Abstract
Aims
This study aims to compare the performance of a handheld fundus camera (Eyer) and standard tabletop fundus cameras (Visucam 500, Visucam 540, and Canon CR-2) for diabetic retinopathy and diabetic macular edema screening.
Methods
This was a multicenter, cross-sectional study that included images from 327 individuals with diabetes. The participants underwent pharmacological mydriasis and fundus photography in two fields (macula and optic disk centered) with both strategies. All images were acquired by trained healthcare professionals, de-identified, and graded independently by two masked ophthalmologists, with a third senior ophthalmologist adjudicating in discordant cases. The International Classification of Diabetic Retinopathy was used for grading, and demographic data, diabetic retinopathy classification, artifacts, and image quality were compared between devices. The tabletop senior ophthalmologist adjudication label was used as the ground truth for comparative analysis. A univariate and stepwise multivariate logistic regression was performed to determine the relationship of each independent factor in referable diabetic retinopathy.
Results
The mean age of participants was 57.03 years (SD 16.82, 9–90 years), and the mean duration of diabetes was 16.35 years (SD 9.69, 1–60 years). Age (P = .005), diabetes duration (P = .004), body mass index (P = .005), and hypertension (P < .001) were statistically different between referable and non-referable patients. Multivariate logistic regression analysis revealed a positive association between male sex (OR 1.687) and hypertension (OR 3.603) with referable diabetic retinopathy. The agreement between devices for diabetic retinopathy classification was 73.18%, with a weighted kappa of 0.808 (almost perfect). The agreement for macular edema was 88.48%, with a kappa of 0.809 (almost perfect). For referable diabetic retinopathy, the agreement was 85.88%, with a kappa of 0.716 (substantial), sensitivity of 0.906, and specificity of 0.808. As for image quality, 84.02% of tabletop fundus camera images were gradable and 85.31% of the Eyer images were gradable.
Conclusions
Our study shows that the handheld retinal camera Eyer performed comparably to standard tabletop fundus cameras for diabetic retinopathy and macular edema screening. The high agreement with tabletop devices, portability, and low costs makes the handheld retinal camera a promising tool for increasing coverage of diabetic retinopathy screening programs, particularly in low-income countries. Early diagnosis and treatment have the potential to prevent avoidable blindness, and the present validation study brings evidence that supports its contribution to diabetic retinopathy early diagnosis and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetes mellitus (DM) is considered a global epidemic [1], with estimated 537 million adult diabetic worldwide and projected 784 million by 2045, according to the International Diabetes Federation [1]. The prevalence and underdiagnosis of DM are particularly higher in low- and middle-income countries (LMICs) due to the economic transition of most nations, the westernization of lifestyle, and improved longevity [1, 2]. Diabetic retinopathy (DR) affects approximately one-third of diabetics and can lead to sight-threatening complications in 10% of them [3].
While individuals with DR experience no symptoms until the onset of diabetic macular edema (DME) or proliferative diabetic retinopathy (PDR), early screening and treatment can effectively prevent irreversible visual impairment [2, 4,5,6]. Screening programs and evidence-based strategies have the potential to prevent 95% of DR-related blindness cases [2, 7, 8], making DR the leading cause of preventable blindness in the working-age population in the industrialized world [4, 7, 9]. However, DR-related blindness has increased by approximately 68% in the last 30 years, mainly in LMICs, due to the increasing prevalence of DM [7].
The International Council of Ophthalmology recommends an annual examination for individuals with type 1 diabetes five years after the onset of the disease and for those with type 2 diabetes from the time of diagnosis [8, 10]. The screening process can be performed through a trained ophthalmologist's clinical examinations or remote retinal fundus photograph image evaluation via teleophthalmology [7]. However, the burden on health systems will continue to increase with the population aging, particularly in LMICs, where health systems are limited in terms of trained specialists or financial resources [10, 11].
The use of mobile fundus cameras is a new, exciting alternative to conventional screening methods due to their favorable cost-effectiveness profile [2, 7, 12, 13]. Handheld imaging devices are typically integrated into a smartphone or mobile computing platform with mobile internet connectivity [2, 7], and they have the potential to allow diagnosis and guide treatment. However, their validation is necessary before they can be widely adopted [7].
