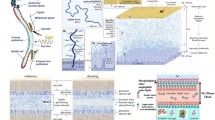
Abstract
Purpose of Review
This review summarizes the major factors currently implicated in the pathogenesis of tear film related ocular disorders, including dysfunctional adhesions, inflammatory factors, and insufficient lubrication by ocular surface mucins, and highlights the potential of in vitro models to propel more effective treatments into the clinic while improving fundamental understanding of ocular surface biology.
Recent Findings
The cornea and conjunctiva form a continuous ocular surface that shields the visual system from environmental threats. Because of its precise composition and organization, the ocular surface also refracts and focuses light rays, enabling clear vision. In many ocular surface diseases such as dry eye, tear film homeostasis is disrupted, causing inflammation, dryness, and epithelial damage. These friction-driven phenomena result in negative feedback loops in the eye that cause significant discomfort and compromise visual acuity over time.
Summary
Despite the prevalence of lubrication-related eye disorders, much remains unknown about disease initiation and progression, a knowledge gap reflected in the limited treatment options currently available. As novel therapeutics are developed to treat these conditions, scalable, cost-effective model systems that sufficiently recapitulate the complexities of the native ocular surface are needed to streamline drug screening and clinical translation.




Similar content being viewed by others
References
Gipson IK. The Ocular Surface: the challenge to enable and protect vision: the Friedenwald lecture. Investig Opthalmol Vis Sci. 2007;48:4391.
Sridhar MS. Anatomy of cornea and ocular surface. Indian J Ophthalmol. 2018;66:190–4.
Fini ME, Jeong S, Gong H, Martinez-Carrasco R, Laver NMV, Hijikata M, et al. Membrane-associated mucins of the ocular surface: new genes, new protein functions and new biological roles in human and mouse. Prog Retin Eye Res. 2020;75:100777.
Franzco IC. Blink-related microtrauma: when the ocular surface harms itself. Clin Exp Ophthalmol. 2003;31:183–90.
Cher I. A new look at lubrication of the ocular surface: fluid mechanics behind the blinking eyelids. Ocul Surf. 2008;6:79–86.
Pult H, Tosatti SGP, Spencer ND, Asfour JM, Ebenhoch M, Murphy PJ. Spontaneous blinking from a tribological viewpoint. Ocul Surf. 2015;13:236–49.
McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and humanistic burden of dry eye disease in Europe, North America, and Asia: a systematic literature review. Ocul Surf. 2016;14:144–67.
Yu J, Asche CV, Fairchild CJ. The economic burden of dry eye disease in the United States: a decision tree analysis. Cornea. 2011;30:379–87.
Govindarajan B, Gipson IK. Membrane-tethered mucins have multiple functions on the ocular surface. Exp Eye Res. 2010;90:655–63.
Pflugfelder SC, Bian F, Paiva CD. Matrix metalloproteinase-9 in the pathophysiology and diagnosis of dry eye syndrome. Metalloprotein Med. 2017;4:37–46 https://www.dovepress.com/matrix-metalloproteinase-9-in-the-pathophysiology-and-diagnosis-of-dry-peer-reviewed-article-MNM.
Xin Y, Lu Q, Li Q. 14-3-3σ controls corneal epithelial cell proliferation and differentiation through the Notch signaling pathway. Biochem Biophys Res Commun. 2010;392:593–8.
Stepp MA, Spurr-Michaud S, Gipson IK. Integrins in the wounded and unwounded stratified squamous epithelium of the cornea. Invest Ophthalmol Vis Sci. 1993;34:1829–44.
Gipson IK. Adhesive mechanisms of the corneal epithelium. Acta Ophthalmol. 1992;70:13–7.
Nemeth G, Felszeghy S, Kenyeres A, Szentmary N, Berta A, Suveges I, et al. Cell adhesion molecules in stromal corneal dystrophies. Histol Histopathol. 2008;23:945–52.
