Abstract
Background
Regular screening for retinopathy and timely intervention reduces blindness from diabetes by 90%. Screening is currently dependent on the interpretation of images captured by trained technicians. Inherent barriers of accessibility and affordability with this approach impede widespread success of retinopathy screening programs. Herein, we report our observations on the potential of a novel approach, Selfie Fundus Imaging (SFI), to enhance diabetic retinopathy screening.
Methods
The study was undertaken over a two-month period during COVID 19 lockdown. 60 diabetic patients participated in the study. Retinal images were captured using three different approaches, handheld smartphone-based photographs captured by patients themselves after a short video-assisted training session (SFI group), and smartphone-based photographs captured by a trained technician and photographs taken on desktop conventional digital fundus camera (Gold standard). Sensitivity and kappa statistics was determined for retinopathy and macular oedema grading.
Findings
Mean age of the study participants was 52.4 years ± 9.8 years and 78% were men. Of 120 images captured using SFI, 90% were centred-gradable, 8% were decentred-gradable and 2% were ungradable. 82% patients captured the image within a minute (majority by 31–45 s). The sensitivity of SFI to detect diabetic retinopathy was 88.39%. Agreement between SFI grading and standard fundus photograph grading was 85.86% with substantial kappa (0.77). For the detection of diabetic macular oedema, the agreement between SFI images and standard images was 93.67, with almost perfect kappa (0.91).
Conclusion
Fundus images were captured by patients using SFI without major difficulty and were comparable to images taken by trained specialist. With greater penetrance, advances, and availability of mobile photographic technology, we believe that SFI would positively impact the success of diabetic retinopathy screening programs by breaking the barriers of availability, accessibility, and affordability. SFI could ensure continuation of screening schedules for diabetic retinopathy, even in the face a highly contagious pandemic.
Similar content being viewed by others
Introduction
Nine percent of the global adult population suffers from diabetes mellitus [1]. By 2030, this would increase to 366 million and the most vulnerable population would be from low-middle income countries [2]. With increasing duration of the disease, microvascular and macrovascular sequelae begin to manifest. Health-related economic burden, due to expenditure on control and treatment of the disease and its complications, as well as working hours lost in the process is substantial. Diabetic retinopathy is one of the three major microvascular complications of diabetes mellitus. In its natural history, diabetic retinopathy develops after a period of latency and progresses slowly but relentlessly, from a stage of mild severity to an advanced stage and to blindness. The disease remains asymptomatic not only in its early stages but also when it has reached a stage of impending vision loss. Regular screening for retinopathy and timely intervention has the potential to reduce diabetes-related blindness by almost 90% [2].
Diabetic retinopathy, being an important public health problem, the presence of an asymptomatic stage and the availability of effective treatment, mandates measures to improve regular screening of patients as recommended by international guidelines [3]. Unfortunately, only 50% to 65% of the total diabetic population undergoes annual DR screening as recommended by the American Academy of Ophthalmology [4]. Barriers to achieving targets for retinopathy screening, other than the disease being asymptomatic, include poor patient education, lack of access and affordability and dependency on technicians or specialists. The global COVID 19 pandemic has further compounded problems for diabetic retinopathy screening, for both patients and retinal physicians due to prolonged lockdown measures.
Available methods for diabetic retinopathy screening include ophthalmoscopy, biomicroscopy and/or fundus photography [5]. The gold standard for detection and grading of diabetic retinopathy is by capturing seven field 30° stereoscopic photographic images of the fundus [6]. Fundus photography has a sensitivity and specificity that is superior to ophthalmoscopic methods [7, 8]. Over the past few decades, fundus photography using desktop analogue and digital cameras has been supplanted by handheld digital and smartphone-based cameras [9]. Some of these newer devices have also gained acceptance from national agencies as acceptable tools for regular diabetic retinopathy screening. However, all these devices still necessitate a trained technician to capture the retinal photographs.
