Abstract
Purpose
To evaluate the choriocapillaris (CC) flow deficit (FD) in eyes with stable intermediate age-related macular degeneration (AMD) eyes over 12 months of follow-up.
Methods
Thirty four patients with intermediate AMD were prospectively enrolled and evaluated by swept-source optical coherence tomography (SS-OCT) and OCT-angiography (OCTA) using the PLEX-Elite 9000. A 6 × 6 mm foveal-centered scan was used for both modalities and the study eyes were scanned twice to allow subsequent averaging. En face OCTA CC slabs (31–41 µm below the RPE-band) were exported and compensated for signal attenuation. Two compensated CC en-face images were registered and averaged prior to binarization and CC FD computation. The CC FD of the entire 6 × 6 macular region was quantified at baseline and at 12-months. The presence of high-risk features, namely intraretinal hyper-reflective foci (HRF), subretinal drusenoid deposits (SDD), and hyporeflective-core-drusen, were evaluated using SS-OCT volume scans.
Results
Among the 34 eyes, 25 eyes from 25 patients were noted on exam and OCT to remain stable as intermediate AMD at 12-months without the development of late AMD. Eleven eyes had high-risk features at baseline compared to 14 eyes at the end of the follow-up (p = 0.094). The mean ± SD FD% across the whole 6 × 6 macular region at baseline was 19.32 ± 4.64% and significantly increased to 28.62 ± 4.71% at the end of the study (p = 0.001). The CC FD progressed significantly both in non-HR and HR-eyes.
Conclusions
Choriocapillaris flow impairment significantly deteriorated over one year in relatively stable intermediate AMD. This might suggest that underlying progression of CC dysfunction occurs before structural changes appears on OCT and lead to the progression to late-stage AMD.
Similar content being viewed by others
Introduction
Age-related macular degeneration (AMD) is the leading cause of vision loss among the elderly population in developed countries [1]. It is estimated that 200 million people worldwide having some degree of macular degeneration. Furthermore, up to half of the patients who present with intermediate AMD in one eye and already have advanced AMD in the fellow eye will progress to advanced AMD (i.e., geographic atrophy (GA) and choroidal neovascularization (CNV)) within 5 years [2]. Although intravitreal injections of agents that target vascular endothelial growth factor are now available for treatment of CNV, currently, there is no effective therapy for GA. Several agents under study are hopeful of demonstrating a slowing of the progression of GA, but it may be preferable to intervene in AMD patients at an earlier stage, before irreversible atrophic changes or exudative CNV lesions appear. To enable such early intervention studies, identification of individuals with the greatest risk for progression to advanced AMD would appear to be of importance.
Over the last two decades, optical coherence tomography (OCT) has evolved into an essential tool for evaluating and managing patients with AMD. Various studies using OCT have identified several novel OCT-based features such as high central drusen volume (>0.03 mm3), intraretinal hyperreflective foci (HRF), drusen with hyporeflective core, and subretinal drusenoid deposits (SDD), which appear to be associated with a higher risk for progression to advanced AMD [3, 4].
Recently, optical coherence tomography angiography (OCTA) has evolved into a useful noninvasive imaging technology that allows the retinal and choriocapillaris circulations to be evaluated and quantified in vivo. Swept source (SS) OCTA has some particular advantages for the evaluation of the CC in diseases such as AMD as it may have less attenuation of signal due to pathologic features such as drusen [5]. Several studies have utilized OCTA to study the choriocapillaris (CC) in early and intermediate AMD [5,6,7,8,9,10,11]. For example, Borrelli et al. demonstrated CC flow attenuation immediately around drusen, and later Nassisi et al showed even more severe impairment directly below drusen using SS-OCTA. Nassisi et al. also demonstrated that the regional severity of CC flow deficit could also predict the location of incident drusen and the enlargement of the existing drusen [12,13,14]. Recently we also identified a greater flow deficit underneath and surrounding HRF drusen and drusen with hyporeflective core [15, 16]. In addition, Nassisi et al. and Alagorie el al. demonstrated even more severe impairment of CC flow in areas surrounding GA lesions [3, 17].
