Abstract
There is increasing interest in solid particle-stabilized soft dispersed systems, including bubbles/foams (a gas-in-liquid dispersed system) and liquid marbles (a liquid-in-gas dispersed system). The studies on these dispersed systems have mainly been conducted utilizing inorganic particles and research using polymer particles has recently gained attention. Synthetic colloidal polymer particles are attractive stabilizers, as their hydrophilic–hydrophobic character and surface chemistries can be designed and controlled on demand via polymerization with various functional monomers and post polymer reactions. The successful synthesis of polymer particles would inspire the construction of well-defined and functionalized particle-stabilized gas–liquid soft dispersed systems. In this review article, bubbles/foams and liquid marbles stabilized solely with stimulus-responsive polymer particles will be reviewed. The stabilities, structures, and motions of these dispersed systems can be controlled by external stimuli.
Similar content being viewed by others
Introduction
Colloidal polymer particles have mainly been utilized in the form of films in various industries, including the paint, adhesive, and paper industries. In addition to their usage as films, there has been great interest in using polymer particles in their particulate form [1,2,3,4]. Polymer particles have been used as catalysts [5], column beads [6], calibration standards for the determination of particle size [7], toner [8], marker particles in immunodiagnostic assays [9], antistatic and/or anticorrosion coatings [10], pigments [11], synthetic mimics for cosmic dusts [12], building blocks for colloidal crystals [13,14,15], and spacers for polymer films to prevent electrostatic problems [16]. The last two decades have witnessed significant progress in the synthesis, characterization, and application of stimuli-responsive polymer particles, whose size, hydrophilic–hydrophobic character, and softness can be controlled on demand by external stimuli [17,18,19].
Solid colloidal particles are known to be adsorbed at liquid/liquid and gas/liquid soft interfaces and form network structures at the interfaces [20,21,22,23,24]. These phenomena have led to emerging concepts and novel materials, such as Pickering emulsions [20,21,22,23,24,25], colloidosomes [26, 27], armored bubbles [28,29,30], liquid marbles [31,32,33], dry water [34, 35], and gas marbles [36]. Bubbles/foams are gas-in-liquid soft dispersed systems and are known to be stabilized with solid particles adsorbed at gas–liquid interfaces [20, 28,29,30]. These particle-stabilized dispersions have been used in various industrial fields, including food, cleaning, ore flotation, radioactive material processing, water purification, and crude oil refining industries [37, 38]. In the food industry, particle-stabilized bubbles/foams are important for obtaining highly stable creams [39]. Bubbles/foams stabilized with solid particles have been utilized in ore flotation and ink removal research to recover target particulate materials. In addition, foams were demonstrated to work as precursors for porous materials [40]. On the other hand, particle-stabilized bubbles/foams can also work in a harmful manner. Mixing liquids and gases in the presence of solid particles using pumps, homogenizers, or agitators often generates undesirable bubbles/foams. In papermaking industries, unwanted bubbles/foams stabilized with fibers, tars, or clays are generated, which could lead to problems such as pipe and filter clogging. Furthermore, bubbles/foams stabilized by asphaltene particles are problematic in the petroleum industry. Liquid marbles are liquid droplets dispersed in the gas phase, which are stabilized by solid particles adsorbed at the gas–liquid interfaces. Liquid marbles are a unique soft dispersed system which cannot be stabilized by surfactants adsorbed at the interfaces at a molecular level. Liquid marbles have remarkable properties that cannot be realized using bare liquid droplets. The solid particles existing at the liquid marble surfaces can work as a shield, which renders the droplets as non-wetting to any solid substrates. As a result, the encapsulated liquid phase can avoid contamination and the evaporation of the inner volatile liquids can be minimized. The liquid phase inside the liquid marbles can be mixed and released by disrupting the liquid marbles. By taking advantage of the abovementioned unique properties, an increasing number of potential applications have been proposed for using the liquid marbles in a wide range of research areas; e.g., liquid marbles have been proposed for use in sensors [41,42,43,44], actuators [45, 46], accelerometers [47], transport and microfluidic applications [48,49,50,51], miniature reactors [52,53,54,55], cosmetics [56, 57], and personal and health care products [58].
Although solid particle-stabilized gas–liquid soft dispersed systems have a long history, the particulate stabilizers studied previously are mainly composed of inorganic particles with ill-defined shapes, inhomogeneous surface chemistries, and polydisperse size distributions, and it is difficult to obtain precise and reproducible results regarding the formation, stability, and structure of the soft dispersed systems. In this context, synthetic colloidal polymer particles are particularly attractive for use as stabilizers in soft dispersed systems, because their size and size distribution can be controlled by utilizing various heterogeneous polymerization methods (e.g., emulsion polymerization, dispersion polymerization, and seeded polymerization) and the hydrophilic–hydrophobic character, surface chemistries, and softness of synthetic colloidal polymer particles can be designed and modified on demand via polymerizing various functional monomers and performing post polymer reactions (e.g., esterification and hydrolysis) [1,2,3,4]. Furthermore, modifying the stimuli-responsive character of the polymer particles could introduce novel functions to the soft dispersed systems (i.e., bubbles/foams and liquid marbles). Stimulus-responsive soft dispersed systems are one of the most rapidly emerging and exciting research fields, and there are many unexplored topics that could create commercial applications. Many exciting challenges exist in this evolving scientific research field, which can be addressed by designing, fabricating, and functionalizing stimulus-responsive materials. This review article highlights the progress made in the research area of recently developed stimuli-responsive bubbles/foams and liquid marbles stabilized with stimuli-responsive polymer particles. First, the physicochemistry of the polymer particle adsorbed at the air–water interface is discussed. Next, bubbles/foams and liquid marbles stabilized with stimulus-responsive polymer particles, whose stability, structure, and motion can be controlled by external stimuli, are described. Then, future research directions are indicated.
Particles at gas–liquid interfaces
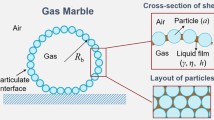
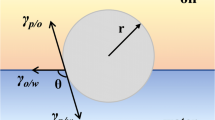
To design and fabricate particle-stabilized soft dispersed systems and control their stability and structure, the hydrophilic–hydrophobic character of the particles (i.e., their wettability at air–water interfaces) is a crucial factor, as it determine the adsorption energy of the particles at the interfaces (Fig. 1). The interfacial free energy decreases when a portion of the air–water interface is replaced by a particle–water interface by attaching a solid particle [59]. The adsorption energy ΔG required to detach the spherical solid particle from the air–water interface and move it to the air phase can be expressed using equation 1: [60]
where γaw is the surface energy of the water, a is the radius of the solid particle, and θ is the contact angle of the water on the particle surface (as measured through the aqueous phase). From equation 1, ΔG is at a maximum when θ = 90° and estimated to be several times greater than the thermal energy of solid particles with diameters >10 nm at the air–water interface, which leads to the irreversible adsorption of the particles to the interface. Due to the high adsorption energy, soft air–water dispersed systems stabilized by solid particles with a suitable surface wettability are very stable for long periods of time, which is in stark contrast to those stabilized by molecular-scale stabilizers, which can reversibly adsorb to or desorb from the air–water interface. Particles with relatively hydrophilic surfaces, which exhibit θ < 90°, are suitable to stabilize air-in-water-type bubbles/foams. In contrast, particles with relatively hydrophobic surfaces, which exhibit θ > 90°, have a tendency to stabilize water-in-air-type liquid marbles. When the surfaces of the particles are highly hydrophilic (θ ~ 0°), aqueous particle dispersions tend to form instead of soft dispersed systems, because the particles do not adsorb on the air–water interfaces. Considering the abovementioned behavior of the particles, it is expected that the formability and stability of the soft dispersed systems can be controlled using stimulus-responsive particles, which can change the surface wettability of the air–water interface by external stimuli.
Polymer particle-stabilized soft dispersed systems comprising air and water. Bubbles/foams (air-in-water dispersed systems) are preferably formed using relatively hydrophilic polymer particles and liquid marbles (water-in-air dispersed systems) are preferably formed using relatively hydrophobic polymer particles. An aqueous dispersion of polymer particles is obtained using highly hydrophilic polymer particles
The properties of particle-stabilized soft dispersed systems, such as the stability and stimulus responsiveness, arise from the complex coupling of multiple size scales. At the nanometer scale, the surface chemistry of the solid particulate stabilizer (i.e., the functional groups that determine the mobility of the molecules at surfaces) is an important factor for controlling the adsorption/desorption of the particles to/from the air–water interface. At the micrometer scale, the wettability of the particulate stabilizers at the air–water interface is crucial. Chemical and physical changes of the air and water phases, such as pH, temperature, and salt concentration, at larger scales (> a micrometer) can influence the properties of the particulate stabilizers and the air–water interface, resulting in changes to the structure and stability of the soft dispersed systems.