Recently, in a small pilot study of 3 patients, we reported our initial observations on the ability of diabetic patients to capture images of their retina, themselves [10, 11]. We designated this innovative approach to capturing retinal images as Selfie Fundus Imaging (SFI). During the ongoing lockdown restrictions due to COVID-19 and the increased need for precautions to be taken by physicians and technicians while interacting with patients, we studied the utility of SFI in a larger cohort of patients with diabetes. Our observations indicate that images obtained by SFI are non-inferior to those captured using standard fundus camera and has the potential to compliment traditional methods of retinal screening being currently practiced. In difficult periods, like the ongoing pandemic, SFI would enable continued success of retinopathy screening programs without increasing the risk of disease spread. With further advances in smartphone image capture capabilities and its integration with machine learning, we contemplate that SFI may be able to significantly enhance current approaches to diabetic retinopathy screening.
Methods
This prospective, comparative study was initiated after obtaining due ethical clearance (IECPG-646/19/12/2018) from the institutional ethics committee and followed the tenets of the Helsinki Declaration. Written informed consent was taken from all patients stating their voluntary participation in the study [after reading the patient information sheet provided to each participant]. 60 diabetic patients with clear ocular media were recruited. Inclusion criteria were diabetic patients above 18 years of age, clear ocular media, good visual acuity and fixation and willing to provide informed consent. One eyed patients and those with other ocular conditions like cataract, ocular hypertension, and retinal pathology other than diabetic retinopathy were not considered for study enrolment.
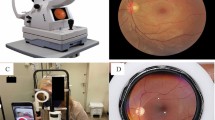
Preliminary visual acuity measurement, anterior segment evaluation and dilated indirect ophthalmoscopy were undertaken for all patients. Demographic history and pertinent details regarding diabetes were also recorded. Patients who fulfilled the inclusion and exclusion criteria were given instructions about the approach to SFI. Instructions on obtaining images using SFI was provided using a tutorial video created for the purpose of the study [supplementary material 1] and reinforced by a mock demonstration. Once the patient indicated that they had comprehended the instructions, they were given the camera and asked to perform SFI. During, the same hospital visit, fundus images of each patient were then also captured by a trained technician using two additional approaches, first, with the same handheld smartphone camera and second, using standard digital tabletop fundus camera (gold standard). All 360 images (2 images of both eyes of each patient, using three different approaches) were then graded for severity of retinopathy and diabetic macular oedema by a retina specialist/trained ophthalmologist. Treatment was offered as necessitated, based on severity and as per recommended guidelines.
Retinal photography
Eyes were dilated with eyedrop Tropicamide 1%, one drop, three times at 10 min intervals. A short video training session, ranging from two to five minutes, was carried out where it was demonstrated to the patients, how to hold the camera, align it and move it slowly towards the eye. This was followed by a live, mock demonstration. Patients were taught to hold the camera firmly with both the hands at the distal and proximal end with the eyepiece facing them and positioned approximately 4 inches away from the eye to be imaged. They were to then move in the camera slowly, whence, upon sensing the red retinal reflex, the camera automatically begins image capture. While the image is being captured, a snap sound is produced with every click from the device and the patient is advised to hold the camera steady at this point of time. Hence, multiple images get captured with one take. These were reviewed, and the best images archived for later grading. If the patient found it difficult to align, dynamic external fixation (tip of finger) was provided by the technician.
Once the patient seemed familiar with the instructions and felt comfortable with the technique, they were seated with elbow resting on the table and camera was handed over to them. They were then encouraged to replicate what they had learnt from the training session and to independently capture photographs of the right and the left eye separately. Time taken to capture these images was also noted. Assistance provided to the patient, if any, for improving fixation, retracting the eyelids etc., and discomfort or difficulty of any kind, expressed by the patients was also made note of.