Mantelli F, Mauris J, Argüeso P. The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol. 2013;13:563–8.
AbuSamra DB, Argüeso P. Lectin-glycan interactions in corneal infection and inflammation. Front Immunol. 2018;9:2338.
Labbé A, Alalwani H, van Went C, Brasnu E, Georgescu D, Baudouin C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest Ophthalmol Vis Sci. 2012;53:4926–31.
Rosenthal P, Borsook D. The corneal pain system. Part I: the missing piece of the dry eye puzzle∗. Ocul Surf. 2012;10:2–14.
Galor A. Painful dry eye symptoms: a nerve problem or a tear problem? Ophthalmology. 2019;126:648–51.
Wei ZG, Wu RL, Lavker RM, Sun TT. In vitro growth and differentiation of rabbit bulbar, fornix, and palpebral conjunctival epithelia. Implications on conjunctival epithelial transdifferentiation and stem cells. Invest Ophthalmol Vis Sci. 1993;34:1814–28.
Shafaie S, Hutter V, Cook MT, Brown MB, Chau DYS. In vitro cell models for ophthalmic drug development applications. BioRes Open Access. 2016;5:94–108.
Uchino Y. The ocular surface glycocalyx and its alteration in dry eye disease: a review. Invest Ophthalmol Vis Sci. 2018;59:DES157–62.
Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013;183:35–48.
Gipson IK. Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res. 2016;54:49–63.
Gipson IK, Hori Y, Argüeso P. Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf. 2004;2:131–48.
Rosenfeld L, Fuller GG. Consequences of interfacial viscoelasticity on thin film stability. Langmuir. 2012;28:14238–44.
Bhamla MS, Chai C, Rabiah NI, Frostad JM, Fuller GG. Instability and breakup of model tear films. Invest Ophthalmol Vis Sci. 2016;57:949–58.
Leiske DL, Raju SR, Ketelson HA, Millar TJ, Fuller GG. The interfacial viscoelastic properties and structures of human and animal meibomian lipids. Exp Eye Res. 2010;90:598–604.
Leiske DL, Leiske CI, Leiske DR, Toney MF, Senchyna M, Ketelson HA, et al. Temperature-induced transitions in the structure and interfacial rheology of human meibum. Biophys J. 2012;102:369–76.
Millar TJ, Schuett BS. The real reason for having a meibomian lipid layer covering the outer surface of the tear film – a review. Exp Eye Res. 2015;137:125–38.
Baudouin C, Rolando M, Benitez del Castillo JM, Messmer EM, Figueiredo FC, Irkec M, et al. Reconsidering the central role of mucins in dry eye and ocular surface diseases. Prog Retin Eye Res. 2019;71:68–87.
Spurr-Michaud S, Argüeso P, Gipson I. Assay of mucins in human tear fluid. Exp Eye Res. 2007;84:939–50.
Bansil R, Stanley E, Lamont JT. Mucin biophysics. Annu Rev Physiol. 1995;57:635–57.
Mantelli F, Argüeso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008;8:477–83.
Argüeso P. Glycobiology of the ocular surface: mucins and lectins. Jpn J Ophthalmol. 2013;57:150–5.
Sumiyoshi M, Ricciuto J, Tisdale A, Gipson IK, Mantelli F, Argu¨eso P. Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:197–203.
Gipson IK. Distribution of mucins at the ocular surface. Exp Eye Res. 2004;78:379–88.
Gipson IK, Spurr-Michaud S, Argu¨eso P, Tisdale A, Ng TF, Russo CL. Mucin gene expression in immortalized human corneal–limbal and conjunctival epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2496–506.
Pitenis AA, Urueña JM, Hormel TT, Bhattacharjee T, Niemi SR, Marshall SL, et al. Corneal cell friction: survival, lubricity, tear films, and mucin production over extended duration in vitro studies. Biotribology. 2017;11:77–83.
Ablamowicz AF, Nichols JJ. Ocular surface membrane-associated mucins. Ocul Surf. 2016;14:331–41.