Stimulus-responsive polymer particles
This section introduces stimulus-responsive polymer particles that have been utilized as particulate stabilizers for bubbles/foams and liquid marbles. The properties of these particles, such as the hydrophilic–hydrophobic character of the surfaces and generation of heat, can change in response to various external stimuli (e.g., pH, temperature, and light). The stimulus-responsive polymers that have been introduced into particulate stabilizers for soft dispersed systems are shown in Fig. 2. To synthesize these particles, several heterogeneous polymerization methods, including emulsion polymerization, dispersion polymerization, and precipitation polymerization, have been applied [1,2,3,4]. The surface grafting of seed particles, postmodification methods, and the self-assembly of block polymers have also been utilized [1,2,3,4].
pH-responsive particles
The pH of the aqueous phase can be simply tuned by adding an acid and a base, resulting in changes to the surface charge of the pH-responsive polymer particles due to the protonation/deprotonation of the functional groups of the particles. The surface charge changes correlate with the wettability of the polymer particles at the air–water interface and therefore the hydrophilic–hydrophobic character of such particulate stabilizers can be controlled by changing the pH of the aqueous media. The stability of soft dispersed systems stabilized with such pH-responsive polymer particles can be controlled by an external pH stimulus. Some stabilizers have utilized acidic functional groups, e.g., polymer particles with carboxylic acid groups, including polystyrene (PS) particles with poly(acrylic acid) (PAA) colloidal stabilizers (PAA-PS particles) [61], silica particles with poly[6-(acrylamido)hexanoic acid] hairy colloidal stabilizers [62], PS particles with succinic anhydride-esterified poly(2-hydroxypropyl methacrylate) hairy colloidal stabilizers [63], and poly(styrene-co-acrylic acid-co-2,2,3,4,4,4-hexafluorobutyl methacrylate) particles [64]. Other stabilizers have utilized basic functional groups, e.g., PS particles with poly(4-vinylpyridine) (P4VP) colloidal stabilizers (P4VP-PS particles) [65], poly(2-vinylpyridine) (P2VP) particles with poly(ethylene glycol) (PEG) hairy colloidal stabilizers (PEG-P2VP particles) [66], and P2VP particles with polydimethylsiloxane (PDMS) hairy colloidal stabilizers (PDMS-P2VP particles) [67].
Temperature-responsive particles
Temperature is another attractive stimulus that can be easily controlled without any direct addition of chemicals to the systems (note that the addition of an acid/base to the systems is required in the case of using pH as the stimulus). As a consequence, temperature could be used to steadily control systems that are influenced by pH or ionic strength. Poly(N-isopropylacrylamide) (PNIPAM) is a well-known polymer that exhibits a temperature-responsive phase transition behavior in aqueous media [68]. PNIPAM undergoes a sharp reversible phase transition at a lower critical solution temperature (LCST) of 32 °C. PNIPAM is soluble in aqueous media due to the hydrogen bonding of the pendant amide groups with water molecules at temperatures less than the LCST. However, PNIPAM is not soluble in aqueous media at temperatures greater than the LCST due to dehydration. Therefore, polymer particles with a PNIPAM component can change their hydrophilic–hydrophobic character at the LCST in aqueous media and be applied as temperature-responsive stabilizers of bubbles [69] and liquid marbles [70].
pH- and temperature-responsive particles
Polymers that respond to two stimuli are of great interest, because an independent response to several factors might be required in some applications. To synthesize polymers that are responsive to two stimuli, pH-responsive and temperature-responsive monomers are often copolymerized. In addition to these copolymers, there is increasing interest in homopolymers synthesized by polymerizing N,N-dialkylaminoethyl methacrylates, which respond to both pH and temperature in aqueous media. Poly(N,N-(dimethylamino)ethyl methacrylate) (PDMA) is a well-known example of this type of polymer [71,72,73]. PDMA is a pH-responsive polybase with a pKa value of 7.0 and the pH of aqueous media can change its hydrophilic–hydrophobic character. PDMA is soluble in water at room temperature when it is protonated and hydrophilic below the pKa and when it is nonprotonated and relatively hydrophilic above the pKa. PDMA also shows its temperature-responsive character, as it has an LCST of 32 °C when it is nonprotonated due to its hydration and nonhydration behaviors. Although it was once believed to be responsive only to pH, poly(N,N-diethylaminoethyl methacrylate) (PDEA) has been found to be responsive to both pH and temperature [74, 75]. PDEA has a pKa value of 7.3 and an LCST of 41 °C (at a pH of 6.86). In basic media, PDEA is hydrophobic and water insoluble, because it contains amino groups that are nonprotonated and electrostatically neutral. On the other hand, PDEA is water soluble and hydrophilic in acidic media, because it contains amino groups that are protonated and positively charged. PEDA shows LCST-based reversible dissolution/precipitation behaviors at a pH of 6–8. As PDMA and PDEA are responsive to two stimuli, it is possible to achieve extensive control over the hydrophilic–hydrophobic character of these polymers and polymer particles containing these polymer components reflect these properties. PS particles with PDMA hairy colloidal stabilizers (PDMA-PS particles) and PS particles with PDEA hairy colloidal stabilizers (PDEA-PS particles) have been utilized as a stabilizers for bubbles/foams [76,77,78,79] and liquid marbles [80,81,82,83,84,85].
Light-responsive particles
Light stimuli can be remotely applied to systems, which is an advantage over other stimuli such as pH and temperature, which require direct application or contact. Furthermore, the precise control of the timing, direction, position, area, and intensity makes light a unique stimulus. Until now, various light-responsive polymers whose color, hydrophilic–hydrophobic character, and conductivity can be changed by applying light have been studied [86,87,88]. Among these light-responsive polymers, conjugated polymers, such as polypyrrole (PPy), polyaniline (PANI), poly(3,4-ethylenedioxythiophene) (PEDOT), and poly(3-hexylthiophene) (P3HT), are fascinating light-responsive polymers that have light-to-heat photothermal character. Due to the low luminescence efficiency of such conjugated polymers, most of the energy absorbed from near-infrared (NIR) light is converted into heat [89, 90]. It has been reported that the surface temperatures of these polymers increase from room temperature to over several hundreds of degree Celsius within a few seconds by NIR irradiation. Using such conjugated polymer particles as stabilizers, photothermal character can be imparted to the soft dispersed systems.
Stimulus-responsive bubbles/foams
Bubbles and foams stabilized with polymer particles can be fabricated using methods similar to those utilized to fabricate bubbles and foams stabilized with molecular-scale surfactants. Bubbles/foams can be fabricated by mixing air, water, and polymer particles (or air and an aqueous dispersion of polymer particles); specifically, air has been introduced into aqueous dispersions of polymer particles by manual shaking, blender agitation, and air bubble injection. Another method used to prepare bubbles/foams is an in situ bubble generation method: (1) air is dissolved in the aqueous dispersion of polymer particles under high pressure and bubbles are subsequently generated in the continuous water phase by sharply reducing the pressure and (2) a foaming agent (i.e., a gas generating compound) is introduced into the aqueous dispersion of polymer particles. Note that bubbles stabilized by polymer particles have been observed in the Challenger space shuttle (STS 7, June 1983) [91]. Gas bubbles stabilized in an aqueous medium with micrometer-sized PS particles were fabricated on the Challenger space shuttle during seeded emulsion polymerization under zero gravity. These nitrogen gas bubbles formed as a byproduct of the decomposition of a 2,2′-azobis(isobutyronitrile) azoinitiator, which was expected to generate free radicals, were stabilized by polymer particles. Using microfluidic devices, highly monodisperse particle-stabilized bubbles have been produced in microchannels [92]. A method used to fabricate nonspherical bubbles stabilized by solid particles (named as the air pocket-trapping technique) has been developed [93]. In this method, air is trapped in a layer of dried particle powder by quickly adding water onto the powder layer. The water penetrates the particle layer containing air until the pressure of the trapped air is balanced by the capillary pressure of the advancing air–water interface.
The hand-shaking method has been generally utilized to generate stimulus-responsive bubbles/foams stabilized with polymer particles, because it is simple, quick, and easy, and does not require any special equipment. In this section, stimulus-responsive bubbles/foams stabilized with the polymer particles are introduced. Articles reviewing stimulus-responsive bubbles/foams that are stabilized with functional particles, including inorganic particles, have been recently published [94, 95] and are available for reference.
pH-responsive bubbles/foams
Polymer particle-stabilized bubbles/foams with stabilities that can be tuned by contact with an acid or a base have been developed (Fig. 3a). The acid and base could be either a liquid or vapor, and the phase of the acid and base can change the wettability of the particles on air–water interfaces. By controlling the hydrophilic–hydrophobic character of the particle surfaces, the adsorption/desorption of the particles to/from the interfaces can be attained, which can lead to stabilization (foaming) and destabilization (defoaming).