Histopathologic studies have also suggested that CC loss may be an important early finding in the evolution of AMD [18, 19]. Bhutto and Lutty postulated that RPE dysfunction may represent the trigger for atrophic AMD, whereas in exudative AMD, a primary insult to the choroidal vasculature might lead to the subsequent disruption of the RPE/Bruch’s membrane/choroidal vascular complex [20]. At present, it is unknown whether this CC impairment is primary or secondary to associated RPE dysfunction. Regardless, these various studies highlight the relevance of the CC in the pathophysiology of AMD, and they also show that the severity of CC loss appears to mirror the overall severity or stage of AMD. We hypothesized that CC dysfunction might progress continuously during the progression of AMD and that progressive loss of CC flow might be apparent before progression of structural changes on OCT or clinical exam.
Thus, the aim of the present study was to longitudinally evaluate CC flow deficit in eyes with otherwise clinically stable intermediate AMD over a period of 12 months.
Methods
In this prospective study, we enrolled patients at the Doheny-UCLA Eye Centers who had evidence of intermediate AMD in at least one eye, and were willing to undergo SS-OCTA imaging and be followed over time. The study was approved by the institutional review board of the University of California—Los Angeles, and the research adhered to the tenets set forth in the Declaration of Helsinki. The study was performed in accordance with the Health Insurance Portability and Accountability Act and all patients provided written informed consent.
Intermediate AMD was defined on exam in accordance with the Beckman classification and the presence of drusen was also confirmed on structural OCT [21]. To be eligible for the analysis for the present study, eyes were required to remain at the stage of intermediate AMD for a 12 month period, without the appearance of late AMD on clinical exam or structural OCT. Late AMD was defined as the presence of incomplete or complete RPE and photoreceptor atrophy (iRORA or cRORA) or the development of CNV. Only one eye of each patient included in the study even if both eyes were eligible. By convention, for patients with bilateral intermediate AMD, only the right eye was scanned and enrolled in the study. Patients were also excluded if they had other macular pathology aside from intermediate AMD on exam or OCT, with the exception of non-visually significant vitreoretinal interface disease, such as an epiretinal membrane only visible by OCT.
All patients underwent a comprehensive ophthalmic examination including measurement of best corrected visual acuity, tonometry, slit lamp biomicroscopy, dilated ophthalmoscopy, and imaging at baseline and at the 12 month follow up visit.
Image acquisition
All patients underwent SS-OCTA imaging using the PLEX Elite 9000 device (Carl Zeiss Meditec Inc, Dublin, California, USA), which uses a swept laser source with a central wavelength of 1050 nm (1000–1100 nm full bandwidth) and operates at 100,000 A-scans per second. This instrument employs a full-width at half-maximum [22] axial resolution of approximately 5 μm in tissue, and a lateral resolution at the retinal surface estimated to be approximately 14 μm. OCTA imaging of the macula was obtained after dilation using a 6 × 6-mm area centered on the fovea (500 A-scans × 500 B-scans). Imaging was repeated until two OCTA volume scans were obtained with sufficient image quality (signal strength index > 7) that fulfilled the acceptance criteria (e.g., absence of motion artifact, shadows) of the Doheny Image Reading Center [23, 24]. The same volume was also used for structural OCT assessments.
The manufacturer’s complex optical microangiopathy algorithm was utilized to generate the motion signal by analysis of variation in intensity and phase between successive B-scans at the same location [25]. The manufacturer’s fully automated retinal layer segmentation algorithm was applied to the three-dimensional structural OCT data, in order to segment the CC slab as defined previously (10 μm thick starting 31 μm posterior to the RPE-band centreline) [26]. The RPE band reference was manually adjusted in all B-scans in which the fully automated algorithm failed to select the correct segmentation. This segmentation was then applied to OCTA flow intensity and structural data to obtain vascular and structural images of the choriocapillaris, respectively. It should be noted that the anatomic position of the slab is presumed to be located below the anatomic location of the CC and is thought to primarily capture the projection artifact of the CC [26]. This slab has been used in several previous publications and has been shown to be repeatable. It may be more appropriately termed an inner choroidal slab, but we continue to refer to this as the CC slab in this report in order to maintain consistency with prior descriptions. En face slabs of the CC were generated from both the flow and structural data in the OCTA images, in order to allow for subsequent signal loss compensation, image registration and averaging using FIJI (an expanded version of ImageJ version 1.51a, available at fiji.sc, National Institutes of Health, Bethesda, Maryland, USA).
