Abstract
Objectives
Compare the outcomes of two surgical techniques, lamellar hole–associated epiretinal proliferation (LHEP) embedding and LHEP sparing, in treating idiopathic lamellar macular holes (LMHs).
Methods
Retrospective consecutive case series with 34 LMHs with LHEP that underwent operation. LHEP-sparing technique was used before July 2015 and LHEP-embedding after July 2015. Morphological features in optical coherence tomography (OCT) images were identified, including the presence of LHEP, ellipsoid zone (EZ) defects, and types of LMH closure, along with best-corrected visual acuity (BCVA) before and after surgery.
Results
No baseline differences were observed between the embedding (17 patients) and sparing (17 patients) groups in LMH size, retinal defect depth, or preoperative BCVA. The two groups’ mean postoperative BCVAs were similar (embedding vs sparing: 0.388 ± 0.337 vs 0.465 ± 0.418 [Snellen: 20/49 and 20/58], P = 0.812). Postoperatively, a U-type closure was observed in 77 and 65% of patients in the embedding and sparing groups, respectively. Both groups exhibited V-type and T-type closures in half of the remaining patients (P = 0.753). Older age, postoperative external limiting membrane defect, postoperative EZ disruption, and non–U-type closure were associated with worse final BCVA.
Conclusions
Both the LHEP-embedding and LHEP-sparing techniques significantly improved vision in patients with LMHs and produced similar visual and anatomical outcomes. Most patients achieved a normal U-type closure with either technique. Preservation of LHEP during surgery is vital and could facilitates successful surgery.
Similar content being viewed by others
Introduction
Lamellar hole–associated epiretinal proliferation (LHEP), first described by Witkin et al. [1] as thickened epiretinal membrane (ERM) and later as LHEP by Pang et al. [2] in 2014, is epiretinal tissue with homogenous moderate reflectivity that is visible through optical coherence tomography (OCT) in patients with lamellar macular holes (LMHs) or full-thickness macular holes (FTMHs) [2, 3]. LHEP was found to originate from the retinal glial cells in the middle retinal layers of retinal defects in a clinicopathological study [4]. Other studies further supported this finding by demonstrating a deeper and larger retinal defect in LMHs with coexisting LHEP than in those without LHEP [3, 5,6,7]. The presence of LHEP was later used as one criterion for the classification of LMHs into two types in a new classification system proposed by Govetto et al. [8]. The morphological difference observed with OCT between LMHs with and without LHEP, along with the functional difference in visual acuity observed, indicated that LMHs with LHEP might result from different aetiologies as opposed to LMHs without LHEP or be associated with a different LMH stage and therefore potentially require different treatments [8,9,10].
Varied surgical outcomes have been reported for LMHs with LHEP. Some studies have described limited visual improvement in patients with coexisting LMHs and LHEP [6, 7, 10, 11]. Another study we conducted demonstrated significantly improved vision in patients who had LMHs with LHEP, with visual gain similar to that among patients who had LMHs without LHEP [3]. The preservation of LHEP during surgery was proposed by Shiraga et al. [12] and emphasized in our previous report [3] to potentially be the most critical step in the improvement of surgical outcomes. Further studies adopting this concept of LHEP preservation have all demonstrated significant visual improvements [13,14,15,16], despite different surgical approaches being used. Currently, two surgical approaches are used to preserve LHEP during operation: the LHEP-sparing technique, in which the LHEP remains untouched at the LMH edge [3, 13], and the LHEP-embedding technique, in which the LHEP is inverted into the LMH and fills the defect [12, 14,15,16]. However, the visual outcomes of these two techniques have not been evaluated or compared in a single study. Furthermore, the morphological changes identified with OCT that result from these two approaches are not fully understood.
The purpose of this study was to investigate the visual and anatomical outcomes for LMHs with LHEP resulting from operation with either the LHEP-sparing or LHEP-embedding technique and to optimize the treatment for LMHs with LHEP.