pH-responsive bubbles/foams: a Schematic of pH-induced disruption of bubbles/foams stabilized with pH-responsive polymer particles. The bubbles/foams are stable for long period of time at pH values where the surfaces of the polymer particles are hydrophobic enough for the particles to adsorb at the air–water interface. In contrast, bubbles/foams are immediately disrupted and the polymer particles spontaneously disperse into continuous aqueous media by controlling the pH via adding an acid/base, as the surfaces become hydrophilic by protonation/deprotonation. Reproduced with permission [77]. Copyright 2011, American Chemical Society. b Effects of the length of the PDEA hairy stabilizer on the PDEA-PS particles (polymerization degree = 30, 60, and 90) on the foamability and foam stability. The heights of the foam layers as functions of the pH of the aqueous dispersions recorded at different times: (●) immediately after preparation, (■) 24 h after preparation, (◆) 1 week after preparation, and (▲) 1 month after preparation. Reproduced with permission [79]. Copyright 2016, The Royal Society of Chemistry
Bubbles/foams stabilized using polymer particles with acid groups on their surfaces have been demonstrated to be destabilized by exposure to a base. In acidic aqueous media, hydrophobic particles with acid groups in protonated forms can adsorb at air–water interfaces to stabilize aqueous bubbles/foams. On the other hand, particles with hydrophilic surfaces functionalized with deprotonated negatively charged acid groups do not adsorb on the air–water interface. The ability of particles to adsorb on the air–water interface can be controlled by pH, which is reflected by the change in the hydrophilic–hydrophobic character of the particle surfaces. PAA-PS particles have been demonstrated to work as effective base-responsive bubble/foam stabilizers [61]. The PAA-PS particle surfaces are highly hydrophilic at and above a pH of 6, where >90% of the carboxylic acid groups in the PAA stabilizer are deprotonated and negatively charged. As the particles are highly hydrophilic, it is unlikely to be that the PAA-PS particles will adsorb at the air–water interface and instead the particles tend to disperse in the continuous aqueous media via both steric and electrostatic stabilization mechanisms. On the other hand, PAA-PS particles flocculate at and below a pH of 4.5, because the PAA-PS particles lose their colloidal stability via steric and electrostatic stabilization mechanisms. The particle surfaces were shown to be hydrophobic enough for the PAA-PS particles to adsorb at the air–water interface at and below a pH of 3.5, where >90% of the carboxylic acid groups in the PAA stabilizer are protonated. Under these conditions, particle-stabilized bubbles/foams that are stable for >1 month are generated. It is noteworthy that the in situ pH control can tune the adsorption/desorption behavior of the PAA-PS particles to/from air–water interfaces and therefore control the stabilization/destabilization of the bubbles/foams.
In the abovementioned bubble/foam systems, bases are used to render the particles with acidic groups hydrophilic, thus disrupting the particles. If particles with basic groups are applied as stabilizers, bubbles/foams exhibiting a complementary behavior, which is disrupted by adding acids, can be realized. The particles can adsorb at the air–water interfaces when the basic groups on the particle surfaces are nonprotonated and when the particle surfaces are hydrophobic. Conversely, the addition of an acid causes the particles to become hydrophilic due to the protonated, positively charged basic groups on the particle surfaces, thus resulting in defoaming. It has been demonstrated that PDEA-PS particles [73, 96] can function as acid-responsive stabilizers for bubbles/foams [77,78,79]. The PDEA-PS particle surfaces are hydrophobic at pH values > 8.0, which are higher than the pKa value of the PDEA homopolymer. Under these pH conditions, flocs of PDEA-PS particles can adsorb at the air–water interface, which stabilizes the aqueous bubbles/foams; these aqueous bubbles/foams are stable for over 1 month, even though creaming occurred. Relatively stable bubbles/foams, which are stable for at least 24 h, can be prepared at pH values of 6.1 and 7.1. Bubbles/foams prepared at pH values of 6.1 and 7.1 are less stable than those formed at a pH > 8.0, because the surfaces of bubbles/foams prepared at pH values of 6.1 and 7.1 have thinner particle layer thicknesses: monolayers and multilayers are formed on the bubble surfaces at pH values of 6.1 and 10, respectively. Thicker particle layers can hinder contact of the bare air bubble surfaces due to the existence of a larger space between the bubbles. At pH values of 5.1 and 3.1, the PDEA stabilizers on the particle surfaces are protonated and become water soluble, as they are cationic and hydrophilic, and the PDEA-PS particles tend to disperse in aqueous media via electrostatic and steric stabilization mechanisms. Under these conditions, macrophase separation of the air and aqueous dispersion of the particles occurs. Bubbles/foams stabilized with PDEA-PS particles at a pH of 9.0 are pH-responsive: the foam prepared at a pH of 9.0 could be disrupted by adding an acid to achieve a pH of 4.0. Multiple foaming-defoaming cycles have also been demonstrated by adding a base and an acid, accompanied by shaking. Foams stabilized by PDEA-PS particles with longer PDEA hairy colloidal stabilizers, which remained stable for over 1 month, were shown to be more stable than those with shorter PDEA hairs (Fig. 3b) [79]. Bubbles stabilized by PS particles with longer PDEA hairs are coated by thicker PDEA-PS particle layers due to stronger particle–particle interactions, which protect the bubbles from coalescence. Using PDEA-PS particles with longer hairs, foams were formed in a narrower pH range due to both image charge and entropic effects. The mixing of air and the aqueous dispersion of PDEA-PS particles (at a pH of 10) at a high solid concentration (40 wt% solid concentration) using a homogenizer resulted in the formation of a foam with a cream-like texture. Interestingly, the cream-like foam transformed into an aqueous dispersion by exposure to HCl vapor [79].
Other acid-responsive foams can be constructed by a method similar to that used to stabilize bubbles/foams with PDEA-PS particles, whereby polymer particles with basic groups on their surfaces, namely, PDMA-PS particles [76] and P4VP-PS particles [65], are used. PS particles with amidine groups synthesized by free radical precipitation polymerization in the absence of any colloidal stabilizer could also be another candidate for use as an acid-responsive foam stabilizer [97]. Controlling the pKa of the base groups on the particle surfaces makes it possible to tune the foaming/non-foaming threshold pH.
The pH-dependent adsorption of PDMA-PS particles to air–water interfaces was examined in detail to study the resulting particle-stabilized bubbles/foams [98, 99]. A Langmuir−Blodgett trough, an X-ray reflectometer, and a surface tensiometer were utilized to study the pH-dependent adsorption of the PDMA-PS particles at the air–water interface at different pH values [99]. Experiments have shown that the PDMA-PS particles are adsorbed at the air–water interface under basic pH conditions, whereas at acidic pH values the majority of PDMA-PS particles are dispersed in the aqueous media with only a small number of particles adsorbed at the interface. The X-ray reflectometry analysis indicated that a monolayer of PDMA-PS particles forms at the air–water interface and an increase in the surface pressure leads to the dense packing of the PDMA-PS particles. A direct visualization method confirmed that the contact angle of the particles at the air–water interface was 34° at a pH of 10.
In addition, polymer particles with a pH-responsive core and a non-pH-responsive stabilizer can also function as a pH-responsive foam stabilizer. PEG-P2VP particles are one such candidate that can be used as an effective pH-responsive bubble/foam stabilizer [66]. PEGMA-P2VP particles are composed of non-pH-responsive PEG and pH-responsive P2VP, leading to dual surface characteristics, and, therefore, the hydrophilic–hydrophobic character can be controlled by external pH stimulus. The PEGMA-P2VP particles adsorb at the air–water interface to stabilize aqueous bubbles/foams at a pH of 10, but the bubbles/foams are destabilized at a pH of 3 by the desorption of the particles from the air–water interface. These particles undergo a latex-to-microgel transition by decreasing the pH and it could be possible to release the functional compounds contained within the latex particles by acid stimulus while simultaneously destabilizing the bubbles and foams.
pH is an attractive stimulus, because acids/bases are diverse, simple, and easy to handle; however, there is an unfortunate disadvantage regarding the usage of acids/bases. The application of acids/bases to bubble/foam systems could be limited, as the neutralization cycles of acids/bases produce an undesirable accumulation of nonvolatile salts. Carbon dioxide is a unique stimulus that is benign, biocompatible, and easy to be applied. Carbonic acid forms when carbon dioxide is introduced into and reacts with water and carbonic acid can be removed from the aqueous solution by bubbling with an inert gas, such as argon or nitrogen. Therefore, multiple acid-base neutralization cycles can be attained without increasing the ionic strength. Furthermore, this neutralization cycling process is easily conducted and non-accumulative.
Temperature-responsive bubbles/foams
Using polymer particles whose surface hydrophilic–hydrophobic character can be tuned by temperature stimulus as a stabilizer, bubbles and foams whose stabilities and structures can be controlled by temperature can be fabricated (Fig. 4a). PDEA-PS particles [77,78,79] and PDMA-PS particles [76] can be used as particulate bubble/foam stabilizers that are responsive to both temperature and pH. The hydrophilic–hydrophobic character of the particle surfaces can be tuned by dual stimuli, and thus the stability and structure of the resulting bubbles/foams could be well controlled.
pH- and temperature-responsive bubbles/foams: a Schematic of the pH- and temperature-induced disruption and structural change of bubbles/foams stabilized with PDMA-PS particles. The hydrophilic–hydrophobic character of the PDMA-PS particles could be controlled by varying both the pH and temperature, and therefore the particles acted as a dual stimuli-responsive stabilizers for aqueous foams by adsorbing and desorbing to/from the air–water interface. b SEM images of foams stabilized with PDMA-PS particles at 23 °C and 55 °C, and pH values of 6.0 and 8.9. Reproduced with permission [76]. Copyright 2015, The Royal Society of Chemistry
The hydrophilic–hydrophobic character of PDEA-PS particle surfaces could be controlled by temperature stimulus at a nearly neutral pH (where the LCST of PDEA at a pH of 6.86 was 41 °C). The PDEA-PS particles have hydrated PDEA hairs at 25 °C and foams that coalesce with time formed. At 40 °C and 45 °C, the PDEA-PS particles have partially nonhydrated PDEA hairs and relatively stable foams were produced. At and above 50 °C, the PDEA-PS particles have nonhydrated PDEA hairs and highly stable bubbles/foams that are stable for over 1 week formed. The PDEA-PS particles adsorbed at the air–water interface, mainly as a monolayer at 25 °C and as multilayers at and above 40 °C, which has been confirmed by scanning electron microscopy.