Gipson IK, Spurr-Michaud S, Tisdale A, Menon BB. Comparison of the transmembrane mucins MUC1 and MUC16 in epithelial barrier function. PLoS One. 2014;9:e100393.
Guzman-Aranguez A, Argüeso P. Structure and biological roles of mucin-type O-glycans at the ocular surface. Ocul Surf. 2010;8:8–17.
Fini ME, Bauskar A, Jeong S, Wilson MR. Clusterin in the eye: an old dog with new tricks at the ocular surface. Exp Eye Res. 2016;147:57–71.
Taniguchi T, Woodward AM, Magnelli P, McColgan NM, Lehoux S, Jacobo SMP, et al. N-glycosylation affects the stability and barrier function of the MUC16 mucin. J Biol Chem. 2017;292:11079–90.
Inatomi T, Spurr-Michaud S, Tisdale AS, Gipson IK. Human corneal and conjunctival epithelia express MUC1 mucin. Invest Ophthalmol Vis Sci. 1995;36:1818–27.
Blalock TD, Spurr-Michaud SJ, Tisdale AS, Gipson IK. Release of membrane-associated mucins from ocular surface epithelia. Invest Ophthalmol Vis Sci. 2008;49:1864–71.
Blalock TD, Spurr-Michaud SJ, Tisdale AS, Heimer SR, Gilmore MS, Ramesh V, et al. Functions of MUC16 in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:4509–18.
Hori Y, Spurr-Michaud S, Russo CL, Argüeso P, Gipson IK. Differential regulation of membrane-associated mucins in the human ocular surface epithelium. Invest Ophthalmol Vis Sci. 2004;45:114–22.
Schmidt TA, Sullivan DA, Knop E, Richards SM, Knop N, Liu S, et al. Transcription, translation, and function of lubricin, a boundary lubricant, at the ocular surface. JAMA Ophthalmol. 2013;131:766–76.
Das N, Schmidt TA, Krawetz RJ, Dufour A. Proteoglycan 4: from mere lubricant to regulator of tissue homeostasis and inflammation. BioEssays. 2019;41:1800166.
Regmi SC, Samsom ML, Heynen ML, Jay GD, Sullivan BD, Srinivasan S, et al. Degradation of proteoglycan 4/lubricin by cathepsin S: potential mechanism for diminished ocular surface lubrication in Sjögren’s syndrome. Exp Eye Res. 2017;161:1–9.
Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746–55.
Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci. 2013;110:5852–7.
Jay GD, Waller KA. The biology of lubricin: near frictionless joint motion. Matrix Biol. 2014;39:17–24.
Jay GD, Torres JR, Warman ML, Laderer MC, Breuer KS. The role of lubricin in the mechanical behavior of synovial fluid. Proc Natl Acad Sci. 2007;104:6194–9.
Reesink HL, Bonnevie ED, Liu S, Shurer CR, Hollander MJ, Bonassar LJ, et al. Galectin-3 binds to lubricin and reinforces the lubricating boundary layer of articular cartilage. Sci Rep. 2016;6:25463.
Rhee DK, Marcelino J, Baker MA, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115:622–31.
Rhee DK, Marcelino J, al-Mayouf S, Schelling DK, Bartels CF, Cui Y, et al. Consequences of disease-causing mutations on lubricin protein synthesis, secretion, and post-translational processing. J Biol Chem. 2005;280:31325–32.
Schaefer DB, Wendt D, Moretti M, Jakob M, Jay GD, Heberer M, et al. Lubricin reduces cartilage-cartilage integration. Biorheology. 2004;41:503–8.
Greene GW, Martin LL, Tabor RF, Michalczyk A, Ackland LM, Horn R. Lubricin: a versatile, biological anti-adhesive with properties comparable to polyethylene glycol. Biomaterials. 2015;53:127–36.