Subjects and Methods
In this consecutive case series, we perform a retrospective review of all patients who received surgical intervention by a single surgeon for idiopathic LMHs containing LHEP at National Taiwan University Hospital (NTUH) between August 2012 and February 2019. The patients were further divided into two groups according to the surgical procedure used during the two periods. All patients underwent vitrectomy; before July 2015, LHEP was managed with the LHEP-sparing technique, and after July 2015, LHEP was managed with the LHEP-embedding technique. LMH diagnoses were based on the criteria proposed by the International Vitreomacular Traction Study group [17], and the presence of LHEP was determined through careful evaluation of preoperative OCT images with reference to the OCT characteristics defined by Pang et al. [2]. A minimum of 3 months of follow-up after operation was required for enrollment in our analysis. Patients with high myopia, proliferative diabetic retinopathy, a history of trauma, or retinal dystrophy were excluded. The study adhered to the tenets of the Declaration of Helsinki, and the Institutional Review Board of the NTUH approved the study.
Data collection
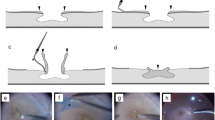
We collected baseline clinical data, including patient age, sex, lens status, and best-corrected visual acuity (BCVA) before operation. The duration of postoperative follow-up and BCVA at the last follow-up visit were also recorded. The high-resolution OCT images (RTVue®, Optovue, Inc., Fremont, CA, USA) captured before and after surgery were independently evaluated by two investigators (T.T.L. and Y.L.) for the presence of LHEP and the integrity of the ellipsoid zone (EZ) and external limiting membrane (ELM). The maximum width of the LMH and the minimum retinal thickness of the base of the LMH were measured with the built-in caliper of the OCT machine in accordance with the protocol described in another study we conducted [3]. The minimum retinal thickness at the base of the LMH after operation was also measured. Using OCT, the morphology of the closed LMH after surgery was also recorded according to our classification, modified from the system [18] used in FTMHs (Fig. 1): U-type closure exhibited a normal foveal contour with a smooth circular inner retinal surface; V-type closure exhibited central foveal thinning with a steep foveal contour; T-type closure exhibited a flat retinal surface without foveal depression. Whether LHEP filled the LMH defect was also documented (Fig. 2).
a Preoperative OCT of a 68-year-old man exhibiting LMH and coexisting lamellar hole–associated epiretinal proliferation (LHEP, asterisk). b After operation, the retinal defect was closed and filled with LHEP, forming a U-type closure that resembles a normal foveal depression (arrow). Visual acuity improved from 20/50 to 20/25. c A 49-year-old woman with LHEP at the edge of LMH before operation (asterisk). d Postoperative OCT revealed a V-type closure with a steep foveal contour (arrow). Visual acuity remained unchanged at 20/50 after surgery. e OCT image of an 80-year-old woman exhibiting LMH with LHEP (asterisk) preoperatively. f T-type closure was observed after surgery, with a flat macular surface without foveal depression (arrow).
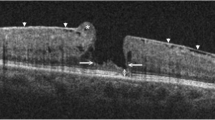
a Prominent LHEP (asterisk) was observed at the hole edge before operation. Deep retinal defects with coexisting ellipsoid zone (EZ) defects (arrow) were also found in the baseline optical coherence tomography image. b After LHEP embedding during surgery, confluent LHEP filled the retinal defect (asterisk), with the inner surface resembling a normal foveal depression. The EZ integrity improved, with only a small area of the EZ defect remaining (arrow).