In a similar manner, PDMA-PS particles can also function as a particulate bubble/foam stabilizer that is responsive to both temperature and pH stimuli (Fig. 4b) [76]. At and above a pH of 6.0, the PDMA hairy stabilizers are either partially protonated or nonprotonated, and bubbles/foams are produced at both 23 °C (below the LCST) and 55 °C (above the LCST). The stability of the bubbles/foams generated at 55 °C is better than those generated at 23 °C. The PDMA-PS particles adsorb on the bubble surfaces as a monolayer at 23 °C and as multilayers at 55 °C, and thicker layers are more effective protectors for the bubbles. At and below a pH of 5, the PDEA-PS particles have positively charged hairy stabilizers with hydrophilic character and no foam is formed, regardless of temperature. At both temperatures, the bubbles/foams are rapidly disrupted on demand by lowering the solution pH, which indicates that the PDMA-PS particles desorb from the bubble surfaces and disperse into the aqueous media via in situ protonation of the PDMA hairy colloidal stabilizers.
Recently, PNIPAM particles were utilized as a temperature-responsive foam stabilizer [69]. The surface tensions of the aqueous dispersions of PNIPAM particles at temperatures less than the LCST of PNIPAM were much lower than those of water. Foams could be prepared at temperature less than the LCST by bubbling nitrogen through the aqueous dispersion of the PNIPAM particles, whereas no/little foam was produced at temperatures greater than the LCST. Furthermore, the foams produced at temperatures below the LCST were quickly disrupted by heating the foams above the LCST. It was claimed that this rapid defoaming occurred by desorbing the PNIPAM particles from the air–water interface, which was achieved by switching off the surface activity of the PNIPAM particles. However, a report has described that the surface tension of an aqueous dispersion of PNIPAM particles decreased from ∼44 mN/m to 39 mN/m when the temperature increased from 20 °C to the LCST and was almost constant above the LSCT [100]. From these results, it is expected that the PNIPAM particles remained at the air–water interface, even at temperatures at and above the LCST, which does not correspond with the results on the foam systems. To understand this phenomena, further systematic studies are required.
Stimulus-responsive liquid marbles
In nature, liquid marbles can form when rain droplets fall on hydrophobic soil produced after forest fires [101]. Some aphids are known to fabricate solid wax particle-stabilized liquid marbles containing honeydew as the inner liquid and these liquid droplets behave as non-wetting materials [102, 103]. Liquid marbles can also be created in an artificial manner by coating droplets with solid particles and several methods have been developed. The most general method is the rolling method [31], where a droplet is gently rolled on a dried polymer particle powder bed, forming a liquid marble. The volume of liquid marbles can be easily tuned by changing the droplet volume using a micropipette. Multiple liquid marbles can be simultaneously fabricated by spraying droplets onto a powder bed and coating the droplets with particles. It has also been demonstrated that liquid marbles can be fabricated in a one-step manner by the impact of depositing droplets onto a dried particle powder bed [104]. Simply drying droplets on a dried particle powder bed has also been demonstrated to fabricate liquid marbles via self-coating with particles [101]. Recently, electrostatic fields have been successfully utilized to fabricate liquid marbles [105,106,107,108]. Dried polymer particles placed on an electrically biased substrate jump to electrically grounded pendant water droplets when the electrostatic field is strong enough. This electrostatic field method has an advantage, as the morphology of the resulting liquid marbles can be controlled. Polymer particles with hydrophilic surfaces tend to disperse in droplets, whereas those with hydrophobic surfaces tend to adsorb at the air–water interface of a droplet, which leads to the formation of a composite liquid marble with a core/shell morphology; the core is an aqueous dispersion of hydrophilic particles and the shell is a hydrophobic particle layer [108]. Stimulus-responsive liquid marbles have been mainly fabricated by the rolling method, which is simple, quick, and only needs a pipette. In this section, stimulus-responsive liquid marbles stabilized with polymer particles are described. Recently, stimuli-responsive liquid marbles that are stabilized with functional particles, including inorganic particles, has been reviewed and this review can be used as a reference [109].
pH-responsive liquid marbles
As in the case of bubbles and foams systems, liquid marbles whose stability can be tuned by pH stimulus can be fabricated using polymer particles as a stabilizer, which changes their wettability to the air–water interface by a pH stimulus (Fig. 5).
pH-responsive liquid marbles: Schematic of the pH-induced disruption of a liquid marble placed at a planar air–water interface. The liquid marbles are stable for long periods of time on planar air–water interface at pH values where the surfaces of the polymer particles are hydrophobic enough for the particles to adsorb at the air–water interface. In contrast, adding an acid/base induces immediate liquid marble disruption and spontaneous desorption of the polymer particles into the aqueous media, as the particle surface becomes hydrophilic by protonation/deprotonation. Reproduced with permission [80]. Copyright 2009, American Chemical Society
Liquid marbles stabilized with PDEA-PS particles were the first to be demonstrated as stimulus responsive [80,81,82,83,84,85]. Liquid marbles consisting of PDEA-PS particles as the stabilizer and distilled water as the inner liquid are stable for over 90 min on the planar air–water interface when the pH of the aqueous subphase is >8. On the other hand, these liquid marbles immediately disintegrate when transferred onto acidic air–water interfaces. Furthermore, liquid marbles that are stable on an air–water interface at a pH of 8 can be disrupted by adding acidic aqueous solutions close to the liquid marble, which indicates that the spontaneous desorption of PDEA-PS particles from the air–water interface and their relocation to the bulk acidic aqueous phase is induced by the protonation of the PDEA stabilizer on the PDEA-PS particle surfaces. In addition, the PDEA-PS particle-stabilized liquid marbles can disrupted by using an acid as the gas (e.g., HCl gas) [82]. This acid-induced disruption mechanism can be applied to liquid marbles that are stabilized with PDMS-P2VP particles [67]. The minimum pH required for liquid marbles to be stable on planar air–water interfaces closely correlates with the pKa of the P2VP particles (4.7). The PDMS-P2VP particles swell with water via protonation by lowering the pH of the aqueous media and it is possible to release chemicals from the inside of the particles and from the inner liquid of the liquid marble.
The interactions between a liquid marble and a bare water droplet have been investigated using a combined apparatus comprising a coalescence rig and a high-speed video camera, which visualizes the dynamics of droplet coalescence with a millisecond resolution [84]. The induction times before coalescence, which measured the interaction between a liquid marble containing water (with a pH of 3 or 10) and stabilized with PDEA-PS particles and the bare water droplet (with a pH of 3), are longer than those observed for the interaction between two bare water droplets (with a pH of 3 or 10). The combination of the liquid marble (with a pH of 10) and the bare water droplet has a longer induction time than the combination of the liquid marble (with a pH of 3) and the bare water droplet. From the obtained results, spatiotemporal information on the liquid marble stability could be acquired.
Liquid marbles that can be disrupted by adding bases have also been fabricated (Fig. 5). To construct such a liquid marble system, polymer particles having acidic groups on their surfaces have been utilized (e.g., poly(styrene-co-acrylic acid-co-2,2,3,4,4,4-hexafluorobutyl methacrylate) [64], succinic anhydride-esterified poly(2-hydroxypropyl methacrylate) [63], and poly[6-(acrylamido)hexanoic acid] [62]). Liquid marbles stabilized by these polymer particles are stable on the planar water surfaces with pH values above the pKa of the acidic groups on the particle surfaces. This occurs because the polymer particle surfaces are hydrophobic when the acidic groups on the particle surfaces are protonated and remain adsorbed to the air–water interface of the liquid marbles. On the other hand, the liquid marbles become unstable and are disrupted once the base is added to the water surfaces near them. This occurs because the surfaces of the polymer particles become anionic and hydrophilic due to the deprotonation of the acidic groups. The transition pH for the disruption of the liquid marbles can be precisely tuned by controlling the pKa of the acidic groups on the particle surfaces.
