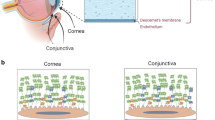
Abstract
The classic model of tear film is composed of mucin layer, aqueous layer and the outermost tear film lipid layer (TFLL). The complex mixture of different classes of lipids, mainly secreted by meibomian glands, gives the TFLL unique physicochemical properties. Based on these properties, several functions of TFLL have been found and/or proposed such as the resistance to evaporation and facilitating the formation of a thin film. However, the role of TFLL in the oxygenation of the cornea, a transparent avascular tissue, has never been discussed in the literature. The continuous metabolic activity of the corneal surface and the replenishment of atmospheric gas creates an O2 gradient in the tear film. The molecules of O2 must therefore be transferred from the gas phase to the liquid phase through the TFLL. This process is a function of the diffusion and solubility of the lipid layer as well as interface transfer, which is influenced by alterations in the physical state and lipid composition. In the absence of research on TFLL, the present paper aims to bring the topic into the spotlight for the first time based on existing knowledge on O2 permeability of the lipid membranes and evaporation resistance of the lipid layers. The oxidative stress generated in perturbed lipid layers and the consequent adverse effects are also covered. The function of the TFLL proposed here intends to encourage future research in both basic and clinical sciences, e.g., opening new avenues for the diagnosis and treatment of ocular surface conditions.
摘要
泪膜的经典模型由粘蛋白层、水液层和最外层的泪膜脂质层 (TFLL) 组成。不同类别脂质的复杂混合物, 主要由睑板腺分泌, 赋予了TFLL独特的理化性质。基于这些性质, 人们发现并/或提出了TFLL的一些功能, 例如防止蒸发和促进薄膜的形成。然而, TFLL在角膜 (一层透明无血管的组织) 的氧合中的作用在文献中从未被讨论过。角膜表面持续的代谢活动与空气环境使得泪膜产生了氧气梯度。因此, 氧分子必须通过TFLL从气相转移至液相。这个过程是脂质层扩散和溶解以及界面转移的功能, 这一功能受到物理状态和脂质成分变化的影响。在缺乏对TFLL研究的情况下, 本文基于脂质膜氧渗透和脂质层防止蒸发的现有知识, 首次将该主题进行探讨并引起大家的关注。本文讨论了紊乱的脂质层产生的氧化应激和后续的不良影响。本文在此提出TFLL的功能, 旨在鼓励进一步进行基础科学和临床方面的研究, 眼表疾病的诊疗开拓新途径。


Similar content being viewed by others
References
de Souza GA, de Godoy LM, Mann M. Identification of 491 proteins in the tear fluid proteome reveals a large number of proteases and protease inhibitors. Genome Biol. 2006;7:R72.
Yazdani M, Elgstøen KBP, Rootwelt H, Shahdadfar A, Utheim ØA, Utheim TP. Tear Metabolomics in Dry Eye Disease: A Review. Int J Mol Sci. 2019;20:3755.
Woodcock B, Bullock J, Shore R, Heard M, Pereira M, Redhead J, et al. Country-specific effects of neonicotinoid pesticides on honey bees and wild bees. Science. 2017;356:1393–5.
Teixeira L & Dubielzig RR. Chapter 53 - Eye. In: Haschek WM, Rousseaux CG, Wallig MA (eds.). Haschek and Rousseaux’s Handbook of Toxicologic Pathology (Third Edition). Academic Press: Boston; 2013. pp 2095–185.
Butovich IA. Tear film lipids. Exp Eye Res. 2013;117:4–27.
Cwiklik L. Tear film lipid layer: A molecular level view. BBA-Biomembranes. 2016;1858:2421–30.
Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film–A review. Exp Eye Res. 2015;137:125–38.
Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66:190.
Avtar R, Tandon D. Mathematical analysis of corneal oxygenation. Int J Health Res. 2008;1:129–38.