Surgical techniques
Surgical intervention for LMHs was indicated for all patients with BCVA worse than 20/40 and OCT-evident LMHs. All surgeries were performed using standard 23- or 25-gauge vitrectomy (CONSTELLATION® Vision System, Alcon, Fort Worth, TX, USA). Complete removal of the posterior hyaloid (PH) and ERM was performed in all cases with or without the use of triamcinolone acetonide after posterior vitreous detachment was confirmed or induced. The internal limiting membrane (ILM) around the hole was also removed with the assistance of diluted indocyanine green dye staining (25 mg in 15 mL of 5% glucose water). LHEP, identified as epiretinal tissue with a yellowish pigment and connection to the LMH defect, was carefully preserved during ERM–PH removal and ILM peeling without forceful removal. For patients who underwent the LHEP-embedding technique, LHEP was trimmed to a size similar to or slightly larger than that of LMH. The LHEP was then flipped over and embedded into the LMH defect with microforceps. In patients who underwent the LHEP-sparing technique, LHEP was also trimmed with microscissors, and the remaining LHEP was left untouched at the hole edge without further manipulation. The surgery was concluded with air–fluid exchange. All patients were advised to maintain the prone position for 3 to 5 days after surgery.
Statistical analysis
Data before and after operation were compared between the LHEP-sparing and LHEP-embedding groups. For continuous variables, the Mann–Whitney test was performed for intergroup comparison, and the Wilcoxon signed-rank test was applied for comparison of the changes in preoperative and postoperative BCVA. For categorical variables, the chi-square test or Fisher’s exact test were used for intergroup comparison and for OCT biomarker changes in the same patient before and after surgery. We used stepwise multivariate linear regression to identify potential prognostic factors for final visual acuity. Preoperative factors such as patient age, the baseline LMH width, the remaining LMH thickness, the integrity of the EZ and ELM, baseline BCVA, and surgical techniques, and postoperative factors such as the retinal thickness after surgery, postoperative integrity of the EZ and ELM, and LMH-closure type were included as independent variables. The data were analysed using SPSS software (SPSS 22.0; SPSS Inc., Chicago, IL, USA). A P value of less than 0.05 was considered statistically significant.
Results
Thirty-four patients who had LMHs with LHEP and underwent operation were enrolled in our study; of these, 17 each underwent the LHEP-sparing technique and LHEP-embedding technique. The baseline demographics of the two groups of patients are summarized in Table 1. No significant differences existed in the mean age at operation, sex distribution, and proportion of pseudophakic patients between the two groups. Before operation, the average BCVA values were similar in the LHEP-embedding (0.624 ± 0.230, Snellen: 20/84) and LHEP-sparing groups (0.791 ± 0.406, 20/124, P = 0.306). In baseline OCT images, the LMH widths were 822.0 ± 263.4 and 637.1 ± 325.3 μm in LHEP-embedding and LHEP-sparing groups, respectively (P = 0.062); the base thicknesses of the LMHs were 92.4 ± 29.1 and 101.4 ± 37.0 μm in LHEP-embedding and LHEP-sparing groups, respectively (P = 0.563). ELM disruption was noted before surgery in eight eyes (47%) in each group (P = 1.0), and EZ defects were observed in eight (47%) and ten (59%) eyes in the LHEP-embedding and LHEP-sparing groups (P = 0.731), respectively. The average postoperative follow-up durations were 23.2 ± 16.4 months in the LHEP-embedding group and 20.4 ± 16.9 months in the LHEP-sparing group (P = 0.231).
The visual and anatomical outcomes for the two surgical techniques are compared in Table 1. The mean final BCVA values were 0.388 ± 0.337 (20/49) in patients who underwent the LHEP-embedding technique and 0.465 ± 0.418 (20/58) in patients who received the LHEP-sparing technique (P = 0.812). Both groups exhibited significantly improved BCVA after operation (P = 0.012 in both groups, compared with baseline), and the average BCVA improvements were −0.235 ± 0.337 and −0.326 ± 0.456 (Early Treatment Diabetic Retinopathy Study [ETDRS] Letters: 11.8 and 16.3) in the LHEP-embedding and LHEP-sparing groups, respectively (P = 0.563). In our study, six patients (three in each group) received combined phacovitrectomy and 12 patients (six in each group) received cataract surgery after vitrectomy. Both patients who underwent vitrectomy alone and those who received phacoemulsification (either in combined or delayed operation) had significantly improved vision at the final visit compared with baseline (BCVA change: −0.230 ± 0.478 and −0.325 ± 0.317 [ETDRS letters: 11.5 and 16.3], respectively; P = 0.403). Most patients had stable vision after 6 months of vitrectomy, or 3 months after the final cataract surgery.