Greene GW, Ortiz V, Pozo-Gonzalo C, Moulton SE, Wang X, Martin LL, et al. Lubricin antiadhesive coatings exhibit size-selective transport properties that inhibit biofouling of electrode surfaces with minimal loss in electrochemical activity. Adv Mater Interfaces. 2018;5:1701296.
John T, Greene GW, Patil NA, Dealey TJA, Hossain MA, Abel B, et al. Adsorption of amyloidogenic peptides to functionalized surfaces is biased by charge and hydrophilicity. Langmuir. 2019;35:14522–31.
Huang S, et al. Cathepsin g degrades both glycosylated and unglycosylated regions of lubricin, a synovial mucin. Sci Rep. 2020;10:1–12.
Samsom ML, Morrison S, Masala N, Sullivan BD, Sullivan DA, Sheardown H, et al. Characterization of full-length recombinant human proteoglycan 4 as an ocular surface boundary lubricant. Exp Eye Res. 2014;127:14–9.
Seo J, Byun WY, Alisafaei F, Georgescu A, Yi YS, Massaro-Giordano M, et al. Multiscale reverse engineering of the human ocular surface. Nat Med. 2019;25:1310–8.
Lambiase A, et al. A two-week, randomized, double-masked study to evaluate safety and efficacy of lubricin (150 μg/mL) eye drops versus sodium hyaluronate (HA) 0.18% eye drops (Vismed®) in patients with moderate dry eye disease. Ocul Surf. 2017;15:77–87.
Zappone B, Greene GW, Oroudjev E, Jay GD, Israelachvili JN. Molecular aspects of boundary lubrication by human lubricin: effect of disulfide bonds and enzymatic digestion. Langmuir. 2008;24:1495–508.
Coles JM, Chang DP, Zauscher S. Molecular mechanisms of aqueous boundary lubrication by mucinous glycoproteins. Curr Opin Colloid Interface Sci. 2010;15:406–16.
Jay GD, Harris DA, Cha C-J. Boundary lubrication by lubricin is mediated by O-linked β(1-3)Gal-GalNAc oligosaccharides. Glycoconj J. 2001;18:807–15.
Flowers SA, Zieba A, Örnros J, Jin C, Rolfson O, Björkman LI, et al. Lubricin binds cartilage proteins, cartilage oligomeric matrix protein, fibronectin and collagen II at the cartilage surface. Sci Rep. 2017;7:13149.
Chang DP, Abu-Lail NI, Guilak F, Jay GD, Zauscher S. Conformational mechanics, adsorption, and normal force interactions of lubricin and hyaluronic acid on model surfaces. Langmuir. 2008;24:1183–93.
Murphy G, Knäuper V. Relating matrix metalloproteinase structure to function: why the “hemopexin” domain? Matrix Biol. 1997;15:511–8.
Gleghorn JP, Jones ARC, Flannery CR, Bonassar LJ. Boundary mode frictional properties of engineered cartilaginous tissues. Eur Cell Mater. 2007;14:20–9.
Jones ARC, Gleghorn JP, Hughes CE, Fitz LJ, Zollner R, Wainwright SD, et al. Binding and localization of recombinant lubricin to articular cartilage surfaces. J Orthop Res. 2007;25:283–92.
Han M, Silva SM, Lei W, Quigley A, Kapsa RMI, Moulton SE, et al. Adhesion and self-assembly of lubricin (PRG4) brush layers on different substrate surfaces. Langmuir. 2019;35:15834–48.
Zappone B, Ruths M, Greene GW, Jay GD, Israelachvili JN. Adsorption, lubrication, and wear of lubricin on model surfaces: polymer brush-like behavior of a glycoprotein. Biophys J. 2007;92:1693–708.
Majd SE, Kuijer R, Köwitsch A, Groth T, Schmidt TA, Sharma PK. Both hyaluronan and collagen type II keep proteoglycan 4 (lubricin) at the cartilage surface in a condition that provides low friction during boundary lubrication. Langmuir. 2014;30:14566–72.