Signal compensation on OCTA en face CC images
To compensate for potential signal loss below drusen and HRF, the inverse of the en face structural OCT image derived from the slab at the same level as the CC en face flow image was multiplied with the CC en face flow image using the previously described method [27].
Image registration and averaging
Two compensated CC en face images generated from two different OCTA cube scan sets were stacked to create a two-frame video and were registered before multiple image averaging. Registration was first performed on the two-frame video based on the superficial capillary plexus en face images, as previously shown. This same transformation was then applied to the CC layer, as described in detail in a previous publication [23]. After registration, the two frames of the choriocapillaris were compounded into a single image by projecting the average intensity. This averaged compensated CC image was used for the subsequent analysis.
Computation of CC flow deficit percentage
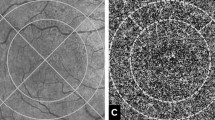
The resultant en face compensated CC image (1024 × 1024 pixels) was binarized for quantitative analysis of CC FD% using the Phansalkar method (90.82 µm radius) [3, 27, 28]. This radius was chosen to maintain consistency with prior reports. The image was than processed with the “Analyze Particles” command in order to calculate the signal deficit as a percentage of the entire 6 × 6 macular area. The CC directly beneath major superficial retinal vessels was excluded from analysis to eliminate potentially confounding shadow or projection artifacts, as previously described [24]. We designed the plugin to measure the described above. Figure 1 summarizes this processing approach for the CC.
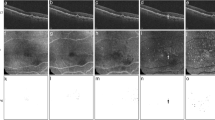
Images (A–D) shows the baseline findings and (E–H) at 12 months. The retinal thickness map superimposed on the infrared reflectance image shows little apparent change between baseline (A) and at 12 months (E). Horizontal B-scan through the fovea shows drusen characteristic of age-related macular degeneration (B, F). En face “choriocapillaris” OCTA slab after compensation for signal loss and averaging of two OCTA scan (C, G), along with the corresponding binarized compensated and averaged choriocapillaris image used for computation of the flow deficit (D, H).
Structural biomarkers
Several structural OCT biomarkers previously reported to increase the risk for progression to late AMD were assessed. Specifically, the presence of intra-retinal HRF, hypo-reflective core drusen, and SDD, both at baseline and at 12 months were noted.
The entire procedure was performed by two independent, experienced Doheny Image Reading Center graders (LT and GC) in order to investigate the repeatability of all measurements. All values were then averaged to perform the statistical analysis.
Statistical analysis
The CC FD% at baseline and 12 months were compared. Statistical analyses were performed using SPSS (version 25.0; IBM Corp., Armonk, NY). Test for normality of data were first performed using the Kolmogorov–Smirnov and Shapiro–Wilk analysis. Measurements of CC FD% were summarized using means, standard deviation (SD), median, and range. As the data were normally distributed, the comparison between baseline and end of study CC FD% was performed using a student’s t test. Intraclass correlation coefficients (ICC) between graders was calculated for the CC flow deficits and the agreement for the presence of structural OCT biomarkers was assessed by Kappa statistics. P value <0.05 was considered as statistically significant.
Results
Thirty four patients had iAMD at baseline. Nine eyes were excluded from the analysis: four because they progressed to late AMD, three had OCTA only at baseline, and two had inadequate image quality. 25 eyes of 25 patients (female/male: 13/12) completed the 12 ± 3 months follow-up and were included in the primary analysis. The mean ± SD age was 78.84 ± 8.95 years. The mean ± SD visual acuity at baseline was 0.14 ± 0.08 LogMAR and 0.16 ± 0.09 LogMAR at the 12 month follow up (p = 0.22), and the mean ± SD central subfield thickness was 269.04 ± 16.53 µm at baseline and 270.95 ± 16.27 µm at the 12 month follow-up (p = 0.218).
At baseline, 11 (44%) eyes had evidence of structural OCT high-risk features for progression. Nine (36%) eyes had one biomarker (either HRF or SDD) and two (8%) eyes had both HRF and SDD. The remaining 14 (56%) eyes showed only large drusen on OCT. None of the eyes in this cohort showed evidence of hyporeflective core drusen at baseline or at the 12 month follow-up. During the study period three (12%) eyes developed HRF, and at the end of the follow-up 14 (56%) eyes demonstrated high-risk features (p = 0.094, χ2 test, comparing to total eyes with high-risk features at baseline).