In postoperative OCT images, two patients (12%) in the LHEP- embedding group and three patients (18%) in the LHEP-sparing group exhibited persistent ELM disruption (P = 1.0), and six (35%) and eight (47%) patients in the two groups retained EZ disruption (P = 0.728). In 13 (77%) and 11 (65%) eyes in the LHEP-embedding and LHEP-sparing groups, visible LHEP-like tissue was observed through OCT to fill the LMH defect. One patient in the LHEP-sparing group exhibited visible LHEP tissue remaining at the hole edge but not filling the LMH defect (Fig. 3). After operation, U-type closure was the most common OCT morphology in the LHEP-embedding and LHEP-sparing groups (13 [77%] vs 11 [65%], respectively), followed by V-type closure (2 [12%] vs 3 [18%], respectively) and T-type closure (2 [12%] vs 3 [18%], respectively). No difference was observed through OCT between the two techniques regarding closure type (P = 0.753).
a Before operation, LHEP (asterisks) was observed at the edge of the LMH, along with a coexisting epiretinal membrane. b LHEP (asterisks) remained on the retinal surface at the edge of hole after surgery with the LHEP-sparing technique. The retinal defect remained after operation without visible LHEP filling the gap.
We performed multivariate linear regression to determine the prognostic factors for final visual outcomes. Among all preoperative and postoperative variables, four factors, including older age, presence of postoperative ELM defect, presence of postoperative EZ defect, and a non-U-type closure, were associated with worse final BCVA (Table 2), and the results held true after adjustment for baseline BCVA. When only preoperative factors were included for analysis, baseline BCVA was the only factor associated with final BCVA (coefficient: 0.427 ± 0.183, P = 0.026). The surgical technique adopted did not affect final visual acuity both before and after adjustment for baseline BCVA. Greater mean visual improvements were observed in patients with U-type closure (−0.343 ± 0.337) than in patients with non–U-type closure (−0.128 ± 0.503, P = 0.152), but the difference was statistically nonsignificant.
Discussion
This retrospective study identified similar anatomical and visual outcomes in patients undergoing one of two surgical procedures, LHEP-sparing and LHEP-embedding, for the treatment of LMHs with LHEP. We observed both techniques to be equally effective in restoring foveal morphology and achieving significantly improved visual acuity.
LHEP differs from a traditional ERM in terms of OCT morphology and cell origin [2,3,4]. During surgery, It should be managed with particular caution and in a different manner from the ERM. LHEP originates from Muller glial cells [4, 13], the major supportive cells in the retina that help to maintain foveal structures and response to retinal injuries [19, 20], and might facilitate repair by Muller glial cells in response to severe defects in macula. The observation that FTMHs with LHEP are often associated with spontaneous hole closure further supports the idea that LHEP is involved in retinal repair and could facilitate the restoration of foveal structures in LMH after surgery [3, 21, 22]. In addition, the connection of LHEP with middle retinal layers, demonstrated by OCT morphology as well as intraoperative findings, was associated with increased risk of additional retinal tissue loss when LHEP was forcefully removed during surgery. Therefore, preservation of LHEP during LMH surgery can prevent unnecessary retinal damage and help to restore foveal structures, thus further promoting favorable surgical outcomes.