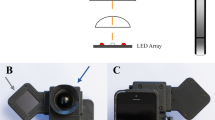
The smartphone-based handheld camera used in this study was the VOLK iNview [Volk, USA]. This is a mydriatic (minimum 5 mm dilatation) fundus camera capable of capturing images of the central 50° of the retina. It combines volk optics, auto capture technology and special software app compatible with iPhone5s, 6 or 6 s or iPod Touch. This device is CE certified for diabetic retinopathy screening. The desktop standard digital camera used as gold standard was Zeiss, FF450 Visupac, Germany.
In brief, three levels of retinal photographic images (50 degree field of view) of both eyes was captured-
-
1.
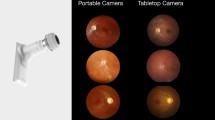
By patient (after appropriate instruction and training videos) using handheld smartphone based single field camera. (Volk iNview, USA) (Fig. 1)
-
2.
By trained technician using the same handheld smartphone based single field camera. (Volk iNview, USA)
-
3.
By technician using standard digital desktop camera that is usually considered as gold standard (Zeiss, FF450 Visupac, Germany)
Image quality was first noted as gradable-centred, gradable-not centred and ungradable. Retinopathy severity was then categorized by a retina specialist/ trained ophthalmologist according to the International Council of Ophthalmology (ICO) classification [12]. The categories were no DR, mild NPDR, moderate NPDR, severe NPDR and PDR. Diabetic macular oedema was classified as no DMO, non-central involved DMO or central involved DMO. Determination of sensitivity and kappa statistics for the level of agreement between the three different methods of image capture, was calculated using STATA 12.1 software. Analysis considered the quality of images obtained and severity of diabetic retinopathy and diabetic macular oedema.
Funding source information—we have no financial interest in any of the products mentioned. Also, the study was not funded or sponsored by the manufacturer/company and was independently carried out by the investigators. No company/manufacturer was involved in the design of the study or analysis of data.
Results
Mean age of the study participants was 52.4 years ± 9.8 years and 78% were men. Using images captured using standard desktop digital camera (gold standard), severity grading of diabetic retinopathy as no DR, mild NPDR, moderate NPDR, severe NPDR and PDR was 2%, 6%, 55%, 18% and 16% respectively. 3% of images were ungradable. Corresponding severity grading using images captured using SFI was 3.3%, 5.8%, 56.7%, 13.3% and 15%. 5.8% of images (n = 7) obtained by SFI were not gradable. The severity grading based on images clicked on smartphone handheld device by the trained technician was 3.3% with no DR, 5.8% with mild NPDR, 58.3% with moderate NPDR, 13.3% with severe NPDR and 15%, PDR. 4.1% of these images were ungradable.
Seven ungradable images on SFI were excluded from sensitivity analysis. Of the remaining 113 images, 99 images were comparable for correct grading of diabetic retinopathy severity to images taken on the standard photographs (true positive), while 13 images failed to show the lesions that were picked up on the standard fundus photography (false negative). Hence, the sensitivity of SFI images for grading of DR severity was 88.4%. The accuracy of the classification was substantial as shown by a positive kappa coefficient (0.77). Agreement between images captured using these two modalities was 85.9%. When SFI images were compared in a similar manner with those obtained by a trained technician using the same handheld camera, the agreement was much higher (95.8%), with an almost perfect kappa coefficient (0.93%).
Images were also graded according to the presence or absence of DMO. On standard photography, percentage of eyes with no DMO, non-centre involved DMO and centre involved DMO was 32.5%, 25% and 39.2% respectively. Corresponding values for SFI images was 32.5%, 24.1% and 37.5%. DMO categorization of images captured by the technician using the handheld camera was 32.5%, no DMO; 25% non-centre involved DMO and 38.3%, centre involved DMO.