Analysis of the choriocapillaris demonstrated a mean ± SD% flow deficit of 19.32 ± 4.64% across the entire 6 × 6 macular region at baseline, and 28.62 ± 4.71% by the end of the study (p = 0.001) (Table 1, Fig. 2A). While one eye showed an apparent decrease in the CC FD% of 2.45%, the 24 (96%) remaining eyes showed an increase or worsening of the flow deficit over the 12 months, among which 13 (52%) eyes showed an increase in CC flow deficit >40% (9% absolute value) compared to baseline (Fig. 3).
(A) Distribution of choriocapillaris flow deficits at baseline versus 12-month follow-up. (B) Baseline distribution of choriocapillaris flow deficits in 11 eyes with high-risk (HR) features versus 14 eyes with no-HR features. [No high-risk features at baseline, High-risk features at baseline, No high-risk features at 12 months, High-risk features at 12 months. *p < 0.05.] High-risk features in this cohort included intraretinal hyper-reflective foci and subretinal drusenoid deposits.
 ) and at 12 months (
) and at 12 months ( ) for each individual patient in this study.
) for each individual patient in this study.Subgroup analysis of the CC FD for 11 eyes which had high-risk OCT biomarkers at baseline showed a trend for a greater CC FD at baseline compared to the 14 non-high-risk eyes (21.43 ± 5.65 vs. 17.91 ± 2.85, respectively. p = 0.061). However, at the end of the follow-up, the CC FD was similar (p = 0.191, Table 1, Fig. 2B).
Finally, the CCFD of the 4 eyes that were excluded from the primary analysis because they had progressed to late AMD showed a significantly greater CCFD at 12 months compared to the 25 iAMD study eyes (30.22 ± 4.68% vs. 28.62 ± 4.71%, respectively. p = 0.02, Wilcoxon test).
The unweighted ƙ value for intergrader repeatability was 0.875 (7/8) for SDD presence and 0.8 (4/5) for HRF at baseline, and 0.875 (7/8) for SDD and 1(8/8) for HRF at the 12 month follow-up. In the only case in which there was a discrepancy between graders, consensus could be achieved following open adjudication and discussion between the two graders, without need for reading center medical director review. Good agreement was also observed for quantitative calculation of the FD in the entire 6 × 6 macular area with ICC = 0.951 (95%CI 0.931–0.983).
Discussion
In this prospective study, we longitudinally evaluated CC flow deficits in intermediate AMD eyes which did not progress to late AMD over a 12 month follow-up period. Furthermore, the central subfield thickness and visual acuity remained similar during the entire study, and evidence of new structural HRF appeared in only three (12%) eyes during the follow-up. Despite this apparent stability, the choriocapillaris flow impairment significantly increased over the course of one year, both in eyes with and without other high-risk biomarkers at baseline. Of note, consistent with previous studies, there was a trend for a greater CC FD in eyes which had these high-risk features at baseline, though CC FD was similar in both groups by the end of the follow-up.
Nassisi et al. and Zheng et al. quantitatively studied the CC FD in healthy eyes using SS-OCTA [3, 29]. Both studies demonstrated an increase in CC FD with age. This was especially prominent in the central 1 mm macular region. Even though an age-dependent generalized reduction of the vessel density of all the retinal vascular layers has been already demonstrated, the physiologic mechanism remains unknown. It also unknown why the CC FD appears to worsen more severely centrally with age. Ramrattan et al. histologically demonstrated that CC density decreases with age together with a significant increase in Bruch’s membrane thickness [30]. Whether these changes are uniform within the macula, however, was not specified in this study.