This study aims to evaluate the quality and reliability of a handheld retinal imaging system and compare it with tabletop retinal fundus photograph cameras for DR and DME screening.
Materials and methods
Ethics and patients
This was a multicenter, prospective, cross-sectional study for a handheld retinal imaging system validation in detecting and classifying DR and DME. One center is a tertiary referral ophthalmological hospital (São Paulo, Brazil), and the other two are diabetic centers that regularly screen their patients for DR (São Paulo and Sergipe, Brazil). The study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Research Ethics Committee of the Federal University of São Paulo (33,842,220.7.0000.5505). All participants gave written informed consent prior to collection of any data.
Inclusion criteria were patients with type 1 or 2 DM who agreed with the study terms. Exclusion criteria were any contraindication for mydriasis and ocular surgery in the past six months.
Retinal cameras
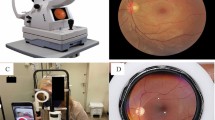
The included cameras consisted of Eyer, Visucam 500, Visucam 524, and Canon CR-2 Digital Retinal Camera.
Tabletop camera
The included tabletop cameras were the Visucam 500 and 524 (Carl Zeiss Meditec, Inc, Jena, Germany) and Canon CR-2 Digital Retinal Camera (Canon Medical Systems Corporation, Otawara, Japan). The Visucam captures retinal fundus photographs with 30° and 45° field angles. It has compensation for ametropia of -35 to + 35 diopters. The Canon CR-2 captures retina fundus photographs with a 45° field angle and has a 24-megapixel resolution in an external Canon camera. The approximate price of a tabletop camera in Brazil was around 25,000 USD in March 2023.
Smartphone-based retinal camera
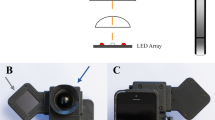
The Eyer (Phelcom Technologies, LLC, Massachusetts, USA) is a smartphone-based camera built using a Samsung Galaxy S10 (Android 11) smartphone. The camera captures retinal fundus photographs with a 45° field angle and a 12-megapixel sensor, producing an image resolution of 1600 × 1600 pixels. It has an autofocus range from −20 to + 20 diopters. The approximate price of an Eyer in Brazil was around 4,5000 USD in march 2023.
Capture and mydriasis protocol
The participants followed a mydriasis protocol, consisting of two drops of 0.5% tropicamide administered every 5 min, followed by a fundus photograph in two fields centered on the macula and optic disk (Fig. 1). The imaging was acquired by trained healthcare professionals using a standardized protocol [14]. All images were de-identified and reviewed for personal health information.
All participants self-reported gender, age, race, weight, height, duration of diabetes, insulin use, and other associated diseases.
Grading protocol
Labeling was performed independently by two masked, certified ophthalmologists, one of them being a retina specialist, with a third senior retinal specialist adjudicating in discordant cases. The diabetic retinal lesions, including hemorrhages, microaneurysms, venous beading, intraretinal microvascular abnormalities, new vessels, vitreous or preretinal hemorrhage, and the presence of retinal tractional membranes, were evaluated, according to the International Classification of Diabetic Retinopathy (ICDR). DR severity was classified as no DR, mild nonproliferative DR, moderate nonproliferative DR, severe nonproliferative DR, proliferative DR, or ungradable. The presence of DME was identified as retinal thickening of at least one disk area from the foveal center as present or absent, as per ICDR criteria [15]. In the presence of pan-retinal photocoagulation scars, images were graded as proliferative DR, even if new vessels were not visible [14].
Referable DR was defined as moderate nonproliferative DR or worse, any DME, or ungradable images. Vision-threatening diabetic retinopathy was defined as severe nonproliferative DR or worse, DME or ungradable images [7]. Images were considered gradable when at least eighty percent of the image was visible, and the assessment of at least the third retinal vascular branch was possible [16].