It is noteworthy that the drying conditions of the aqueous dispersions of polymer particles play a crucial role in the subsequent formation of the liquid marbles. For instance, powdered poly(styrene-co-methacrylic acid) particles prepared by freeze-drying an acidic aqueous dispersion (with a pH of 3), which have protonated and relatively hydrophobic surfaces, can function as an effective liquid marble stabilizer. On the other hand, liquid marbles are difficult to be prepared using particles prepared by freeze-drying a basic aqueous dispersions (with a pH of 10) [110]. The same phenomenon has been observed for liquid marbles stabilized with PDEA-PS particles [85]; liquid marbles can be fabricated using powdered PDEA-PS particles prepared by drying a dispersion with a pH of 10.0, whereas the water droplets were absorbed by the powdered PDEA-PS particles prepared by drying a dispersion with a pH of 3.0 (Fig. 6). The dried PDEA homopolymers prepared from the aqueous solutions with pH values of 3.0 and 10.0 are partially protonated and nonprotonated, which was confirmed by elemental microanalysis, pulse nuclear magnetic resonance, thermogravimetric analysis, and contact angle measurements. The difference in the chemical state can cause a difference in the hydrophilic–hydrophobic character of the dried PDEA-PS particle surfaces. These results indicate that the surface hydrophilic–hydrophobic character of the dried polymer particles strongly depends on the drying conditions and the initial state of the dried particle surfaces (protonated or nonprotonated), which plays a significant role in the formulation of liquid marbles, even though the same particles are used. In addition, it has been confirmed that the grain structures of the PDEA-PS particle powder, which should affect the wettability of the water, also depend on the drying conditions [111, 112]. An investigation of the relationship between the grain structures of the powder and the formability, stability, and structure of the liquid marbles will be an interesting research topic.
Effect of the drying conditions of pH-responsive polymer particles on the formability of liquid marbles: the PDEA-PS particle powder obtained from an aqueous dispersion with a pH of 3.0 consists of irregular-shaped colloidal crystal grains that are hydrophilic. In contrast, the PDEA-PS particle powder obtained from an aqueous dispersion with a pH of 10.0 consists of amorphous and disordered colloidal aggregate grains that are hydrophobic. Due to the hydrophilic–hydrophobic character of the dried polymer particle powders, liquid marbles are prepared using the powder prepared from the dispersion with a pH of 10.0, whereas water droplets are absorbed into the powders prepared from dispersion with a pH of 3.0. Reproduced with permission [85]. Copyright 2018, American Chemical Society
Temperature-responsive liquid marbles
Liquid marbles, whose stabilities can be controlled by temperature, have been prepared using temperature-responsive polymer particles [70] (Fig. 7). PNIPAM is the most extensively studied temperature-responsive polymer and undergoes a reversible LCST phase transition. The LCST of PNIPAM depends on the type and concentration of salts introduced to the aqueous medium [113], and due to salting out effects the LCST of PNIPAM decreases with an increase in the salt concentration. Thus, the disintegration temperature of the PNIPAM particle-stabilized liquid marbles, which is close to the LCST of PNIPAM, can be controlled by tuning the Na2SO4 concentration in water. For example, liquid marbles can float on planar interface between air and an aqueous solution of Na2SO4 at a temperature greater than the LCST, and this behavior is disrupted by cooling below the LCST. The disintegration temperature of the liquid marble decreases with an increase in the Na2SO4 concentration. By maintaining the Na2SO4 concentrations between 0 and 0.5 M, the disintegration temperature of the liquid marbles can be tuned between 5 °C and 24 °C (Fig. 7d). The temperature-sensitive formation of liquid marbles was studied using PS particles coated with a crosslinked PNIPAM shell by electrostatic liquid marble formation experiments [114]. PNIPAM-coated PS particles tend to disperse into water droplets due to the high hydrophilicity of the particles at temperature less than the LCST. On the other hand, these particles attach to the droplet surfaces and liquid marbles with rough surfaces are produced using water at 40 °C or a saturated KCl aqueous solution at 25 °C.
Temperature-responsive liquid marbles: a Schematic of a liquid marble consisting of a 0.5 M Na2SO4 aqueous solution, which acts as the inner liquid phase, and PNIPAM, which acts as the stabilizer, and the liquid marble floats on the planar interface between air and the aqueous 0.5 M Na2SO4 solution. The liquid marble can be disrupted by cooling. b, c Digital photographs illustrating the disruption of the PNIPAM particle-stabilized liquid marble floating on the planar interface between air and the aqueous 0.5 M Na2SO4, solution (b) before and (c) after cooling to 0 °C from room temperature. d Disruption temperatures of the PNIPAM particle-stabilized liquid marbles containing 10 μL water with various concentrations of Na2SO4 floated on bulk water with the same Na2SO4 concentration as that in the liquid marble. Reproduced with permission [70]. Copyright 2014, Nature Publishing Group
Other temperature-responsive polymers demonstrate LCST phase-transition character, including poly(N-vinylcaprolactam) [115], chitosan [116], PDEA, and PDMA, and these polymers are possible candidates for use as temperature-responsive liquid marble stabilizers. Until now, temperature-responsive liquid marbles that can be disrupted by a temperature increase have not been realized. For such a system, particles that contain polymer components that exhibit upper critical solution temperatures, such as poly(N-acryloylglycinamide)-co-poly(N-acetylacrylamide) [117], poly(N-acryloylasparaginamide) [118], and ureido-derivatized polymers [119], are expected to function as temperature-responsive liquid marble stabilizers.
Light-responsive liquid marbles
There have been reports indicating that Marangoni flow can work as a propulsion force to move liquid marbles on planar air–water interfaces. When a liquid marble containing a water–alcohol mixture is placed on a planar air–water interface, self-propulsions of the liquid marble can be observed [120, 121]. This phenomenon is caused by the generation of a surface tension gradient on the air–water interface via preferential evaporation of the alcohol component from the liquid marble (i.e., the solutocapillary effect). Although solutocapillary Marangoni flow is an interesting propulsion force, it is difficult to control the direction and timing of the liquid marble movements. This occurs because the alcohol starts to evaporate, generating the surface tension gradient on the water surface; the direction and speed of the liquid marble are difficult to control once the liquid marble is placed on the interface. To control the motion of the liquid marbles, light-induced Marangoni flow has been applied as a powerful and effective propulsion force [122,123,124,125,126,127,128]. Using light as a stimulus, it is possible to control the position, area, direction, and timing of the stimulus application, and, therefore, precise motion control of the liquid marble can be realized. Conjugated polymers such as PPy, PANI, PEDOT, and P3HT are known to function as light-to-heat transducing materials [129,130,131]. PPy is one of the most studied conjugated polymer materials that has light-to-heat photothermal properties and its temperature can increase rapidly (within a few seconds) to up to several hundreds of degree Celsius under NIR (808 nm) laser irradiation. Hydrophobic PPy particle-stabilized liquid marbles can move on a planar air–water interface by site-selective NIR laser irradiation (Fig. 8a–c) [122,123,124]. The manual NIR laser irradiation of the three-phase contact line formed by the liquid marble, air, and water at an angle of ∼45° generates a surface tension gradient that functions as a propulsion force, causing the liquid marbles to move on the planar air–water interface. Due to the surface tension gradient, the liquid marbles immediately move forward on the air–water interface, away from the point of irradiation. Thermography studies indicate that the temperature of the water surface near the liquid marble is ∼31 °C, which contrasts with the temperature of the bulk water (21 °C). This temperature difference generates a surface tension difference of a few mN/m, which drives the movement of the liquid marble (Fig. 8c). Numerical analyses confirmed that the velocity and acceleration of the liquid marble movements are ∼0.01 m s−1 and 0.1 m s−2, respectively, and the generated force is estimated to be a few μN using the Newtonian equation of motion. The locomotion of the liquid marble on the air–water interface is ideal in that the object gains force from the viscous liquid phase and moves in the gas phase with a low viscosity, where the drag force is low [132].
a Schematic illustrating the locomotion of a PPy particle-stabilized liquid marble driven by light-induced Marangoni propulsion. Liquid marbles can be moved on planar air–water interfaces by NIR laser irradiation and the stimuli-induced disruption of the liquid marbles cause it to release its inner liquid. b, c The drift motion of the PPy-stabilized liquid marble observed by (b) a digital camera and (c) thermography. d Delivery of materials by sunlight irradiation of liquid marbles. The liquid marbles are bound to a plastic boat due to lateral capillary forces. Nonlinear movements could be attained using multiple liquid marbles. e The relationship between the tilt angle of the boat based on the horizontal axis and the time, which depends on the application of the focused sunlight on each liquid marble. Reproduced with permission [122]. Copyright 2016, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. f Side view digital photographs illustrating the transfer of the PPy-stabilized liquid marble from the air–water interface to the air–solid interface. The application of mechanical stress to the liquid marble using a cover glass disrupts the liquid marble and releases the internal liquid. Reproduced with permission [124]. Copyright 2017, American Chemical Society
Other conjugated polymers such as PANI [125], PEDOT [126], and P3HT [127] can also be used as stabilizers to enable the light-driven motion of liquid marbles. It is advantageous that various excitation wavelengths can be utilized as a light source due to the broad adsorption spectrum of the conjugated polymers. For example, sunlight, which provides clean, free, and inexhaustible energy, can work as an effective light source to induce the environmentally friendly locomotion of liquid marbles. To synthesize the PPy, PANI, and PEDOT particles, which can function as liquid marble stabilizers, perfluoroalkyl dopants should be doped to render the conjugated polymer particles hydrophobic. Considering the bioaccumulation and toxicity of perfluoroalkyl dopants, P3HT is a fascinating material, because it is hydrophobic enough to stabilize liquid marbles without the use of a perfluoroalkyl dopant.