To the best of our knowledge, this is the first study to report progression of the underlying CC flow impairment over a relatively short period of time without apparent clinical progression of the AMD, at least with respect to our commonly used AMD staging systems. Taken together with other CC studies in AMD which show greater CC FDs with high-risk OCT features and even greater impairment with late AMD, our findings would suggest that the level of CC flow impairment may serve as a more sensitive and quantitative biomarker to monitor subtle AMD progression which may be inapparent clinically. This will of course need to be validated by much longer-term longitudinal studies which are underway. Our findings add to the evolving hypothesized story of the CC in the setting of ageing and AMD. As a result of normal aging, the CC shows a progressive attenuation, which is accentuated in the central macula [3, 29]. When the impairment of the CC reaches a certain “critical point” (which may vary among individuals), drusen start to accumulate and further accentuation of the CC flow impairment continues. Several studies using different approaches have demonstrated a strong association between microvascular choroidal changes and the evolution of AMD from early to advanced stages. Histopathological studies have highlighted increasing CC alterations with age and the presence of drusen. It has been suggested that the location in which drusen appear may not be stochastic but may be influenced by the anatomy of the underlying CC. For example, Lengyel et al. demonstrated a spatial relationship between equatorial drusen and intercapillary pillars of the CC, which may represent an initial site of drusen deposition. Furthermore, an increased sub-RPE deposit density has been correlated with CC loss and the development of drusen over areas of the choroid with ghost vessels [14, 18, 19, 30]. Whether the drusen accumulate as a result of reduced clearance of debris secondary to CC insufficiency remains to be established. Regardless, the accumulation of drusen may exacerbate the disease process, by creating a further barrier for metabolic exchange between the RPE and CC. Accentuation of the RPE impairment may further worsen the CC and later yield other more severe iAMD features such as intraretinal RPE migration visible as HRF on OCT. As the CC impairment progresses, the eye may respond by the initiation of the neovascular response. Indeed, several recent studies have hypothesized that CNV lesions may evolve as a last-ditch attempt to rescue the RPE and photoreceptors and prevent atrophy [31]. This “CNV response” attempt is unsuccessful in many cases or the CNV response never materializes, and CC impairment progresses and regions of RPE and photoreceptor atrophy develop and enlarge leading to the end-stage manifestations of the disease. It is our hope that ongoing and future larger and longer-term longitudinal studies will either confirm or refute this pathophysiologic hypothesis.
If this hypothesis could be validated in future studies, it is possible that the monitoring of CC flow as an individual ages could prove to be useful tool to identify patients at risk for reaching this “critical tipping point” and potentially could be used as a biomarker to measure the effectiveness of a therapeutic intervention.
In this study we used swept-source OCTA devices featuring a longer light source wavelength, we also used averaging of multiple en face OCTA images to improve image quality and reduce noise that could be misinterpreted as flow [23]. In addition, in all our current CC studies we routinely adapted Zhang et al. suggestions for compensation of signal loss [27]. Also, in order to eliminate potentially confounding shadow or projection beneath major superficial retinal vessels we designed a plugin to exclude those region from the analysis as previously described [12, 32]. Finally, we used the Phansalkar method for thresholding and binarization of the en face CC OCTA image for quantification of the CC FD with a local thresholding radius consistent with prior publications. The optimal local thresholding radius, however, is still a topic of controversy, and could influence CC FD results [28]. In addition, as we relied on a deeper slab (in concordance with previous publications) with probable imaging of the projection artifact related to the CC, our findings may not reflect a pure assessment of the CC, and may better be termed an inner choroidal slab. Regardless, our findings would support that the selected slab is a potentially useful biomarker.
Our study is not without other limitations which should also be considered when assessing our findings, including the relatively small sample size. In addition, as we did not have a companion of longitudinal healthy age-matched control group, we cannot determine how much of the progressive CC impairment was simply normal ageing of CC. However, the slope of CC worsening observed during the short period (12 months) of our study was faster than one might predict based on the previous studies evaluating the impact of age. Indeed, age-dependent contribute to the progression but probably too much lesser extent. The fact that all eyes, both with and without structural high-risk features markedly progressed over a very short period support the hypothesis that on top of normal aging modification, a rapid acceleration of the CC dysfunction occurred in diseased eyes.
Our study also has several strengths including its prospective longitudinal design, the use of SS-OCTA, use of processing methods to optimize image quality and compensate for signal loss and projection artifact, and the use of dual certified reading center graders for all assessments, with confirmation of a high level of reproducibility.
In summary, our finding of progressive CC flow impairment in relatively clinically stable intermediate AMD eyes over short period of time highlights the potentially important role of CC dysfunction in the pathophysiology and progression of AMD. The quantification of choriocapillaris impairment may prove to be a useful biomarker in future early intervention trials for AMD.
Summary
What was known before
-
Advance stages of AMD are associated with worse choriocapillaris flow deficit around CNV and GA.