Statistical analysis
In the statistical analysis, we compared demographic data, DR classification, artifacts; image quality was compared between graders and devices. The tabletop fundus camera senior ophthalmologist adjudication label was considered the ground truth for comparative analysis. Descriptive statistics were used to compare demographic groups with referable diabetic retinopathy as the outcome. Continuous variables were presented as mean and standard deviations and compared using the Mann–Whitney test, while categorical variables were presented as counts and percentages and compared using the Chi-square test. An alpha of 0.05 was used to define statistical significance.
To assess the predictive ability of demographic and clinical characteristics for referable diabetic retinopathy, we conducted univariate and multivariate logistic regression analyses. The stepwise method was used for multicollinearity analysis to determine the relationship of each independent factor.
The interrater reliability was evaluated using the Kappa statistic test. It can range from 0 to + 1, where values ≤ 0 indicate no agreement or agreement that can be expected from random chance, 0.01–0.20 as none to slight, 0.21–0.40 as fair, 0.41– 0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1.00 as almost perfect agreement [17]. The weighted Kappa was calculated based on ICDR classification subgroups. All statistical tests and descriptions were performed using IBM SPSS Statistics for Windows (IBM Corp., version 25.0. Armonk, NY) and Python 3.9.13 (Python Software Foundation, Delaware, USA).
Results
This study included retinal fundus photographs from 327 patients with a mean age of 57.03 years (SD 16.82, 9–90 years) and 45.26% male patients. The baseline participant's demographics and comorbidities are detailed in Table 1. In the patient-level classification, 44% had no retinopathy, 26.47% non-proliferative diabetic retinopathy, and 29.31% proliferative diabetic retinopathy. Age (P = 0.005), diabetes duration (P = 0.004), body mass index (P = 0.005), hypertension (P < 0.001), and type 1 DM (P < 0.001) were statistically different between referable and non-referable patients.
Regarding image quality, 84.02% of the tabletop fundus camera images and 85.31% of the Eyer images were gradable. Diabetic retinopathy and maculopathy classification for each retinal camera image is provided in Table 2. Multivariate logistic regression analysis revealed a positive association between male sex (OR 1.687) and hypertension (OR 3.603) with referable diabetic retinopathy (Table 3).
Intermodality
In the comparison between images obtained with tabletop fundus cameras and the handheld device, the DR classification showed a high level of agreement of 73.18%, with a weighted kappa of 0.808 (almost perfect). The agreement for edema was 88.48% with a kappa of 0.809 (almost perfect), while the agreement for image quality was 87.15% with a kappa of 0.495 (moderate).
For referable DR, the agreement was 85.88% with a kappa of 0.71 (substantial), and for vision-threatening DR, the exact agreement was 84.95% and kappa was 0.699 (substantial).
Regarding the smartphone-based camera, the sensitivity for referable DR was 0.906, and specificity was 0.808, while for vision-threatening DR, the sensitivity was 0.87 and specificity was 0.82 (Table 4).
When comparing different modalities, the agreement rate was highest in the “no retinopathy” group and "proliferative" and lowest within non-proliferative classifications.
Discussion
The aim of this study was to evaluate the effectiveness of a portable handheld camera for the classification of diabetic retinopathy (DR) and diabetic macular edema (DME). The results showed that the portable handheld cameras had a gradability rate of 85.31% and almost perfect agreement with the traditional tabletop imaging protocol for the classification of DR and DME [14], reaching a sensitivity for referable diabetic retinopathy higher than the end-point established by the National Health Service Diabetic Eye Screening Program threshold [18]. Our referable study population was significantly older, had significantly longer diabetes duration, and significantly higher body mass index. Hypertension diagnosis and male sex also had a positive association with referable diabetic retinopathy.
Smartphone-based retinal imaging and teleophthalmology [19, 20] are innovative strategies that could increase coverage rates of DR screening programs which, associated with timely treatment, have been proven important measures to prevent visual loss [7, 19]. Limited access to ophthalmologists in many parts of the world is a significant hurdle to preventing avoidable blindness secondary to diabetes [20]. Traditionally, imaging has been performed with standard tabletop fundus cameras using the seven fields described in the Early Treatment Diabetic Retinopathy Study and with images evaluated by an ophthalmologist or other specifically trained examiner [21, 22]. However, this approach hinders patient access and requires significant physical space, a complex acquisition process, and high capital investment, limiting its use [23, 24].