Greene GW, Thapa R, Holt SA, Wang X, Garvey CJ, Tabor RF. Structure and property changes in self-assembled lubricin layers induced by calcium ion interactions. Langmuir. 2017;33:2559–70.
Chang DP, Abu-Lail NI, Coles JM, Guilak F, Jay GD, Zauscher S. Friction force microscopy of lubricin and hyaluronic acid between hydrophobic and hydrophilic surfaces. Soft Matter. 2009;5:3438–45.
Abubacker S, Ponjevic D, Ham HO, Messersmith PB, Matyas JR, Schmidt TA. Effect of disulfide bonding and multimerisation on proteoglycan 4’s cartilage boundary lubricating ability and adsorption. Connect Tissue Res. 2016;57:113–23.
Swann DA, Hendren RB, Radin EL, Sotman SL. The lubricating activity of synovial fluid glycoproteins. Arthritis Rheum. 1981;24:22–30.
Iqbal SM, Leonard C, C. Regmi S, de Rantere D, Tailor P, Ren G, et al. Lubricin/proteoglycan 4 binds to and regulates the activity of Toll-like receptors in vitro. Sci Rep. 2016;6:18910.
Alquraini A, Jamal M, Zhang L, Schmidt T, Jay GD, Elsaid KA. The autocrine role of proteoglycan-4 (PRG4) in modulating osteoarthritic synoviocyte proliferation and expression of matrix degrading enzymes. Arthritis Res Ther. 2017;19:89.
Al-Sharif A, et al. Lubricin/proteoglycan 4 binding to CD44 receptor: a mechanism of the suppression of proinflammatory cytokine–induced synoviocyte proliferation by lubricin. Arthritis Rheum. 2015;67:1503–13.
Park DSJ, et al. Human pericardial proteoglycan 4 (lubricin): implications for postcardiotomy intrathoracic adhesion formation. J Thorac Cardiovasc Surg. 2018;156:1598–1608.e1.
Ikegawa S, Sano M, Koshizuka Y, Nakamura Y. Isolation, characterization and mapping of the mouse and human PRG4 (proteoglycan 4) genes. Cytogenet Genome Res. 2000;90:291–7.
Jin C, Ekwall AKH, Bylund J, Björkman L, Estrella RP, Whitelock JM, et al. Human synovial Lubricin expresses Sialyl Lewis x determinant and has L-selectin ligand activity. J Biol Chem. 2012;287:35922–33.
Yu-Wai-Man C, Tagalakis AD, Meng J, Bouremel Y, Lee RMH, Virasami A, et al. Genotype-phenotype associations of IL6 and PRG4 with conjunctival fibrosis after glaucoma surgery. JAMA Ophthalmol. 2017;135:1147–55.
Wang J, Chen D, Sullivan DA, Xie H, Li Y, Liu Y. Expression of Lubricin in the human amniotic membrane. Cornea. 2020;39:118–21.
Oh J, Kuan KG, Tiong LU, Trochsler MI, Jay G, Schmidt TA, et al. Recombinant human lubricin for prevention of postoperative intra-abdominal adhesions in a rat model. J Surg Res. 2017;208:20–5.
Navascues-Cornago M, Maldonado-Codina C, Morgan PB. Mechanical sensitivity of the human conjunctiva. Cornea. 2014;33:855–9.
Kato H, Yokoi N, Watanabe A, Komuro A, Sonomura Y, Sotozono C, et al. Relationship between ocular surface epithelial damage, tear abnormalities, and blink in patients with dry eye. Cornea. 2019;38:318–24.
Qin G, Baidouri H, Glasser A, Raghunathan VK, Morris C, Maltseva I, et al. Development of an in vitro model to study the biological effects of blinking. Ocul Surf. 2018;16:226–34.
Vu CHV, Kawashima M, Yamada M, Suwaki K, Uchino M, Shigeyasu C, et al. Influence of meibomian gland dysfunction and friction-related disease on the severity of dry eye. Ophthalmology. 2018;125:1181–8.