What this study adds
-
Progressive worsening of choriocapillaris flow deficits observed in eyes with otherwise clinically stable intermediate age-related macular degeneration eyes, highlighting the importance of the choriocapillaris as a biomarker for progression.
Data availability
Data of this study are available upon request.
References
Bressler NM, Bressler SB, Congdon NG, Ferris FL 3rd, Friedman DS, Klein R, et al. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch Ophthalmol. 2003;121:1621–4.
Eye TA, Study D States U. AREDS Report No. 8. 2013; 119.
Nassisi M, Baghdasaryan E, Tepelus T, Asanad S, Borrelli E, Sadda SR. Topographic distribution of choriocapillaris flow deficits in healthy eyes. PLoS One. 2018;13:1–13.
Lei J, Balasubramanian S, Abdelfattah NS, Nittala MG, Sadda SR. Proposal of a simple optical coherence tomography-based scoring system for progression of age-related macular degeneration. Graefe’s Arch Clin Exp Ophthalmol. 2017;255:1551–8.
Waheed NK, Moult EM, Fujimoto JG, Rosenfeld PJ. Optical coherence tomography angiography of dry age-related macular degeneration. Dev Ophthalmol. 2016;56:91–100.
Lane M, Moult EM, Novais EA, Louzada RN, Cole ED, Lee B, et al. Visualizing the choriocapillaris under drusen: comparing 1050-nm swept-source versus 840-nm spectral-domain optical coherence tomography angiography. Investig Ophthalmol Vis Sci. 2016;57:OCT585–90.
Roisman L, Zhang Q, Wang RK, Gregori G, Zhang A, Chen C-L, et al. Optical coherence tomography angiography of asymptomatic neovascularization in intermediate age-related macular degeneration. Ophthalmology. 2016;123:1309–19.
Moreira-Neto CA, Moult EM, Fujimoto JG, Waheed NK, Ferrara D. Choriocapillaris loss in advanced age-related macular degeneration. J Ophthalmol. 2018;2018:8125267.
Moult EM, Waheed NK, Novais EA, Choi W, Lee B, Ploner SB, et al. Swept-source optical coherence tomography angiography reveals choriocapillaris alterations in eyes with nascent geographic atrophy and drusen-associated geographic atrophy. Retina. 2016;36:S2–S11.
Arya M, Sabrosa AS, Duker JS, Waheed NK. Choriocapillaris changes in dry age-related macular degeneration and geographic atrophy: a review. Eye Vis. 2018;5:22.
Corvi F, Tiosano L, Corradetti G, Nittala MG, Lindenberg S, Alagorie AR, et al. Choriocapillaris Flow Deficit as a risk factor for progression of Age-Related Macular Degeneration. Retina. 2020. https://doi.org/10.1097/IAE.0000000000002990.
Borrelli E, Shi Y, Uji A, Balasubramanian S, Nassisi M, Sarraf D, et al. Topographic analysis of the choriocapillaris in intermediate age-related macular degeneration. Am J Ophthalmol. 2018;196:34–43.
Nassisi M, Shi Y, Fan W, Borrelli E, Uji A, Ip MS, et al. Choriocapillaris impairment around the atrophic lesions in patients with geographic atrophy: A swept-source optical coherence tomography angiography study. Br J Ophthalmol. 2019;103:911–7.
Nassisi M, Tepelus T, Nittala MG, Sadda SR. Choriocapillaris flow impairment predicts the development and enlargement of drusen. Graefes Arch Clin Exp Ophthalmol. 2019;257:2079–85.
Byon I, Ji Y, Alagorie AR, Tiosano L, Sadda SR. Topographic assessment of choriocapillaris flow deficit in the intermediate age-related macular degeneration eyes with hyporeflective core inside drusen. Retina. 2020. https://doi.org/10.1097/IAE.0000000000002906.
Tiosano L, Byon I, Alagorie AR, Ji Y-S, Sadda SR. Choriocapillaris flow deficit associated with intraretinal hyperreflective foci in intermediate age-related macular degeneration. Graefe’s Arch Clin Exp Ophthalmol. 2020. https://doi.org/10.1007/s00417-020-04837-y.
Alagorie AR, Verma A, Nassisi M, Sadda SR. Quantitative assessment of choriocapillaris flow deficits in eyes with advanced age-related macular degeneration versus healthy eyes. Am J Ophthalmol. 2019;205:132–9.
Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33:265–76.
Mullins RF, Johnson MN, Faidley EA, Skeie JM, Huang J. Choriocapillaris vascular dropout related to density of drusen in human eyes with early age-related macular degeneration. Investig Ophthalmol Vis Sci. 2011;52:1606–12.
Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol Asp Med. 2012;33:295–317. http://www.sciencedirect.com/science/article/pii/S0098299712000453
Bird A, Chakravarthy U, Wilkinson CP, Sadda SR, Csaky K, Ferris FL, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51. https://doi.org/10.1016/j.ophtha.2012.10.036
Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13:388–91.
Uji A, Balasubramanian S, Lei J, Baghdasaryan E, Al-Sheikh M, Sadda SR. Impact of multiple en face image averaging on quantitative assessment from optical coherence tomography angiography images. Ophthalmology. 2017;124:944–52.
Borrelli E, Uji A, Sarraf D, Sadda SR. Alterations in the choriocapillaris in intermediate age-related macular degeneration. Investig Ophthalmol Vis Sci. 2017;58:4792–8.
Rosenfeld PJ, Durbin MK, Roisman L, Zheng F, Miller A, Robbins G, et al. ZEISS AngioplexTM spectral domain optical coherence tomography angiography: technical aspects. Dev Ophthalmol. 2016;56:18–29.
Byon I, Nassisi M, Borrelli E, Sadda SR. Impact of slab selection on quantification of choriocapillaris flow deficits by optical coherence tomography angiography. Am J Ophthalmol. 2019;208:397–405.
Zhang Q, Zheng F, Motulsky EH, Gregori G, Chu Z, Chen C-L, et al. A novel strategy for quantifying choriocapillaris flow voids using swept-source OCT angiography. Investig Ophthalmol Vis Sci. 2018;59:203–11.
Chu Z, Gregori G, Rosenfeld PJ, Wang RK. Quantification of choriocapillaris with OCTA: a comparison study. Am J Ophthalmol. 2019;208:111–23.
Zheng F, Zhang Q, Shi Y, Russell JF, Motulsky EH, Banta JT, et al. Age-dependent changes in the macular choriocapillaris of normal eyes imaged with swept-source optical coherence tomography angiography. Am J Ophthalmol. 2019;200:110–22.
Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Investig Ophthalmol Vis Sci. 1994;35:2857–64.
Dhrami-Gavazi E, Balaratnasingam C, Lee W, Freund KB. Type 1 neovascularization may confer resistance to geographic atrophy amongst eyes treated for neovascular age-related macular degeneration. Int J Retin Vitr. 2015;1:15.
Borrelli E, Lonngi M, Balasubramanian S, Tepelus TC, Baghdasaryan E, Iafe NA, et al. Macular microvascular networks in healthy pediatric subjects. Retina. 2018;39:1216–24.
Author information
Authors and Affiliations
Contributions
LT and GC contributed equally to this work. LT, GC, and SRS contributed to the study concept and design, acquisition, analysis or interpretation of data, drafting of the manuscript, and critical revision of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
LT: none; GC: none; SRS: Allergan (C,F), Carl Zeiss Meditec (C, F), CenterVue (C), Genentech (C, F), Heidelberg engineering (C), Iconic (C), 4DMT (C), Novartis (C), Optos (C,F), Topcon (C), Oxurion (C).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tiosano, L., Corradetti, G. & Sadda, S.R. Progression of choriocapillaris flow deficits in clinically stable intermediate age-related macular degeneration. Eye 35, 2991–2998 (2021). https://doi.org/10.1038/s41433-020-01298-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-020-01298-9
- Springer Nature Limited
This article is cited by
-
Central macular choriocapillaris impairment as a manifestation of microvascular disease in eyes with subretinal drusenoid deposits
Eye (2024)
-
Integrity of the Hyperreflective Layer in the Inner Choroid in Eyes with Drusen
Ophthalmology and Therapy (2024)
-
Topographic analysis of local OCT biomarkers which predict progression to atrophy in age-related macular degeneration
Graefe's Archive for Clinical and Experimental Ophthalmology (2024)
-
Biomarkers for the Progression of Intermediate Age-Related Macular Degeneration
Ophthalmology and Therapy (2023)