The adoption of smaller, portable devices, such as the one evaluated in the present study, allows a more affordable, technically simpler, and easier screening process, especially in low-resource settings and hard-to-reach populations [7, 23, 25]. Previous reports have already demonstrated that portable devices such as Aurora, Smartscope, RetinaVue700, and iNview handheld retina cameras obtained adequate rates of sensitivity (83–100%) and specificity (54–99%) for the detection of referable diabetic retinopathy in comparison to tabletop cameras [7, 20, 24, 26]; diagnostic sensitivity increases progressively along with the increase in DR severity [20]. Some handheld devices yield specificity rates lower than the sensitivity [7], and further studies are needed to evaluate optimal screening thresholds for different settings. The trade-off between sensitivity and specificity should be tailored according to several local factors such as disease prevalence, availability of workforce, and economic constraints [27].
A major concern regarding handheld retinal imaging systems is adequate image quality. Automatic focus on a handheld fundus camera has the potential to reduce the rate of ungradable images [28]. In our study, we observed that 85.31% of the images taken with Eyer were gradable, similar to the rate found with standard tabletop fundus cameras. Ocular media opacities are usually reported as major causes of ungradability in this subset of patients, mainly cataracts [25].
Our analysis revealed that the agreement between the devices was higher among groups with no retinopathy and proliferative DR. Disagreements in microaneurysm, small hemorrhages, and intraretinal microvascular abnormalities contributed to the higher discordance within non-proliferative diabetic retinopathy. More detailed studies are required to evaluate the granularity within each classification.
There are several strengths to our study. Firstly, we used a standardized protocol for the capture and grading, making the study reproducible. Secondly, our study was the first to validate a handheld retinal camera for DR screening with a Brazilian population, which has a distinctive diverse demographic profile. Thirdly, our study sample was larger than in other validation studies [7, 20, 24, 25]. Lastly, the high agreement with tabletop fundus cameras allows the portable device to be used in real life for diabetic retinopathy screening, with the potential to expand coverage in poor and remote areas through telemedicine.
However, our study also has limitations. Firstly, the demographic and health data were self-reported, which may lead to inaccuracies. Secondly, we did not have access to optical coherence tomography, which is the gold-standard method for diabetic macular edema evaluation. Additionally, the use of diabetic retinopathy classification systems and referring criteria may not reflect the granularity and differences between devices, and further studies that shall objectively evaluate image quality parameters and lesions are needed. Lastly, although the cameras are described as non-mydriatic, our study included only images collected after pupil dilation, due to our services´ routine, which consists of evaluating diabetic patients after pharmacological mydriasis; it is well recognized that mydriasis improves image quality and increases agreement with the identification of disease [7].
Conclusion
In conclusion, our study shows that the low-cost, handheld imaging device evaluated in the present study presented gradability and imaging comparable to standard tabletop retinal cameras. These findings support the use of this device in DR screening programs, particularly in LMIC and remote areas. Promoting timely diagnosis and treatment has the potential to prevent irreversible visual loss.
Data availability statement
The data supporting this study's findings are available from the corresponding author.