Takatori SC, de la Jara PL, Holden B, Ehrmann K, Ho A, Radke CJ. In vivo oxygen uptake into the human cornea. Invest Ophthalmol Vis Sci. 2012;53:6331–7.
Kayal A. The Physiology of Tear Film. Dry Eye Syndrome-Modern Diagnostic Techniques and Advanced Treatments. IntechOpen. 2021;1–9.
Yazdani M. Technical aspects of oxygen level regulation in primary cell cultures: A review. Interdiscip Toxicol. 2016;9:85.
Möller MN, Li Q, Chinnaraj M, Cheung HC, Lancaster JR Jr, Denicola A. Solubility and diffusion of oxygen in phospholipid membranes. BBA-Biomembranes. 2016;1858:2923–30.
Subczynski WK, Hyde JS, Kusumi A. Oxygen permeability of phosphatidylcholine-cholesterol membranes. Proc Natl Acad Sci USA 1989;86:4474–8.
Dzikovski BG, Livshits VA, Marsh D. Oxygen permeation profile in lipid membranes: comparison with transmembrane polarity profile. Biophys J. 2003;85:1005–12.
Widomska J, Raguz M, Subczynski WK. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. BBA-Biomembranes. 2007;1768:2635–45.
Lemp MA, Foulks GN. The definition and classification of dry eye disease. Ocul Surf. 2007;5:75–92.
Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31:472–8.
Kulovesi P Tear film lipid layer. PhD thesis, University of Helsinki, Finland, 2015.
Dogru M, Kojima T, Simsek C, Tsubota K. Potential role of oxidative stress in ocular surface inflammation and dry eye disease. Invest Ophthalmol Vis Sci. 2018;59:DES163–8.
Perez-Garmendia R, Lopez de Eguileta Rodriguez A, Ramos-Martinez I, Zuñiga NM, Gonzalez-Salinas R, Quiroz-Mercado H et al. Interplay between oxidative stress, inflammation, and amyloidosis in the anterior segment of the eye; its pathological implications. Oxid Med Cell Longev; 2020;2020:6286105.
Greaves JL, Wilson CG. Treatment of diseases of the eye with mucoadhesive delivery systems. Adv Drug Deliv Rev. 1993;11:349–83.
Wolff E. The muco-cutaneous junction of the lidmargin and the distribution of the tear fluid. Trans Ophthalmol Soc UK. 1946;66:291–308.
Meibom H. De vasis palpebrarum novis epistola. Müller; 1666.
Knop E, Knop N, Millar T, Obata H, Sullivan DA. The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci. 2011;52:1938–78.
Davidson HJ, Kuonen VJ. The tear film and ocular mucins. Vet Ophthalmol. 2004;7:71–77.
Bron A, Tiffany J, Gouveia S, Yokoi N, Voon L. Functional aspects of the tear film lipid layer. Exp Eye Res. 2004;78:347–60.
Tawfik HA, Abdulhafez MH, Fouad YA, Dutton JJ. Embryologic and fetal development of the human eyelid. Ophthalmic Plast Reconstr Surg. 2016;32:407.
Schuett BS, Millar TJ. An investigation of the likely role of (O-acyl) ω-hydroxy fatty acids in meibomian lipid films using (O-oleyl) ω-hydroxy palmitic acid as a model. Exp Eye Res. 2013;115:57–64.
Mudgil P. Antimicrobial role of human meibomian lipids at the ocular surface. Invest Ophthalmol Vis Sci. 2014;55:7272–7.
Kaufman PL, Alm A, Adler FH & Levin LA. Adler’s Physiology of the Eye. Elsevier Health Sciences; 2011.
McDonald JE. Surface phenomena of tear films. Trans Am Ophthalmol Soc. 1968;66:905–39.
McDonald JE. Surface phenomena of the tear film. Am J Ophthalmol. 1969;67:56–64.
