2018;197:214–24.
Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX-445-Tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J med. 2018;379:1612–20.
Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, et al. VX-659-tezacaftor-ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med. 2018;379:1599–611.
Bell SC, De Boeck K, Amaral MD. New pharmacological approaches for cystic fibrosis: promises, progress, pitfalls. Pharmacol Ther. 2015;145:19–34.
De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med. 2016;4:662–74.
Bosch B, De Boeck K. Searching for a cure for cystic fibrosis. A 25-year quest in a nutshell. Eur J Pediatr. 2016;175:1–8.
Cholon DM, Esther, Charles R, Gentzsch, Martina. Efficacy of lumacaftor-ivacaftor for the treatment of Cystic Fibrosis patients homozygous for the F508del-CFTR mutation. Expert Rev Precis Med Drug Dev. 2016;1:235–43.
Masson A, Schneider-Futschik EK, Baatallah N, Nguyen-Khoa T, Girodon E, Hatton A et al. Predictive factors for lumacaftor/ivacaftor clinical response. J Cyst Fibros. 2019;18:368–74.
FDA. Sponsor Briefing Document: ORKAMBI (Lumacaftor/Ivacaftor) for the treatment of cystic fibrosis in patients age 12 years and older who are homozygous for the F508del mutation in the CFTR gene. In: commiteeFACBMV-FP-Ada, editors. VERTEX Pharmaceuticals Incorporated; 2015. p. 98.
Schneider EK Cytochrome P450 3A4 induction: lumacaftor versus ivacaftor potentially resulting in significantly reduced plasma concentration of ivacaftor. Drug Metab Lett. 2018;12:71–4. https://doi.org/10.2174/1872312812666180328105259
Schneider EK, Reyes-Ortega F, Wilson JW, Kotsimbos T, Keating D, Li J, et al. Development of HPLC and LC-MS/MS methods for the analysis of ivacaftor, its major metabolites and lumacaftor in plasma and sputum of cystic fibrosis patients treated with ORKAMBI or KALYDECO. J Chromatogr B, Analyt Technol Biomed Life Sci. 2016;1038:57–62.
EMA. Assessment report ORKAMBI (ivacaftor/lumacaftor) European medicines agency EMEA/H/C/003954/0000, 2015.
Cholon DM, Quinney NL, Fulcher ML, Esther CR,Jr, Das J, Dokholyan NV. et al. Potentiator ivacaftor abrogates pharmacological correction of DeltaF508 CFTR in cystic fibrosis. Sci Transl Med. 2014;6:246ra96
Veit G, Avramescu RG, Perdomo D, Phuan PW, Bagdany M, Apaja PM. et al. Some gating potentiators, including VX-770, diminish DeltaF508-CFTR functional expression. Sci Transl Med. 2014;6:246ra97
Foundation CF. Drug Development Pipeline. https://www.cff.org/Trials/Pipeline: cff.org. 2017.
De Boeck K, Davies JC. Where are we with transformational therapies for patients with cystic fibrosis? Current Opin Pharmacol. 2017;34:70–75.
Cholon DM, Gentzsch M, Recent progress in translational cystic fibrosis research using precision medicine strategies. J Cyst Fibros. 2018;17:S52–60. https://doi.org/10.1016/j.jcf.2017.09.005.
Patel AK, Kaczmarek JC, Bose S, Kauffman KJ, Mir F, Heartlein MW, et al. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv Mater. 2019;31:e1805116. https://doi.org/10.1002/adma.201805116.
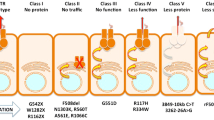
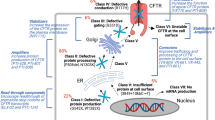
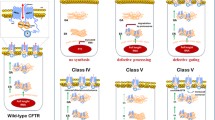
De Boeck K, Zolin A, Cuppens H, Olesen HV, Viviani L. The relative frequency of CFTR mutation classes in European patients with cystic fibrosis. J Cyst Fibros. 2014;13:403–9.
http://www.cftr.info/about-cf/cftr-mutations/the-six-classes-of-cftr-defects/. Classification of CFTR mutations (2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author has received the Vertex Cystic Fibrosis Research Award from the ‘Thoracic Society of Australia and New Zealand (TSANZ)’, which was sponsored by Vertex Pharmaceuticals.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schneider-Futschik, E.K. Beyond cystic fibrosis transmembrane conductance regulator therapy: a perspective on gene therapy and small molecule treatment for cystic fibrosis. Gene Ther 26, 354–362 (2019). https://doi.org/10.1038/s41434-019-0092-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41434-019-0092-5
- Springer Nature Limited
This article is cited by
-
Human β-defensin 3 gene modification promotes the osteogenic differentiation of human periodontal ligament cells and bone repair in periodontitis
International Journal of Oral Science (2020)
-
Cyclodextrins in drug delivery: applications in gene and combination therapy
Drug Delivery and Translational Research (2020)