Meloni M, De Servi B, Marasco D, Del Prete S. Molecular mechanism of ocular surface damage: application to an in vitro dry eye model on human corneal epithelium. Mol Vis. 2011;17:113–26.
Berry M, Pult H, Purslow C, Murphy PJ. Mucins and ocular signs in symptomatic and asymptomatic contact lens wear. Optom Vis Sci. 2008;85:E930–8.
Uchino M, Schaumberg DA. Dry eye disease: impact on quality of life and vision. Curr Ophthalmol Rep. 2013;1:51–7.
Georgiev GA, Eftimov P, Yokoi N. Contribution of mucins towards the physical properties of the tear film: a modern update. Int J Mol Sci. 2019;20:6132.
Pult H, Purslow C, Murphy PJ. The relationship between clinical signs and dry eye symptoms. Eye. 2011;25:502–10.
Holland EJ, Darvish M, Nichols KK, Jones L, Karpecki PM. Efficacy of topical ophthalmic drugs in the treatment of dry eye disease: a systematic literature review. Ocul Surf. 2019;17:412–23.
Nebbioso M, Fameli V, Gharbiya M, Sacchetti M, Zicari AM, Lambiase A. Investigational drugs in dry eye disease. Expert Opin Investig Drugs. 2016;25:1437–46.
Gipson IK, Spurr-Michaud SJ, Senchyna M, Ritter R, Schaumberg D. Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye. Cornea. 2011;30:1346–52.
Aragona P, Aguennouz M'H, Rania L, Postorino E, Sommario MS, Roszkowska AM, et al. Matrix metalloproteinase 9 and transglutaminase 2 expression at the ocular surface in patients with different forms of dry eye disease. Ophthalmology. 2015;122:62–71.
Paiva CSD, et al. Dry eye–induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Invest Ophthalmol Vis Sci. 2007;48:2553–60.
Alex A, Edwards A, Hays JD, Kerkstra M, Shih A, de Paiva CS, et al. Factors predicting the ocular surface response to desiccating environmental stress. Invest Ophthalmol Vis Sci. 2013;54:3325–32.
Krauss AH, Corrales RM, Pelegrino FSA, Tukler-Henriksson J, Pflugfelder SC, de Paiva CS. Improvement of outcome measures of dry eye by a novel integrin antagonist in the murine desiccating stress model. Invest Ophthalmol Vis Sci. 2015;56:5888–95.
Albertsmeyer A-C, Kakkassery V, Spurr-Michaud S, Beeks O, Gipson IK. Effect of pro-inflammatory mediators on membrane-associated mucins expressed by human ocular surface epithelial cells. Exp Eye Res. 2010;90:444–51.
Arafat SN, Suelves AM, Spurr-Michaud S, Chodosh J, Foster CS, Dohlman CH, et al. Neutrophil collagenase, gelatinase, and myeloperoxidase in tears of patients with Stevens-Johnson syndrome and ocular cicatricial pemphigoid. Ophthalmology. 2014;121:79–87.
Chotikavanich S, de Paiva CS, Li DQ, Chen JJ, Bian F, Farley WJ, et al. Production and activity of matrix metalloproteinase-9 on the ocular surface increase in dysfunctional tear syndrome. Invest Ophthalmol Vis Sci. 2009;50:3203–9.
Li D-Q, Lokeshwar BL, Solomon A, Monroy D, Ji Z, Pflugfelder SC. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001;73:449–59.
Yang Y-N, Bauer D, Wasmuth S, Steuhl K-P, Heiligenhaus A. Matrix metalloproteinases (MMP-2 and 9) and tissue inhibitors of matrix metalloproteinases (TIMP-1 and 2) during the course of experimental necrotizing herpetic keratitis. Exp Eye Res. 2003;77:227–37.
Garrana RM, Zieske JD, Assouline M, Gipson IK. Matrix metalloproteinases in epithelia from human recurrent corneal erosion. Invest Ophthalmol Vis Sci. 1999;40:1266–70.