References
Magliano D, Boyko EJ (2021) IDF Diabetes Atlas. International Diabetes Federation. Available: https://play.google.com/store/books/details?id=OG6IzwEACAAJ
Ramasamy K, Mishra C, Kannan NB, Namperumalsamy P, Sen S (2021) Telemedicine in diabetic retinopathy screening in India. Indian J Ophthalmol 69:2977–2986. https://doi.org/10.4103/ijo.IJO_1442_21
Sivaprasad S, Pearce E (2019) The unmet need for better risk stratification of non-proliferative diabetic retinopathy. Diabet Med 36:424–433. https://doi.org/10.1111/dme.13868
Teo ZL, Tham Y-C, Yu M, Chee ML, Rim TH, Cheung N et al (2021) Global prevalence of diabetic retinopathy and projection of burden through 2045: systematic review and meta-analysis. Ophthalmology 128:1580–1591. https://doi.org/10.1016/j.ophtha.2021.04.027
Egunsola O, Dowsett LE, Diaz R, Brent MH, Rac V, Clement FM (2021) Diabetic retinopathy screening: a systematic review of qualitative literature. Can J Diabetes 45:725-733.e12. https://doi.org/10.1016/j.jcjd.2021.01.014
Lin K-Y, Hsih W-H, Lin Y-B, Wen C-Y, Chang T-J (2021) Update in the epidemiology, risk factors, screening, and treatment of diabetic retinopathy. J Diabetes Investig 12:1322–1325. https://doi.org/10.1111/jdi.13480
Salongcay RP, Aquino LAC, Salva CMG, Saunar AV, Alog GP, Sun JK, et al. (2022) Comparison of handheld retinal imaging with ETDRS 7-standard field photography for diabetic retinopathy and diabetic macular edema. Ophthalmol Retina, pp 548–556.https://doi.org/10.1016/j.oret.2022.03.002
Wong TY, Sun J, Kawasaki R, Ruamviboonsuk P, Gupta N, Lansingh VC et al (2018) Guidelines on diabetic eye care: the international council of ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology 125:1608–1622. https://doi.org/10.1016/j.ophtha.2018.04.007
Krause J, Gulshan V, Rahimy E, Karth P, Widner K, Corrado GS et al (2018) Grader variability and the importance of reference standards for evaluating machine learning models for diabetic retinopathy. Ophthalmology 125:1264–1272. https://doi.org/10.1016/j.ophtha.2018.01.034
Benet D, Pellicer-Valero OJ (2022) Artificial intelligence: the unstoppable revolution in ophthalmology. Surv Ophthalmol 67:252–270. https://doi.org/10.1016/j.survophthal.2021.03.003
Grauslund J (2022) Diabetic retinopathy screening in the emerging era of artificial intelligence. Diabetologia 65:1415–1423. https://doi.org/10.1007/s00125-022-05727-0
Abràmoff MD, Lou Y, Erginay A, Clarida W, Amelon R, Folk JC et al (2016) Improved automated detection of diabetic retinopathy on a publicly available dataset through integration of deep learning. Invest Ophthalmol Vis Sci 57:5200–5206. https://doi.org/10.1167/iovs.16-19964
Jain A, Krishnan R, Rogye A, Natarajan S (2021) Use of offline artificial intelligence in a smartphone-based fundus camera for community screening of diabetic retinopathy. Indian J Ophthalmol 69:3150–3154. https://doi.org/10.4103/ijo.IJO_3808_20
Malerbi FK, Morales PH, Farah ME, Drummond KRG, Mattos TCL, Pinheiro AA et al (2015) Comparison between binocular indirect ophthalmoscopy and digital retinography for diabetic retinopathy screening: the multicenter Brazilian type 1 diabetes study. Diabetol Metab Syndr 7:116. https://doi.org/10.1186/s13098-015-0110-8
Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M et al (2003) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110:1677–1682. https://doi.org/10.1016/S0161-6420(03)00475-5
Shi C, Lee J, Wang G, Dou X, Yuan F, Zee B (2022) Assessment of image quality on color fundus retinal images using the automatic retinal image analysis. Sci Rep 12:10455. https://doi.org/10.1038/s41598-022-13919-2
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med 22:276–282. https://doi.org/10.1016/j.jocd.2012.03.005
Scanlon PH (2019) Update on screening for sight-threatening diabetic retinopathy. Ophthalmic Res 62:218–224. https://doi.org/10.1159/000499539
Lim G, Bellemo V, Xie Y, Lee XQ, Yip MYT, Ting DSW (2020) Different fundus imaging modalities and technical factors in AI screening for diabetic retinopathy: a review. Eye Vis (Lond) 7:21. https://doi.org/10.1186/s40662-020-00182-7