Langmuir I, Schaefer VJ. The Effect of Dissolved Salts on Insoluble Monolayers. J Am Chem Soc. 1937;59:2400–14.
King-Smith PE, Bailey MD, Braun RJ. Four characteristics and a model of an effective tear film lipid layer (TFLL). Ocul Surf. 2013;11:236–45.
Recchioni A, Mocciardini E, Ponzini E, Tavazzi S. Viscoelastic properties of the human tear film. Exp Eye Res. 2022;219:109083.
Tiffany J. Normal and abnormal functions of meibomian gland secretions. R Soc Med Int Congr Symp Ser. 1981;40:1061–4.
Tiffany J & Marsden R. The influence of composition on physical properties of meibomian secretion. The Preocular Tear Film in Health, Disease and Contact Lens Wear Lubbock, TX, Dry Eye Institute: 597–608 (1986).
Alio J, Padron M. Influence of age on the temperature of the anterior segment of the eye. Ophthalmic Res. 1982;14:153–9.
Utracki L. Temperature dependence of liquid viscosity. J Macromol Sci, Part B: Phys. 1974;10:477–505.
Vargaftik N, Volkov B, Voljak L. International tables of the surface tension of water. J Phys Chem Ref Data. 1983;12:817–20.
Yañez-Soto B, Mannis MJ, Schwab IR, Li JY, Leonard BC, Abbott NL, et al. Interfacial phenomena and the ocular surface. Ocul Surf. 2014;12:178–201.
Butovich IA. The Meibomian puzzle: combining pieces together. Prog Retin Eye Res. 2009;28:483–98.
Barron GA Tear Film Dynamics Concerning A Lipid Reservoir. Rochester Institute of Technology; 2015.
Georgiev GA, Eftimov P, Yokoi N. Structure-function relationship of tear film lipid layer: A contemporary perspective. Exp Eye Res. 2017;163:17–28.
Efron N, Brennan NA. In search of the critical oxygen requirement of the cornea. Contax. 1987;2:5–11.
Papas EB, Sweeney DF. Interpreting the corneal response to oxygen: Is there a basis for re-evaluating data from gas-goggle studies? Exp Eye Res. 2016;151:222–6.
Helbig H, Hinz J, Kellner U, Foerster M. Oxygen in the anterior chamber of the human eye. Ger J Ophthalmol. 1993;2:161–4.
Morris J. The physiological causes of contact lens complications. Optom Today. 1999;12:3.
Compan V, Oliveira C, Aguilella-Arzo M, Mollá S, Peixoto-de-Matos SC, González-Méijome JM. Oxygen diffusion and edema with modern scleral rigid gas permeable contact lenses. Invest Ophthalmol Vis Sci. 2014;55:6421–9.
Bonanno JA, Stickel T, Nguyen T, Biehl T, Carter D, Benjamin WJ, et al. Estimation of human corneal oxygen consumption by noninvasive measurement of tear oxygen tension while wearing hydrogel lenses. Invest Ophthalmol Vis Sci. 2002;43:371–6.
Srinivas SP, Guidoboni G, Carichino L, Jiang Y, Bonanno JA. Rapid Measurement of Tear Oxygen Tension underneath Soft Contact Lenses by Frequency-Domain Phosphorimetry. Invest Ophthalmol Vis Sci. 2012;53:6106.
Harvitt DM, Bonanno JA. Direct noninvasive measurement of tear oxygen tension beneath gas-permeable contact lenses in rabbits. Invest Ophthalmol Vis Sci. 1996;37:1026–36.
Bonanno JA, Clark C, Pruitt J, Alvord L. Tear oxygen under hydrogel and silicone hydrogel contact lenses in humans. Optom Vis Sci. 2009;86:E936.
Liu Z Microelectrodes in an ophthalmic electrochemical sensor: Google Patents; 2015.