In 2013, Shiraga et al. [12] first reported the preservation of epiretinal tissue with macular pigment, later termed LHEP, through inversion of LHEP to cover the LMH. They noted significant visual improvements along with restored foveal contour in most of their patients. Other studies that have involved a surgical technique for preserving LHEP, either LHEP-sparing [3, 13] or LHEP-embedding [12, 14,15,16], have reported favorable outcomes. In the current study, we further demonstrated that when LHEP was preserved, regardless of the surgical technique used, the patient had a high chance of restoring normal foveal morphology and regaining vision. As expected, LHEP filled the LMH defect in most of our patients undergoing the LHEP-embedding technique; for 65% of our patients receiving operation with the LHEP-sparing technique, LHEP was also observed to fill the retinal defect postoperatively, and in only one patient did LHEP remain at the edge of the hole rather than fill the hole. The proliferative tissue remaining at the hole margin after application of the LHEP-sparing technique was able to settle into the retinal defect, probably as a result of the complete removal of coexisting tractional forces such as the ERM or PH and ILM, followed by the scaffolding of the gas tamponade and the assistance of the face-down position, which facilitated the filling of the defect with LHEP [15]. In addition, the tendency of LHEP fragments to amalgamate may have played a role. However, we did not have immediate postoperative OCT images to support the aforementioned mechanisms. Further studies using intraoperative OCT and serial OCT exams performed during the early postoperative period might help better understand the differences in the positional changes of LHEP after surgery between these two techniques.
The surgical outcomes of patients who have LMHs with LHEP have varied among studies. Some studies have reported no visual improvement in patients who have LMHs with LHEP [6, 7, 11] or a less prominent visual gain compared with that of patients who have LMHs without LHEP [23]. In contrast to studies involving an LHEP-preserving surgical technique, studies involving a surgical procedure that includes LHEP removal [7, 23] or that make no mention of LHEP preservation in their surgical techniques [6, 11] have a higher risk of resulting in less favorable outcomes. A common complication after removal of LHEP during LMH surgery is the formation of FTMHs. Coassin et al. [11] reported three cases of FTMH formation after LMH surgery, and all had LHEP. Parolini et al. [9] also reported three cases of FTMHs after the surgical removal of LHEP for clinicopathologic examination. By contrast, none of the studies involving the use of a LHEP-preservation technique reported postoperative FTMH formation [3, 12, 13, 15, 16]. The difference in surgical techniques and postoperative complications may be the cause of inconsistency in surgical results.
Differences in classification systems used in these studies constitute another potential cause of the variance in surgical outcomes. Although some studies have compared patients with and without LHEP, other studies have reported results according to the OCT morphological classification proposed by Govetto et al. [8] and compared “degenerative” LMHs with “tractional” LMHs. Although the presence of LHEP is a criterion used to diagnose “degenerative” LMHs, not all cases of “degenerative” LMHs involve LHEP. Similarly, not all cases of LMHs with LHEP are considered “degenerative” according to OCT morphology. A recent proposed new OCT classification system also used LHEP (or epiretinal proliferation) as an optional (not mandatary) criterion for the diagnosis of LMH, in contrast to ERM foveoschsis and macular psuedohole [24], thus LMHs without LHEP could still be noted according to this new classification. Therefore, the classification of patients according to the presence or absence of LHEP has different clinical meanings from those of classification based on the system proposed by Govetto et al. [8] or from the most recent OCT classification [24]. The findings of the present study and those of other reports suggest that the management of LHEP during surgery is a key factor for the improvement of surgical results. Hence, determining whether LHEP coexists with LMHs would be more valuable for planned surgical interventions. Therefore, we classified patients according to the presence or absence of LHEP in LMHs, especially in surgically treated cases.
The final visual acuity after operation for LMHs was associated with the integrity of the outer retina and the normalization of foveal contour. A higher incidence of intact EZ and ELM after LHEP-preserving surgeries explain the superior visual improvements [15, 16]. In addition, we found that the morphological classification of foveal contour after FTMH operation reported by Imai et al. [18] could be adopted with minor modification for patients with LMHs undergoing surgery. We removed the W-type closure in our classification because this would be considered a complication of FTMH formation after LMH surgery, and we replaced it with T-type morphology according to our observations. We found, in accordance with the findings of Imai et al., that better postoperative vision was achieved for the U-type closure than for other types. However, limited by the small numbers of patients for V-type and T-type closure, we were unable to discriminate differences between their postoperative visual outcomes. Further studies are required to confirm the clinical usefulness of this classification.