Sengupta S, Sindal MD, Besirli CG, Upadhyaya S, Venkatesh R, Niziol LM et al (2018) Screening for vision-threatening diabetic retinopathy in South India: comparing portable non-mydriatic and standard fundus cameras and clinical exam. Eye 32:375–383. https://doi.org/10.1038/eye.2017.199
Vujosevic S, Benetti E, Massignan F, Pilotto E, Varano M, Cavarzeran F, et al. (2009) Screening for diabetic retinopathy: 1 and 3 nonmydriatic 45-degree digital fundus photographs vs 7 standard early treatment diabetic retinopathy study fields. Am J Ophthalmol, pp 111–118. https://doi.org/10.1016/j.ajo.2009.02.031
Vujosevic S, Aldington SJ, Silva P, Hernández C, Scanlon P, Peto T et al (2020) Screening for diabetic retinopathy: new perspectives and challenges. Lancet Diabetes Endocrinol 8:337–347. https://doi.org/10.1016/S2213-8587(19)30411-5
Piyasena MMPN, Piyasena MMP, Yip JLY, MacLeod D, Kim M, Murthy Gudlavalleti VS (2019) Diagnostic test accuracy of diabetic retinopathy screening by physician graders using a hand-held non-mydriatic retinal camera at a tertiary level medical clinic. BMC Ophthalmol. https://doi.org/10.1186/s12886-019-1092-3
Midena E, Zennaro L, Lapo C, Torresin T, Midena G, Pilotto E, et al. (2022) Handheld fundus camera for diabetic retinopathy screening: a comparison study with table-top fundus camera in real-life setting. J Clin Med Res, 11. https://doi.org/10.3390/jcm11092352
Davila JR, Sengupta SS, Niziol LM, Sindal MD, Besirli CG, Upadhyaya S et al (2017) Predictors of photographic quality with a handheld nonmydriatic fundus camera used for screening of vision-threatening diabetic retinopathy. Ophthalmologica 238:89–99. https://doi.org/10.1159/000475773
Palermo BJ, D’Amico SL, Kim BY, Brady CJ (2022) Sensitivity and specificity of handheld fundus cameras for eye disease: a systematic review and pooled analysis. Surv Ophthalmol 67:1531–1539. https://doi.org/10.1016/j.survophthal.2021.11.006
Malerbi FK, Melo GB (2022) Feasibility of screening for diabetic retinopathy using artificial intelligence, Brazil. Bull World Health Org, pp 643–647. https://doi.org/10.2471/blt.22.288580
Sengupta S, Sindal MD, Baskaran P, Pan U, Venkatesh R (2019) Sensitivity and specificity of smartphone-based retinal imaging for diabetic retinopathy: a comparative study. Ophthalmol Retina 3:146–153. https://doi.org/10.1016/j.oret.2018.09.016
Acknowledgements
LFN is a researcher supported by Lemann Foundation, Instituto da Visão-IPEPO.
Funding
'Open Access funding provided by the MIT Libraries'. Authors declare that they have no financial or non-financial interests that are directly or indirectly related to this work.
Author information
Authors and Affiliations
Contributions
Juliana Angélica Estevão de Oliveira took part in data collection, investigation, methodology, writing—original draft, writing—review and editing. Luis Filipe Nakayama involved in conceptualization, data curation, investigation, methodology, writing—original draft, writing—review and editing. Lucas Zago Ribeiro involved in writing—original draft, review and editing. Talita Virgínia Fernandes de Oliveira took part in data collection, writing—original draft, writing—review and editing. Stefano Neto Jai Hyun Choi involved in data collection. Edgar Menezes Neto took part in data collection. Viviane Santos Cardoso involved in data collection. Sergio Atala Dib took part in supervision, writing—review and editing. Gustavo Barreto de Melo involved in writing—original draft, review and editing. Caio Vinicius Saito Regatieri involved in conceptualization, supervision, data curation, writing—review and editing. Fernando Korn Malerbi involved in writing—original draft, review and editing.
Corresponding author
Ethics declarations
Conflict of interest
FKM is a medical consultant for Phelcom Technologies.
Additional information
This article belongs to the Topical Collection "Diabetic Eye Disease", managed by Giuseppe Querques.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Oliveira, J.A.E., Nakayama, L.F., Zago Ribeiro, L. et al. Clinical validation of a smartphone-based retinal camera for diabetic retinopathy screening. Acta Diabetol 60, 1075–1081 (2023). https://doi.org/10.1007/s00592-023-02105-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-023-02105-z