The light-driven locomotion of liquid marbles enables the transport of liquid materials encapsulated within the liquid marbles and polymer particles can be used as liquid marble stabilizers. In addition, the on-demand and on-site release of the inner liquid materials by external stimuli can also be attained after/during the liquid marble locomotion. For this purpose, liquid marbles stabilized with a mixture of conjugated polymer particles and polymer particles that respond to other stimuli, such as pH (e.g., PDEA-PS particles) and temperature (e.g., PNIPAM particles), can be utilized [122]; in this case, liquid marbles can move via NIR laser irradiation and can be disrupted by tuning the pH/temperature. Furthermore, liquid marbles can be used as light-driven vehicles to carry loads (Fig. 8d, e). If liquid marbles are attached to floating objects (with centimeter sizes) via capillary forces and irradiated by NIR or sunlight, the floating objects (even those that are 150 times heavier than the liquid marbles) can be pushed or pulled on the planar air–water interface [122].
It is also possible to transfer liquid marbles between liquid and solid surfaces, which should drive the development of novel material transportation technologies (Fig. 8f) [124]. Light-induced Marangoni flow or an air stream can be utilized as propulsion forces to move liquid marbles on a planar water film surface. When the liquid marbles are close to the rim of the water film, they slide down the rim due to gravity and move onto the solid surface. High-speed camera observations indicate that liquid marbles move on water and solid surfaces by sliding and rolling modes, respectively. Interestingly, liquid marbles can be transferred back to a water surface from a solid surface by an air stream.
The ability of liquid marbles to transport, push/pull, transfer between liquid and solid surfaces, and release the inner liquid by stimuli, on demand, should make liquid marbles useful for a wide range of conceivable applications, including light-controlled drug delivery and microfluidics systems.
Conclusions and future research directions
The development of air–water soft dispersed systems whose stability, structure, and motion can respond to various stimuli should broaden the potential applications of these soft dispersed systems. Therefore, it is particularly crucial to develop a broad range of functional polymer particles that can function as effective stimulus-responsive stabilizers for soft dispersed systems. Synthetic biodegradable polymer particles and natural polymer particles produced from woods and grass should be attractive stabilizers that can disrupt the soft dispersion systems, in vivo and in nature. Polymer particles that can respond to specific molecules and biorelated polymer-based materials, including cells, bacteria, proteins, and viruses, can also be used as stimulus-responsive stabilizers. (Nano) Composites [133] and element-block polymers [134] have unique optical and magnetic properties, and are expected to add interesting functionality to soft dispersed systems if they are utilized in the form of particles and used as stabilizers. Molecularly imprinted polymers are interesting materials [135], as they can selectively absorb/adsorb and recover target molecules from the environment. Soft dispersed systems that are stabilized with molecularly imprinted polymer particles are expected to be applied as a key technology for water purification and ion flotation.
Until now, bubbles and foams that are responsive to external stimuli, such as magnetic fields [136,137,138] and mechanical stress [93], have been realized using inorganic particles. Liquid marbles whose stabilities and structures are responsive to various stimuli, including mechanical stress [139, 140], oxidizing agents [141], UV [142], specific molecules [143], and magnetic fields [52], as well as pH [144], temperature [145], and NIR [146], have also been developed using inorganic particles and small molecule powders. By combining/incorporating these functions of inorganic compounds and small molecules with/into the polymer particles, more sophisticated soft dispersed systems can be attained.
It is crucial to explicate the correlations between the size and shape of the particulate stabilizers and the formability, stability, structure, and stimulus-responsiveness of the soft dispersed systems [147,148,149,150,151]. Polymer particles are advantageous, as they can be synthesized and modified to have various sizes from a few tens of nanometers to a few millimeters, and the polymer particles can be prepared in many different shapes, including bowls, disks, rods, snowmen, octopuses, jellyfish, and worms [152,153,154]. Recently, studies have examined the effects of the size and shape of polymer particles on the formability and structure of soft dispersed systems [150, 155], and introducing stimulus-responsive character to these systems should expand their range of applications. Janus particles with two different surface chemistries are other interesting materials and will be used as stimulus-responsive particulate stabilizers in the future [156].
Future investigations will be conducted to develop stimulus-responsive modes. Stimuli-responsive shape change is an exciting modes that could be pursued using shape memory polymer particles [157]. Stimulus-driven locomotion (e.g., rotation, rising, and falling) are important modes to be controlled. It is also important for multiple stimuli-responsive systems to be developed in a predictable and controllable manner. Such particle-stabilized soft dispersed systems are particularly interesting, because stimuli-responsive controllable ranges could be widened. Precise control of the stable/unstable state transition of the soft dispersed systems by a narrow condition transition range is important and is expected to be attained by fully exploiting the strength of polymer chemistries. To construct and realize multiple stimuli-responsive soft dispersed systems, it is worth revisiting the methods used to apply stimuli to the systems. It is possible to apply a stimulus to the systems in a site-selective manner at a desired time if the sizes of the bubbles and liquid marbles are large enough (e.g., several hundreds of micrometers to the meter scale) for the stimulus to be applied in a small area. The simultaneous and time sequential applications of multiple stimuli to the same/different areas of a single bubble or liquid marble will be realized, which should ultimately contribute to the construction of lab-on-a-bubble/liquid marble systems. In addition, multiple stimuli-responsive soft dispersed systems whose motion and disruption timing can be tuned by an independent external stimulus would enable the development of material delivery and release systems. It is also important to develop multiple air–water soft dispersed systems (e.g., air-in-water-in-air and water-in-air-in-water systems), which should show multiple and stepwise responses.
Polymer particle-stabilized air–water soft dispersed systems can be used as platforms, opening the door for the development of novel functional soft materials. Capsules are attractive materials that can be fabricated from soft dispersed systems. PS particle-stabilized bubbles dispersed in a continuous water medium could be transformed into polymeric capsules via film formation of the PS particles at the bubble surfaces by the addition of dichloromethane and subsequent evaporation of the solvent (Fig. 9) [158]. In a similar manner, polymeric capsules can be prepared from liquid marbles stabilized with polymer particles via a solvent vapor treatment [83, 159]. Film formation can also be attained by heat annealing and subsequent cooling. Capsules fabricated from bubbles and liquid marbles that can be disrupted and can release their inner materials by application of an external stimulus, on demand, function as intelligent materials. Polymer film structures (characterized by various roughness and pore sizes) on the bubble and liquid marble surfaces can be controlled by varying the film formation conditions and it is possible to control the release kinetics of the inner materials (i.e., a digital on/off or a slow release manners).
a Schematic illustrating the fabrication of a polymeric microcapsule by solvent treatment of PS particle-stabilized bubbles in a water medium. b–e SEM images of the PS particle-stabilized bubbles (b, c) before and (d, e) after a dichloromethane solvent treatment. c, e Magnified images of the surfaces of the bubble and the microcapsule. Reproduced with permission [158]. Copyright 2015, The Chemical Society of Japan
There are many similarities between particle-stabilized air–water dispersed systems (bubbles/foams and liquid marbles) and particle-stabilized emulsions consisting of oils and water (Pickering emulsions), because air behaves as a highly hydrophobic oil. Based on this fact, the principles established in the oil-water soft dispersed systems can be applied to the stimulus-responsive air–water soft dispersed systems stabilized with polymer particles. The knowledge acquired in the liquid marble system can be leveraged for understanding the formability, stability, and structure of dry liquids [35].
Polymer particles with well-controlled sizes, shapes, and surface chemistries should be studied to gain deep insight into soft dispersed systems. Interdisciplinary research involving chemists, physicists, chemical engineers, and mathematician should play an important role in developing particle-stabilized gas–liquid soft dispersion science and engineering, and in proposing a wide range of academic and industrial applications.
References
Fitch RM. Polymer colloids: a comprehensive introduction. California: Academic Press Inc; 1997.
Lovell PA, El-Aasser MS. Emulsion polymerization and emulsion polymers. Chichester, UK: John Wiley and Sons; 1997.
Okubo M (ed.). Polymer particles. Part of the Advances in Polymer Science book series (POLYMER, volume 175), Berlin, Heidelberg: Springer; 2005. https://doi.org/10.1007/B14102.
Kawaguchi H. Functional polymer microspheres. Prog Polym Sci. 2000;25:1171–210. https://doi.org/10.1016/S0079-6700(00)00024-1.
Fujii S, Matsuzawa S, Nakamura Y, Ohtaka A, Teratani T, Akamatsu K, et al. Synthesis and characterization of polypyrrole-palladium nanocomposite-coated latex particles and their use as a catalyst for Suzuki coupling reaction in aqueous media. Langmuir. 2010;26:6230–9. https://doi.org/10.1021/la9039545.
Gokmen MT, Du Prez FE. Porous polymer particles-A comprehensive guide to synthesis, characterization, functionalization and applications. Prog Polym Sci. 2012;37:365–405. https://doi.org/10.1016/j.progpolymsci.2011.07.006.
Vanderhoff JW, El-Aasser MS, Micale FJ, Sudol ED, Tseng CM, Silwanowicz A, et al. Preparation of large-particle-size monodisperse latexes in space: Polymerization kinetics and process development. J Disper Sci Technol. 1984;5:231–46. https://doi.org/10.1080/01932698408943220.