As before, of the total of 120 SFI images, 7 ungradable images were excluded from analysis. Of the remaining 113 images, 4 images failed to show lesions suggestive of DMO that were picked up on the standard fundus photography (false negative). Seventy four images were comparable and correctly identified DMO (true positive). Thirty nine eyes had no DMO in both selfie image and standard images (true negative). Hence, the sensitivity of SFI to detect diabetic macular oedema was found to be 93.7%. The accuracy of the classification was almost perfect, as shown by positive kappa coefficient (0.91). In a similar manner, it was found that agreement between SFI images and that captured by the technician using the same handheld camera was much higher at 98.3% with a perfect kappa coefficient (0.97).
Forty nine patients (81.7%) completed the task of SFI within a minute (usually between 31 and 45 s). Younger patients were familiar with technology and they took even less time. The remaining patients, who needed more than 2 min, were provided some form of assistance by the technician like stabilizing the forearm, providing external fixation target, retracting the eyelid etc. Ninety (108 images) SFI images were well centred with adequate view of the macula, optic disc, and major retinal vessels. 8% (9 images) were decentred but gradable, while 2% (3 images) were totally decentred and ungradable. Of the 9 decentred images, only 5 were gradable. Hence, of 120 SFI images taken, 113 gradable ones were considered for analysis. Summary of the comparative results for grading of diabetic retinopathy severity and grade of diabetic macular oedema is depicted in Table 1.
Of 60 patients, 45% (n = 27) took a well centred image without any difficulty (Figs. 2, 3). Sixteen patients (26.7%) needed a fixation target (tip of finger was used as external target by the assistant) to capture an image centred on the posterior pole. Four patients (6.8%) could not focus on their retina for which the cause could not be identified. Five patients had hazy media in one or other eye and found it difficult to achieve sharp focus. Due to senile ptosis, 7 patients (11.7%) needed assistance to retract their upper eyelid (Table 2). Two patients (3.3%) had asteroid hyalosis in one or both eyes, masking the diabetic lesion. The procedure was not comfortable to 4 patients (6.7%) who were very photophobic and blinked often. They needed reassurance and assistance to retract their eyelids. Two patients (3.3%) had frozen shoulder and so could not align the camera properly. It was challenging for 1 patient (1.7%) who had poor dilating pupil and 1 with deep-seated eye (1.7%). Three patients (5%) had some form of weakness and needed support from the assistant to have a proper grip on the camera. One patient had cervical spondylosis and found it difficult to move his neck for positioning the camera. Centred image could not be obtained in one patient with 3rd nerve palsy.
Discussion
Diabetic retinopathy is a severe sight threatening complication of diabetes mellitus and has become the most common cause of blindness in middle aged adults, in several countries [13]. There is a significant lead time between onset of diabetes and the development of retinopathy, and in addition, highly efficacious therapy to prevent visual disability is available. The focus of screening is to detect retinopathy before it has progressed to a stage wherein therapy becomes ineffective or less efficacious. Reports suggest that 90% of blindness resulting from diabetic retinopathy is entirely preventable if major success is achieved in implementing internationally accepted screening guidelines. A high percentage of success in screening translates to lower visual morbidity and hence to reduced health costs and improved health economics. Unfortunately, more than 40% of diabetic patients currently fail to report for recommended screening even in developed nations. The situation is even more alarming in low-middle income countries [14,15,16].
Several approaches to diabetic retinopathy screening have been explored and used in practice [17]. Broadly, these can be categorized into methods based on ophthalmoscopy and those based on photography. The former methods are subjective and so have a wide margin of specificity and sensitivity based on the amount of training. The latter is objective, has high sensitivity and specificity but is technology and cost intensive. Despite these limitations, grading of images captured using fundus cameras is considered the most efficient method for the management of diabetic retinopathy. Since the patient must be at the facility (clinic/ telemedicine), screening by fundus photography is limited by the significant drawbacks of accessibility, affordability, and availability [18]. In this background, an important question that has remained unexplored is “Can screening be taken to the patients themselves?” With SFI, we explored this possibility.