Shimazaki J, et al. A prospective, randomized trial of two mucin secretogogues for the treatment of dry eye syndrome in office workers. Sci Rep. 2017;7:1–9.
Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, et al. Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci. 1998;39:2602–9.
Argüeso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjögren syndrome. Invest Ophthalmol Vis Sci. 2002;43:1004–11.
Mantelli F, Schaffer L, Dana R, Head SR, Argüeso P. Glycogene expression in conjunctiva of patients with dry eye: downregulation of Notch signaling. Invest Ophthalmol Vis Sci. 2009;50:2666–72.
Xiong L, Woodward AM, Argüeso P. Notch signaling modulates MUC16 biosynthesis in an in vitro model of human corneal and conjunctival epithelial cell differentiation. Invest Ophthalmol Vis Sci. 2011;52:5641–6.
Kalangara JP, et al. Burning eye syndrome: do neuropathic pain mechanisms underlie chronic dry eye? Pain Med. 2016;17:746–55.
Mcmonnies CW. The potential role of neuropathic mechanisms in dry eye syndromes. Aust J Optom. 2017;10:5–13.
Lambiase A, et al. Alterations of tear neuromediators in dry eye disease. Arch Ophthalmol. 2011;129:981–6.
Lambiase A, Micera A, Pellegrini G, Merlo D, Rama P, de Luca M, et al. In vitro evidence of nerve growth factor effects on human conjunctival epithelial cell differentiation and mucin gene expression. Invest Ophthalmol Vis Sci. 2009;50:4622–30.
Kheirkhah A, Dohlman TH, Amparo F, Arnoldner MA, Jamali A, Hamrah P, et al. Effects of corneal nerve density on the response to treatment in dry eye disease. Ophthalmology. 2015;122:662–8.
Akpek EK, Bunya VY, Saldanha IJ. Sjögren’s syndrome: more than just dry eye. Cornea. 2019;38:658–61.
Pal-Ghosh S, Pajoohesh-Ganji A, Brown M, Stepp MA. A mouse model for the study of recurrent corneal epithelial erosions: α9β1 integrin implicated in progression of the disease. Invest Ophthalmol Vis Sci. 2004;45:1775–88.
Kang Y, Li S, Liu C, Liu M, Shi S, Xu M, et al. A rabbit model for assessing symblepharon after alkali burn of the superior conjunctival sac. Sci Rep. 2019;9:13857.
Shimazaki J, Shinozaki N, Tsubota K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol. 1998;82:235–40.
Goyal R, Jones SM, Espinosa M, Green V, Nischal KK. Amniotic membrane transplantation in children with symblepharon and massive pannus. Arch Ophthalmol. 2006;124:1435–40.
Wanzeler ACV, Barbosa IAF, Duarte B, Barbosa EB, Borges DA, Alves M. Impact of pterygium on the ocular surface and meibomian glands. PLoS One. 2019;14:e0213956.
Chan CML, Liu YP, Tan DTH. Ocular surface changes in pterygium. Cornea. 2002;21:38–42.
Barabino S, Dana MR. Animal models of dry eye: a critical assessment of opportunities and limitations. Invest Ophthalmol Vis Sci. 2004;45:1641–6.
Li X, Kang B, Woo IH, Eom Y, Lee HK, Kim HM, et al. Effects of topical mucolytic agents on the tears and ocular surface: a plausible animal model of mucin-deficient dry eye. Invest Ophthalmol Vis Sci. 2018;59:3104–14.
Marko CK, Tisdale A, Spurr-Michaud S, Gipson IK. The effect of desiccating environmental stress on Spdef null mice that lack conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2012;53:–2329.
Chung S-H, Lee SK, Cristol SM, Lee ES, Lee DW, Seo KY, et al. Impact of short-term exposure of commercial eyedrops preserved with benzalkonium chloride on precorneal mucin. Mol Vis. 2006;12:415–21.