Kinsyo T, Nakanishi H, Hirai K, Noda H, Takikawa T, Yahiro S. Development of polyester resin particles for toner with a controlled particle size distribution and shape. Polym J. 2017;49:593–600. https://doi.org/10.1038/pj.2017.25.
Ugelstad J, Berge A, Ellingsen T, Schmid R, Nilsen T-N, Mørk PC, et al. Preparation and application of new monosized polymer particles. Prog Polym Sci. 1992;17:87–161. https://doi.org/10.1016/0079-6700(92)90017-S.
Wiersma AE, vd Steeg LMA, Jongeling TJM. Waterborne core-shell dispersions based on intrinsically conducting polymers for coating applications. Synth Met. 1995;71:2269–70. https://doi.org/10.1016/0379-6779(94)03254-4.
Kao Co. Ltd. Japanese Patent No. H4-41410 (1992).
Fielding LA, Hillier JK, Burchell MJ, Armes SP. Space science applications for conducting polymer particles: synthetic mimics for cosmic dust and micrometeorites. Chem Commun. 2015;51:16886–99. https://doi.org/10.1039/C5CC07405C.
Okubo T. Polymer colloidal crystals. Prog Polym Sci 1993;18:481–517. https://doi.org/10.1016/0079-6700(93)90015-5.
Fudouzi H, Xia Y. Colloidal crystals with tunable colors and their use as photonic papers. Langmuir. 2003;19:9653–60. https://doi.org/10.1021/la034918q.
Iwasaki T, Tamai Y, Yamamoto M, Taniguchi T, Kishikawa K, Kohri M. Melanin precursor influence on structural colors from artificial melanin particles: PolyDOPA, polydopamine, and polynorepinephrine. Langmuir. 2018;34:11814–21. https://doi.org/10.1021/acs.langmuir.8b02444.
Louwet F, Clercq RD, Geudens J, Winter WD. Cross-linked homodisperse polymer particles. Des Monomers Polym. 1998;1:433–45. https://doi.org/10.1163/156855598X00251.
Fujii S, Wanless EJ, Yusa S, Webber GB, Ishida N. Stimulus-responsive soft surface/interface toward applications in adhesion, sensor and biomaterial. In: Hozumi A, Jiang L, Lee H, Shimomura M, editors. Stimuli-responsive dewetting/wetting smart surfaces and interfaces. Vol. 12, p. 287–397. Basel, Switzerland: Springer Nature Switzerland AG; 2018. https://doi.org/10.1007/978-3-319-92654-4.
Fu X, Hosta-Rigau L, Chandrawati R, Cui J. Multi-stimuli-responsive polymer particles, films, and hydrogels for drug delivery. Chem. 2018;4:2084–107. https://doi.org/10.1016/j.chempr.2018.07.002.
Lu C, Urban MW. Stimuli-responsive polymer nano-science: shape anisotropy, responsiveness, applications. Prog Polym Sci. 2018;78:24–46. https://doi.org/10.1016/j.progpolymsci.2017.07.005.
Ramsden W. Separation of solids in the surface-layers of solutions and ‘suspensions’ (observations on surface-membranes, bubbles, emulsions, and mechanical coagulation)-preliminary account. Proc R Soc Lond. 1904;72:156–64. https://doi.org/10.1098/rspl.1903.0034.
Pickering SU. CXCVI.-Emulsions. J Chem Soc Trans. 1907;91:2001–21. https://doi.org/10.1039/CT9079102001.
Binks BP, Horozov TS. Colloidal particles at liquid interfaces. Cambridge Univ. Press; 2006.
Tang J, Quinlan PJ, Tam KC. Stimuli-responsive Pickering emulsions: Recent advances and potential applications. Soft Matter. 2015;11:3512–29. https://doi.org/10.1039/C5SM00247H.
Wu J, Ma G-H. Recent studies of Pickering emulsions: particles make the difference. Small. 2016;12:4633–48. https://doi.org/10.1002/smll.201600877.
Binks BP. Particles as surfactants—similarities and differences. Curr Opin Colloid. 2002;7:21–41. https://doi.org/10.1016/S1359-0294(02)00008-0.
Velev OD, Furusawa K, Nagayama K. Assembly of latex particles by using emulsion droplets as templates. Microstructured hollow spheres. Langmuir. 1996;12:2374–84. https://doi.org/10.1021/la9506786.
Dinsmore AD, Hsu MF, Nikolaides MG, Marquez M, Bausch AR, Weitz DA. Colloidosomes: selectively permeable capsules composed of colloidal particles. Science. 2002;298:1006–9. https://doi.org/10.1126/science.1074868.
Studart AR, Gonzenbach UT, Tervoort E, Gauckler LJ. Processing routes to macroporous ceramics: a review. J Am Ceram Soc. 2006;89:1771–89. https://doi.org/10.1111/j.1551-2916.2006.01044.x.
Hunter TN, Pugh RJ, Franks GV, Jameson GJ. The role of particles in stabilising foams and emulsions. Adv Colloid Interface. 2008;137:57–81. https://doi.org/10.1016/j.cis.2007.07.007.
Stevenson P. Foam engineering: fundamentals and applications. New Jersey: John Wiley & Sons, Ltd; 2012. https://doi.org/10.1002/9781119954620.
Aussillous P, Quere D. Properties of liquid marbles. Proc R Soc A. 2006;462:973–99. https://doi.org/10.1098/rspa.2005.1581.
Bormashenko E. Liquid marbles, elastic nonstick droplets: From minireactors to self-propulsion. Langmuir. 2017;33:663–9. https://doi.org/10.1021/acs.langmuir.6b03231.
McHale G, Newton MI. Liquid marbles: topical context within soft matter and recent progress. Soft Matter. 2015;11:2530–46. https://doi.org/10.1039/C5SM00084J.
Binks BP, Murakami R. Phase inversion of particle-stabilized materials from foams to dry water. Nat Mater. 2006;5:865–9. https://doi.org/10.1038/nmat1757.
Kido K, Sumoto T, Yasui Y, Nakamura Y, Fujii S. Droplet size and morphology analyses of dry liquid. Adv Powder Technol. 2017;28:1977–81. https://doi.org/10.1016/j.apt.2017.04.027.
Timounay Y, Pitois O, Rouyer F. Gas marbles: much stronger than liquid marbles. Phys Rev Lett. 2017;118:228001. https://doi.org/10.1103/PhysRevLett.118.228001.
Garrett PR. Defoaming: Theory and industrial applications (Surfactant Science Book 45). New York: Marcel Dekker Inc.; 1993.
Prudhomme RK, Khan SA. Foams: theory, measurements and applications (Surfactant Science Book 57). New York: Marcel Dekker Inc.; 1997.
Murray BS. Stabilization of bubbles and foams. Curr Opin Colloid. 2007;12:232–41. https://doi.org/10.1016/j.cocis.2007.07.009.
Studart AR, Gonzenbach UT, Akartuna I, Tervoort E, Gauckler LJ. Materials from foams and emulsions stabilized by colloidal particles. J Mater Chem. 2007;17:3283–9. https://doi.org/10.1039/B703255B.
Bormashenko E, Musin A. Revealing of water surface pollution with liquid marbles. Appl Surf Sci. 2009;255:6429–31. https://doi.org/10.1016/j.apsusc.2009.02.027.
Tian J, Arbatan T, Li X, Shen W. Liquid marble for gas sensing. Chem Commun. 2010;46:4734–6. https://doi.org/10.1039/C001317J.
Sivan V, Shi-Yang T, O’Mullane AP, Petersen P, Eshtiaghi N, Kalantar-zadeh K, et al. Liquid metal marbles. Adv Funct Mater. 2012;23:144–52. https://doi.org/10.1002/adfm.201200837.
Lee HK, Lee YH, Phang IY, Wei J, Miao Y-E, Liu T, et al. Plasmonic liquid marbles: A miniature substrate-less SERS platform for quantitative and multiplex ultratrace molecular detection. Angew Chem Int Ed. 2014;53:5054–8. https://doi.org/10.1002/anie.201401026.
Tang X, Tang S-Y, Sivan V, Zhang W, Mitchell A, Kalantar-zadeh K, et al. Photochemically induced motion of liquid metal marbles. Appl Phys Lett. 2013;103:174104. https://doi.org/10.1063/1.4826923.
Tang S-Y, Sivan V, Khoshmanesh K, O’Mullane AP, Tang X, Gol B, et al. Electrochemically induced actuation of liquid metal marbles. Nanoscale. 2013;5:5949–57. https://doi.org/10.1039/c3nr00185g.
Zeng H, Zhao Y. Dynamic behavior of a liquid marble based accelerometer. Appl Phys Lett. 2010;96:114104. https://doi.org/10.1063/1.3367704.
Newton MI, Herbertson DL, Elliott SJ, Shirtcliffe NJ, McHale G. Electrowetting of liquid marbles. J Phys D Appl Phys. 2007;40:20–4. https://doi.org/10.1088/0022-3727/40/1/S04.
Brown CV, Al-Shabib W, Wells GG, McHale G, Newton MI. Amplitude scaling of a static wrinkle at an oil-air interface created by dielectrophoresis forces. Appl Phys Lett. 2010;97:242904. https://doi.org/10.1063/1.3525708.