Ninety of the selfie images obtained by the patients themselves were of good quality and appropriately centred on the retina. disc macula and both the vascular arcades. Though there were some initial challenges, a good proportion of patients (48.3%) could independently accomplish the task and the remaining could do so with minimal assistance. After the tutorial session, when the patients became acquainted with the procedure and the device, the majority of them were able to capture adequate images within 30-45 s. When compared with images captured using a standard fundus camera, SFI had a sensitivity of 88.4% to diagnose the severity of diabetic retinopathy and the kappa coefficient was substantial at 0.77. For the identification of DMO, the sensitivity of SFI was 93.7% and the kappa coefficient was almost perfect (0.90) with 93.3% agreement. The quality of SFI was also highly comparable to the photographs taken by the trained specialist on the same device.
An important necessity with SFI using the currently available smartphone camera is the need for pupillary dilatation. This may bring to question the safety of having diabetic patients themselves dilate their pupils. However, this concern may be acceptable given the benefits of successful screening for diabetic retinopathy and the reported low risk (~1%) of severe intraocular pressure elevation (>25 mmHg) after dilatation with Tropicamide 1% even in eyes with narrow anterior chamber angle [19]. Other minor obstacles to SFI include severe senile ptosis, dermatochalasis, deeply set eyeball, senile tremor, cervical spondylosis, senile fatigue, frozen shoulder, and few others. As anticipated, the presence of hazy media is an impediment to image capture with all cameras and so it is with SFI also. However, the inability to capture retinal images using SFI should be construed as the presence of significant cataract, posterior capsular opacification, corneal opacity, asteroid hyalosis or even vitreous haemorrhage and urgent ophthalmology consultation becomes inevitable.
So, in this study, we present our observations on SFI, an innovative approach to diabetic retinopathy screening, wherein a patient independently takes a photograph of one’s own retina. The patient can then save the images and tele-consult with the ophthalmologist for further guidance. This approach would overcome barriers like poor access to healthcare, travel cost and distance, busy schedule, lack of caretaker etc. If individuals do not have access to smartphones, SFI may be made available at other public facilities like post offices, banks etc. as it is not heavily dependent on costly infrastructure. It can even be carried by healthcare workers to the patient’s residence. When amalgamated with the burgeoning field of machine learning and AI, we are optimistic that SFI may have the potential to improve the success of diabetic retinopathy screening programmes of all countries. In addition, during situations like a highly contagious and dangerous pandemic, SFI may help to sustain timely screening efforts for diabetic retinopathy. Some limitations of the study include the hospital-based recruitment of participants, limited sample size, evaluation with only one out of the several commercially available fundus cameras, need for initial tutoring of patients using a training video and necessity of pupillary dilatation. Though the need for pupillary dilatation seems like a drawback, the benefits of successful screening would outweigh the associated low risk of elevated intraocular pressure [19].
To conclude, the present study highlights the feasibility of bringing SFI to the forefront of diabetic retinopathy screening. To the best of our knowledge, this is the first study undertaken with selfie fundus imaging to screen diabetic patients for retinopathy. With greater penetrance, advances, and availability of mobile technology, including camera resolution and specific health-related apps, we believe that SFI would positively impact success of diabetic retinopathy screening programs, both in normal circumstances as well as situations like the ongoing COVID-19 infection.
Summary table
What was known before:
-
Fundus imaging by trained specialist was used for grading and screening for diabetic retinopathy.
What this study adds:
-
Selfie Fundus Imaging (SFI), which is taking photo of the retina by the patient themselves can improve screening by overcoming the barrier of accessibility and affordability, more so in the era of pandemic.
References
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107843.
World Health Organization. Prevention of Blindness from Diabetes Mellitus: Report of WHO Consultation in Geneva, Switzerland; 2005. p. 9–11.