Leonard BC, Yañez-Soto B, Raghunathan VK, Abbott NL, Murphy CJ. Species variation and spatial differences in mucin expression from corneal epithelial cells. Exp Eye Res. 2016;152:43–8.
Meyer AE, Baier RE, Chen H, Chowhan M. Differential tissue-on-tissue lubrication by ophthalmic formulations. J Biomed Mater Res B Appl Biomater. 2007;82B:74–88.
Yáñez-Soto B, Leonard BC, Raghunathan VK, Abbott NL, Murphy CJ. Effect of stratification on surface properties of corneal epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:8340–8.
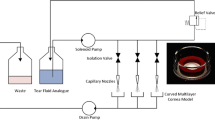
Postnikoff CK, Pintwala R, Williams S, Wright AM, Hileeto D, Gorbet MB. Development of a curved, stratified, in vitro model to assess ocular biocompatibility. PLoS One. 2014;9:e96448.
Robertson DM, Li L, Fisher S, Pearce VP, Shay JW, Wright WE, et al. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005;46:470–8.
Takeji Y, Urashima H, Aoki A, Shinohara H. Rebamipide increases the mucin-like glycoprotein production in corneal epithelial cells. J Ocul Pharmacol Ther. 2012;28:259–63.
Itoh S, Itoh K, Shinohara H. Regulation of human corneal epithelial mucins by rebamipide. Curr Eye Res. 2014;39:133–41.
Uchino Y, Woodward AM, Argüeso P. Differential effect of rebamipide on transmembrane mucin biosynthesis in stratified ocular surface epithelial cells. Exp Eye Res. 2016;153:1–7.
Masterton S, Ahearne M. The effect of calcium and glucose concentration on corneal epithelial cell lines differentiation, proliferation, and focal adhesion expression. BioRes Open Access. 2019;8:74–83.
Barabino S, De Servi B, Aragona S, Manenti D, Meloni M. Efficacy of a new ocular surface modulator in restoring epithelial changes in an in vitro model of dry eye syndrome. Curr Eye Res. 2017;42:358–63.
Kaluzhny Y, Kinuthia MW, Lapointe AM, Truong T, Klausner M, Hayden P. Oxidative stress in corneal injuries of different origin: utilization of 3D human corneal epithelial tissue model. Exp Eye Res. 2020;190:107867.
Lu Q, Yin H, Grant MP, Elisseeff JH. An in vitro model for the ocular surface and tear film system. Sci Rep. 2017;7:1–11.
Govindarajan B, Menon BB, Spurr-Michaud S, Rastogi K, Gilmore MS, Argüeso P, et al. A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PLoS One. 2012;7:e32418.
Yu ACY, Worrall LJ, Strynadka NCJ. Structural insight into the bacterial mucinase StcE essential to adhesion and immune evasion during enterohemorrhagic E. coli infection. Structure. 2012;20:707–17.
Malaker SA, Pedram K, Ferracane MJ, Bensing BA, Krishnan V, Pett C, et al. The mucin-selective protease StcE enables molecular and functional analysis of human cancer-associated mucins. Proc Natl Acad Sci. 2019;116:7278–87.
Grys TE, Walters LL, Welch RA. Characterization of the StcE protease activity of Escherichia coli O157:H7. J Bacteriol. 2006;188:4646–53.
Acknowledgments
The authors and the editors of Current Ophthalmology Reports would like to thank Dr. Matias Soifer, Foster Center for Ocular Immunology, Duke University Eye Center, for his careful review of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Amy C. Madl and Gerald F. Fuller each declares no potential conflicts of interest. David Myung is a section editor of Current Ophthalmology Reports.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Regenerative Medicine in Ophthalmology
Rights and permissions
About this article
Cite this article
Madl, A.C., Fuller, G.F. & Myung, D. Modeling and Restoring the Tear Film. Curr Ophthalmol Rep 8, 281–300 (2020). https://doi.org/10.1007/s40135-020-00258-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40135-020-00258-6