The major limitations of the current study were its relatively small number of patients, variable duration of follow-up, and retrospective nature. The relatively small number of patients might results in insufficient power to detect differences in baseline factors as well as surgical outcomes between the two groups. Nevertheless, we adjusted for baseline factors during multivariate regression and minimized the chance of confounding by undetected baseline differences. In addition, the decision regarding which surgical technique to use during vitrectomy was based on the calendar year in which surgery was performed rather than on patient-related factors. Therefore the possibility of selection bias in the two groups of patients was largely avoided.
To conclude, we demonstrate that eyes that have LMHs with LHEP could achieve significant visual improvement and the restoration of normal foveal contour when LHEP-preserving techniques were adopted, and both LHEP-embedding and LHEP-sparing techniques achieved similar favorable outcomes. Intact EZ and ELM as well as normal foveal contour were associated with better visual outcomes; therefore, the reconstruction of foveal morphology should be the goal of surgery for patients who have LMHs with LHEP.
Summary
What was known before
-
Lamellar hole-associated epiretinal proliferation (LHEP) is different from traditional epiretinal membrane.
-
LHEP can be used to classify lamellar macular holes (LMHs).
-
The surgical outcomes of LMHs with LHEP varied among studies.
What this study adds
-
Both LHEP embedding and LHEP sparing techniques resulted in significantly improved vision in patients with LMHs, and the two techniques had similar visual and anatomical outcomes.
-
Preservation of LHEP during surgery is vital and could facilitate successful surgery.
-
Postoperative OCT findings, including intact ellipsoid zone, external limiting membrane, and U-type hole closure, predict better final visual acuity after surgery in patients with LMH and LHEP.
References
Witkin AJ, Ko TH, Fujimoto JG, Schuman JS, Baumal CR, Rogers AH, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113:388–97.
Pang CE, Spaide RF, Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014;34:1513–23.
Lai TT, Chen SN, Yang CM. Epiretinal proliferation in lamellar macular holes and full-thickness macular holes: clinical and surgical findings. Graefe’s Arch Clin Exp Ophthalmol. 2016;254:629–38.
Pang CE, Maberley DA, Freund KB, White VA, Rasmussen S, To E, et al. Lamellar hole-associated epiretinal proliferation: a clinicopathologic correlation. Retina. 2016;36:1408–12.
Pang CE, Spaide RF, Freund KB. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina. 2015;35:720–6.
Ko J, Kim GA, Lee SC, Lee J, Koh HJ, Kim SS, et al. Surgical outcomes of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Acta Ophthalmologica. 2017;95:e221–e226.
Choi WS, Merlau DJ, Chang S. Vitrectomy for macular disorders associated with lamellar macular hole epiretinal proliferation. Retina. 2018;38:664–9.
Govetto A, Dacquay Y, Farajzadeh M, Platner E, Hirabayashi K, Hosseini H, et al. Lamellar macular hole: two distinct clinical entities? Am J Ophthalmol. 2016;164:99–109.
Parolini B, Schumann RG, Cereda MG, Haritoglou C, Pertile G. Lamellar macular hole: a clinicopathologic correlation of surgically excised epiretinal membranes. Invest Ophthalmol Vis Sci. 2011;52:9074–83.
Schumann RG, Compera D, Schaumberger MM, Wolf A, Fazekas C, Mayer WJ, et al. Epiretinal membrane characteristics correlate with photoreceptor layer defects in lamellar macular holes and macular pseudoholes. Retina. 2015;35:727–35.
Coassin M, Mastrofilippo V, Stewart JM, Fanti A, Belpoliti M, Cimino L, et al. Lamellar macular holes: surgical outcome of 106 patients with long-term follow-up. Graefe’s Arch Clin Exp Ophthalmol. 2018;256:1265–73.