Scanlon PH. Update on screening for sight-threatening diabetic retinopathy. Ophthalmic Res. 2019;62:218–24.
Schoenfeld ER, Greene JM, Wu SY, Leske MC. Patterns of adherence to diabetes vision care guidelines: baseline findings from the Diabetic Retinopathy Awareness Program. Ophthalmology. 2001;108:563–71.
Squirrell DM, Talbot JF. Screening for diabetic retinopathy. J R Soc Med. 2003;96:273–6.
Harding SP, Broadbent DM, Neoh C, White MC, Vora J. Sensitivity and specificity of photography and direct ophthalmoscopy in screening for sight threatening eye disease: the Liverpool Diabetic Eye Study. BMJ. 1995;311:1131–5.
Kalm H, Egertsen R, Blohmé G. Non-stereo fundus photography as a screening procedure for diabetic retinopathy among patients with type II diabetes. Acta Ophthalmologica. 1989;67:546–53.
Garg S, Davis RM. Diabetic retinopathy screening update. Clin Diabetes. 2009;27:140–5.
Rajalakshmi R, Arulmalar S, Usha M, Prathiba V, Kareemuddin KS, Anjana RM, et al. Validation of smartphone based retinal photography for diabetic retinopathy screening. PLoS ONE. 2015;10:e0138285.
Venkatesh P, Kumar S, Tandon N, Takkar B. Selfie fundus imaging: innovative approach to retinopathy screening. Nat Med J Ind. 2018;31:345–6.
Wong TY, Lanzetta P, Bandello F, Eldem B, Navarro R, Lövestam-Adrian M. Current concepts and modalities for monitoring the fellow eye in neovascular age-related macular degeneration: an expert panel consensus. Retina. 2020;40:599–611.
International Council of Ophthalmology. Guidelines for diabetic eye care. International Council of Ophthalmology; 2013.
Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–36.
Wong TY, Sabanayagam C. Strategies to tackle the global burden of diabetic retinopathy: from epidemiology to artificial intelligence. Ophthalmologica. 2020;243:9–20.
Huang OS, Tay WT, Ong PG, Sabanayagam C, Cheng CY, Tan GS, et al. Prevalence and determinants of undiagnosed diabetic retinopathy and vision-threatening retinopathy in a multiethnic Asian cohort: the Singapore Epidemiology of Eye Diseases (SEED) study. Br J Ophthalmol. 2015;99:1614–21.
Verma L, Elankumaran P, Prakash G, Venkatesh P, Tewari Hem K. Awareness of diabetic retinopathy among diabetics. Ind J Ophthalmol. 2002;50:355–355.
Goh JK, Cheung CY, Sim SS, Tan PC, Tan GS, Wong TY. Retinal imaging techniques for diabetic retinopathy screening. J Diabetes Sci Technol. 2016;10:282–94.
Avidor D, Lowenstein A, Waisbourd M, Nutaman A. Cost-effectiveness of diabetic retinopathy screening programs using telemedicine: a systematic review. Cost Eff Resour Alloc. 2020;18:16.
Lavanya R, Baskaran M, Kumar RS, Wong H-T, Chew PTK, Foster PJ, et al. Risk of acute angle closure and changes in intraocular pressure after pupillary dilation in Asian subjects with narrow angles. Ophthalmology. 2012;119(Mar):474–80.
Funding
The study was conducted independently by the authors without any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kumari, S., Venkatesh, P., Tandon, N. et al. Selfie fundus imaging for diabetic retinopathy screening. Eye 36, 1988–1993 (2022). https://doi.org/10.1038/s41433-021-01804-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01804-7
- Springer Nature Limited
This article is cited by
-
Clinical utility of handheld fundus and smartphone-based camera for monitoring diabetic retinal diseases: a review study
International Ophthalmology (2024)
-
The role of endothelial growth factor and tear levels in diabetic retinopathy in type 2 diabetes
International Ophthalmology (2024)