Beebe DC, Shui Y-B, Siegfried CJ, Holekamp NM, Bai F. Preserve the (intraocular) environment: the importance of maintaining normal oxygen gradients in the eye. Jpn J Ophthalmol. 2014;58:225–31.
Zhang L, Tang Y, Tong L. Micro-/nanofiber optics: Merging photonics and material science on nanoscale for advanced sensing technology. Iscience. 2020;23:100810.
Zuniga-Hertz JP, Patel HH. The evolution of cholesterol-rich membrane in oxygen adaption: the respiratory system as a model. Front Physiol. 2019;10:1340.
Al-Samir S, Itel F, Hegermann J, Gros G, Tsiavaliaris G, Endeward V. O2 permeability of lipid bilayers is low, but increases with membrane cholesterol. Cell Mol Life Sci. 2021;78:7649–62.
Phleger CF. Buoyancy in marine fishes: direct and indirect role of lipids. Am Zool. 1998;38:321–30.
Aydemir E, Breward C, Witelski T. The effect of polar lipids on tear film dynamics. Bull Math Biol. 2011;73:1171–201.
Bruna M, Breward C. The influence of non-polar lipids on tear film dynamics. J Fluid Mech. 2014;746:565–605.
Archer RJ, Mer VKL. The rate of evaporation of water through fatty acid monolayers. J Phys Chem. 1955;59:200–8.
McMahon A, Lu H, Butovich IA. The spectrophotometric sulfo-phospho-vanillin assessment of total lipids in human meibomian gland secretions. Lipids. 2013;48:513–25.
Paananen RO, Javanainen M, Holopainen JM, Vattulainen I. Crystalline wax esters regulate the evaporation resistance of tear film lipid layers associated with dry eye syndrome. J Phys Chem Lett. 2019;10:3893–8.
Viitaja T, Moilanen J, Svedstrom KJ, Ekholm FS, Paananen RO. Tear film lipid layer structure: self-assembly of O-Acyl-ω-Hydroxy fatty acids and wax esters into evaporation-resistant monolayers. Nano Lett. 2021;21:7676–83.
Paananen RO, Rantamäki AH, Holopainen JM. Antievaporative mechanism of wax esters: implications for the function of tear fluid. Langmuir. 2014;30:5897–902.
Leedale JA, Lucendo-Villarin B, Meseguer-Ripolles J, Kasarinaite A, Webb SD, Hay DC. Mathematical modelling of oxygen gradients in stem cell-derived liver tissue. Plos One. 2021;16:e0244070.
Yazdani M. Concerns in the application of fluorescent probes DCDHF-DA, DHR 123 and DHE to measure reactive oxygen species in vitro. Toxicol Vitr. 2015;30:578–82.
Wojcik KA, Kaminska A, Blasiak J, Szaflik J, Szaflik JP. Oxidative stress in the pathogenesis of keratoconus and Fuchs endothelial corneal dystrophy. Int J Mol Sci. 2013;14:19294–308.
Hua X, Chi W, Su L, Li J, Zhang Z, Yuan X. ROS-induced oxidative injury involved in pathogenesis of fungal keratitis via p38 MAPK activation. Sci Rep. 2017;7:10421.
Jurkunas UV, Bitar MS, Funaki T, Azizi B. Evidence of oxidative stress in the pathogenesis of fuchs endothelial corneal dystrophy. Am J Pathol. 2010;177:2278–89.
Navel V, Sapin V, Henrioux F, Blanchon L, Labbé A, Chiambaretta F, et al. Oxidative and antioxidative stress markers in dry eye disease: A systematic review and meta‐analysis. Acta Ophthalmol. 2022;100:45–57.
Goda TE, Mohamed FA, Mohamed AA. Therapeutic Effect and Complaints for Patients Receiving Supplemental Oxygen Therapy at Zagazig University. Hospitals Zagazig Nurs J. 2021;17:26–41.
Craig JP, Nichols KK, Akpek EK, Caffery B, Dua HS, Joo C-K, et al. TFOS DEWS II definition and classification report. Ocul Surf. 2017;15:276–83.