Shiraga F, Takasu I, Fukuda K, Fujita T, Yamashita A, Hirooka K, et al. Modified vitreous surgery for symptomatic lamellar macular hole with epiretinal membrane containing macular pigment. Retina. 2013;33:1263–9.
Yang YS, Lee JS, Son G, Sohn J. Epiretinal proliferation associated with lamellar hole or macular hole: origin and surgical prognosis. Korean J Ophthalmol. 2019;33:142–9.
Shiode Y, Morizane Y, Takahashi K, Kimura S, Hosokawa M, Hirano M, et al. Embedding of lamellar hole-associated epiretinal proliferation combined with internal limiting membrane inversion for the treatment of lamellar macular hole: a case report. BMC Ophthalmol. 2018;18:257.
Ho TC, Ho AY, Chen MS. Reconstructing foveola by foveolar internal limiting membrane non-peeling and tissue repositioning for lamellar hole-related epiretinal proliferation. Sci Rep. 2019;9:16030.
Takahashi K, Morizane Y, Kimura S, Shiode Y, Doi S, Okanouchi T, et al. Results of lamellar macular hole-associated epiretinal proliferation embedding technique for the treatment of degenerative lamellar macular hole. Graefe’s Arch Clin Exp Ophthalmol. 2019;257:2147–54.
Duker JS, Kaiser PK, Binder S, de Smet MD, Gaudric A, Reichel E, et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–9.
Imai M, Iijima H, Gotoh T, Tsukahara S. Optical coherence tomography of successfully repaired idiopathic macular holes. Am J Ophthalmol. 1999;128:621–7.
Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci. 2014;15:431–42.
Bringmann A, Syrbe S, Gorner K, Kacza J, Francke M, Wiedemann P, et al. The primate fovea: structure, function and development. Prog Retin Eye Res. 2018;66:49–84.
Lai TT, Yang CM. Lamellar hole-associated epiretinal proliferation in lamellar macular hole and full-thickness macular hole in high myopia. Retina. 2018;38:1316–23.
Compera D, Cereda MG, Schumann RG, Bottoni F. Development and progression of a lamellar macular hole with lamellar hole-associated epiretinal proliferation. Retin Cases Brief Rep. 2019;13:371–5.
Figueroa MS, Govetto A, Steel DH, Sebag J, Virgili G, Hubschman JP. Pars plana vitrectomy for the treatment of tractional and degenerative lamellar macular holes: functional and anatomical results. Retina. 2019;39:2090–8.
Hubschman JP, Govetto A, Spaide RF, Schumann R, Steel D, Figueroa MS, et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br J Ophthalmol. 2020;104:1741–7.
Author information
Authors and Affiliations
Contributions
T.-T.L. helped designing the study, carried out data acquisition, analysis, and interpretation, drafted the manuscript and approved final draft submission. Y.-T.H. was involved in data analysis, provided statistical consultation and critical revision of the manuscript, and approved final draft submission. Y.L. helped in data acquisition and analysis and helped drafting of the manuscript and approved final draft submission. C.-M.Y. conceived and designed the study, carried out data acquisition, analysis, and interpretation, helped draft and critically revise the manuscript and approved final draft submission. All authors agreed to be accountable for all aspects of the work submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lai, TT., Hsieh, YT., Lee, Y. et al. Embedding and sparing of lamellar hole–associated epiretinal proliferation in the treatment of lamellar macular holes. Eye 36, 1308–1313 (2022). https://doi.org/10.1038/s41433-021-01631-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-021-01631-w
- Springer Nature Limited
This article is cited by
-
Outcomes of epiretinal proliferation embedding technique in the surgery for full-thickness macular hole
Scientific Reports (2024)
-
Lamellar macular hole in highly myopic eyes and insights into its development, evolution, and treatment: a mini-review
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
The clinical and pathogenic significance of atypical epiretinal tissue in macular hole
Graefe's Archive for Clinical and Experimental Ophthalmology (2022)