Bartlett JD, Keith MS, Sudharshan L, Snedecor SJ. Associations between signs and symptoms of dry eye disease: a systematic review. Clin Ophthalmol. 2015;9:1719.
Shine WE, McCulley JP. The role of cholesterol in chronic blepharitis. Invest Ophthalmol Vis Sci. 1991;32:2272–80.
Kopacz D, Niezgoda Ł, Fudalej E, Nowak A & Maciejewicz P. Tear Film–Physiology and Disturbances in Various Diseases and Disorders. Ocular Surface Diseases—Some Current Date on Tear Film Problem and Keratoconic Diagnosis: 137–44 (2020).
Rantamäki AH, Wiedmer SK, Holopainen JM. Melting points—the key to the anti-evaporative effect of the tear film wax esters. Invest Ophthalmol Vis Sci. 2013;54:5211–7.
Borchman D, Yappert MC, Milliner SE, Duran D, Cox GW, Smith RJ, et al. 13C and 1H NMR ester region resonance assignments and the composition of human infant and child meibum. Exp Eye Res. 2013;112:151–9.
Mohamed HB, Abd El-Hamid BN, Fathalla D & Fouad EA. Current trends in pharmaceutical treatment of Dry Eye Disease: a review. Eur J Pharm Sci. 2022;175:106206.
Garrigue J-S, Amrane M, Faure M-O, Holopainen JM, Tong L. Relevance of lipid-based products in the management of dry eye disease. J Ocul Pharm Ther. 2017;33:647–61.
Dogru M, Karakaya H, Özçetin H, Ertürk H, Yücel A, Özmen A, et al. Tear function and ocular surface changes in keratoconus. Ophthalmol. 2003;110:1110–8.
Vaidyanathan U, Hopping GC, Liu HY, Somani AN, Ronquillo YC, Hoopes PC, et al. Persistent corneal epithelial defects: a review article. Med Hypothesis, Discov Innov Ophthalmol. 2019;8:163.
Labetoulle M, Benitez-del-Castillo JM, Barabino S, Herrero Vanrell R, Daull P, Garrigue J-S, et al. Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease. Int J Mol Sci. 2022;23:2434.
Fogagnolo P, De Cilla’ S, Alkabes M, Sabella P, Rossetti L. A review of topical and systemic vitamin supplementation in ocular surface diseases. Nutrients. 2021;13:1998.
Huang J-Y, Yeh P-T & Hou Y-C. A randomized, double-blind, placebo-controlled study of oral antioxidant supplement therapy in patients with dry eye syndrome. Clin Ophthalmol. 2016;10:813–20
Yokoi N, Georgiev GA. Tear-film-oriented diagnosis for dry eye. Jpn J Ophthalmol. 2019;63:127–36.
Matossian C, Crowley M, Periman L & Sorkin S. Personalized Management of Dry Eye Disease: Beyond Artificial Tears. Clin Ophthalmol. 2022;39:11–8.
Khanna RK, Catanese S, Emond P, Corcia P, Blasco H, Pisella P-J. Metabolomics and lipidomics approaches in human tears: A systematic review. Surv Ophthalmol. 2022; 67:1229–43.
Lam SM, Tong L, Duan X, Petznick A, Wenk MR, Shui G. Extensive characterization of human tear fluid collected using different techniques unravels the presence of novel lipid amphiphiles1 [S]. J Lipid Res. 2014;55:289–98.
Miyamoto M, Sassa T, Sawai M, Kihara A. Lipid polarity gradient formed by ω-hydroxy lipids in tear film prevents dry eye disease. Elife. 2020;9:e53582.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yazdani, M. Tear film lipid layer and corneal oxygenation: a new function?. Eye 37, 3534–3541 (2023). https://doi.org/10.1038/s41433-023-02557-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-023-02557-1
- Springer Nature Limited