Bormashenko E, Pogreb R, Bormashenko Y, Musin A, Stein T. New investigations on ferrofluidics: ferrofluidic marbles and magnetic-field-driven drops on superhydrophobic surfaces. Langmuir. 2008;24:12119–22. https://doi.org/10.1021/la802355y.
Dorvee JR, Sailor MJ, Miskelly GM. Digital microfluidics and delivery of molecular payloads with magnetic porous silicon chaperones. Dalton T. 2008;6:721–30. https://doi.org/10.1039/B714594B.
Xue Y, Wang H, Zhao Y, Dai L, Feng L, Wang X, et al. Magnetic liquid marbles: a “precise” miniature reactor. Adv Mater. 2010;22:4814–8. https://doi.org/10.1002/adma.201001898.
Arbatan T, Al-Abboodi A, Sarvi F, Chan PPY, Shen W. Tumor inside a pearl drop. Adv Healthc Mater. 2012;1:467–9. https://doi.org/10.1002/adhm.201200050.
Sato E, Yuri M, Nishiyama T, Horibe H, Fujii S, Nakamura Y. Liquid marbles as a micro-reactor for efficient radical alternating copolymerization of diene monomer and oxygen. Chem Commun. 2015;51:17241–4. https://doi.org/10.1039/C5CC07421E.
Sato E, Yuri M, Fujii S, Nishiyama T, Nakamura Y, Horibe H. Liquid marble containing degradable polyperoxides for adhesion force-changeable pressure-sensitive adhesives. RSC Adv. 2016;6:56475–81. https://doi.org/10.1039/C6RA10677C.
Lahanas KM, Vrabie N, Santos E, Miklean S. Powder to liquid compositions. US Patent 6290941 B1; 2001.
Dampeirou C. Hydrophobic silica-based powder containing xylitol or trehalose and water for base of cosmetics. French patent FR2860435A1; 2005.
Arbatan T, Li L, Tian J, Shen W. Microreactors: liquid marbles as micro-bioreactors for rapid blood typing. Adv Health Mater. 2012;1:80–3. https://doi.org/10.1002/adhm.201290002.
McHale G. All solids, including teflon, are hydrophilic (to some extent), but some have roughness induced hydrophobic tendencies. Langmuir. 2009;25:7185–7. https://doi.org/10.1021/la900597a.
Levine S, Bowen BD, Partridge SJ. Stabilization of emulsions by fine particles I. Partitioning of particles between continuous phase and oil/water interface. Colloid Surf. 1989;38:325–43. https://doi.org/10.1016/0166-6622(89)80271-9.
Binks BP, Murakami R, Armes SP, Fujii S, Schmid A. pH-Responsive aqueous foams stabilized by ionizable latex particles. Langmuir. 2007;23:8691–4. https://doi.org/10.1021/la700444a.
Inoue M, Fujii S, Nakamura Y, Iwasaki Y, Yusa S. pH-Responsive disruption of ‘liquid marbles’ prepared from water and poly(6-(acrylamido) hexanoic acid)-grafted silica particles. Polym J. 2011;43:778–84. https://doi.org/10.1038/pj.2011.55.
Dupin D, Thompson KL, Armes SP. Preparation of stimulus-responsive liquid marbles using a polyacid-stabilised polystyrene latex. Soft Matter. 2011;7:6797–800. https://doi.org/10.1039/C1SM05889D.
Sun J, Wei W, Zhao D, Hu Q, Liu X. Liquid marbles prepared from pH-responsive self-assembled micelles. Soft Matter. 2015;11:1954–61. https://doi.org/10.1039/C4SM02832E.
Ito M, Takano K, Hanochi H, Asaumi Y, Yusa S, Nakamura Y, et al. pH-responsive aqueous bubbles stabilized with polymer particles carrying poly(4-vinylpyridine) colloidal stabilizer. Front Chem. 2018;6:269. https://doi.org/10.3389/fchem.2018.00269.
Dupin D, Howse JR, Armes SP, Randall DP. Preparation of stable foams using sterically stabilized pH-responsive latexes synthesized by emulsion polymerization. J Mater Chem. 2008;18:545–52. https://doi.org/10.1039/B714261G.
Fujii S, Kameyama S, Dupin D, Armes SP, Suzaki M, Nakamura Y. pH-Responsive liquid marbles stabilized with poly(2-vinylpyridine) particles. Soft Matter. 2010;6:635–40. https://doi.org/10.1039/B914997J.
Halperin A, Kröeger M, Winnik FM. Poly(N-isopropylacrylamide) phase diagrams: fifty years of research. Angew Chem Int Ed. 2015;54:15342–67. https://doi.org/10.1002/anie.201506663.
Horiguchi Y, Kawakita H, Ohto K, Morisada S. Temperature-responsive Pickering foams stabilized by poly(N-isopropylacrylamide) nanogels. Adv Powder Technol. 2018;29:266–72. https://doi.org/10.1016/j.apt.2017.11.010.
Yusa S, Morihara M, Nakai K, Fujii S, Nakamura Y, Maruyama A, et al. Thermo-responsive liquid marbles. Polym J. 2014;46:145–8. https://doi.org/10.1038/pj.2013.84.
Matsumoto T, Nakamae K, Okubo M, Sue M, Shimao M, Komura M. Kanonseigyousyuuzainiyoru hedoronogyousyuu. Kobunshi Ronbunshu. 1974;31:669–75. https://doi.org/10.1295/koron.31.669.
Bütün V, Armes SP, Billingham NC. Synthesis and aqueous solution properties of near-monodisperse tertiary amine methacrylate homopolymers and diblock copolymers. Polymer. 2001;42:5993–6008. https://doi.org/10.1016/S0032-3861(01)00066-0.
Fujii S, Kakigi Y, Suzaki M, Yusa S, Muraoka M, Nakamura Y. Synthesis of stimuli-responsive macroazoinitiators and their use as an inistab toward hairy polymer latex particles. J Polym Sci. 2009;47:3431–43. https://doi.org/10.1002/pola.23424.
Schmalz A, Hanisch M, Schmalz H, Müller AHE. Double stimuli-responsive behavior of linear and star-shaped poly(N,N-diethylaminoethyl methacrylate) in aqueous solution. Polymer. 2010;51:1213–7. https://doi.org/10.1016/j.polymer.2009.11.023.
Thavanesan T, Herbert C, Plamper FA. Insight in the phase separation peculiarities of poly(dialkylaminoethyl methacrylate)s. Langmuir. 2014;30:5609–19. https://doi.org/10.1021/la5007583.
Fujii S, Akiyama K, Nakayama S, Hamasaki S, Yusa S, Nakamura Y. pH- and temperature-responsive aqueous foams stabilized by hairy latex particles. Soft Matter. 2015;11:572–9. https://doi.org/10.1039/C4SM02236J.
Fujii S, Mochizuki M, Aono K, Hamasaki S, Murakami R, Nakamura Y. pH-Responsive aqueous foams stabilized by hairy latex particles. Langmuir. 2011;27:12902–9. https://doi.org/10.1021/la203062b.
Nakayama S, Yusa S, Nakamura Y, Fujii S. Aqueous foams stabilized by temperature-sensitive hairy polymer particles. Soft Matter. 2015;11:9099–106. https://doi.org/10.1039/C5SM02187.
Nakayama S, Hamasaki S, Ueno K, Mochizuki M, Yusa S, Nakamura Y, et al. Foams stabilized with solid particles carrying stimuli-responsive polymer hairs. Soft Matter. 2016;12:4794–804. https://doi.org/10.1039/C6SM00425C.
Dupin D, Armes SP, Fujii S. Stimulus-responsive liquid marbles. J Am Chem Soc. 2009;131:5386–7. https://doi.org/10.1021/ja901641v.
Fujii S, Suzaki M, Armes SP, Dupin D, Hamasaki S, Aono K, et al. Liquid marbles prepared from pH-responsive sterically-stabilized latex particles. Langmuir. 2011;27:8067–74. https://doi.org/10.1021/la201317b.
Fujii S, Aono K, Suzaki M, Hamasaki S, Yusa S, Nakamura Y. pH-Responsive hairy particles synthesized by dispersion polymerization with a macroinitiator as an inistab and their use as a gas-sensitive liquid marble stabilizer. Macromolecules. 2012;45:2863–73. https://doi.org/10.1021/ma300048m.
Ueno K, Hamasaki S, Wanless EJ, Nakamura Y, Fujii S. Microcapsules fabricated from liquid marbles stabilized with latex particles. Langmuir. 2014;30:3051–9. https://doi.org/10.1021/la5003435.
Ueno K, Bournival G, Wanless EJ, Nakayama S, Giakoumatos EC, Nakamura Y, et al. Liquid marble and water droplet interactions and stability. Soft Matter. 2015;11:7728–38. https://doi.org/10.1039/C5SM01584G.
Kido K, Ireland PM, Sekido T, Wanless EJ, Webber GB, Nakamura Y, et al. Formation of liquid marbles using pH-responsive particles: rolling vs electrostatic methods. Langmuir. 2018;34:4970–9. https://doi.org/10.1021/acs.langmuir.7b04204.
Bertrand O, Gohy J-F. Photo-responsive polymers: synthesis and applications. Polym Chem. 2017;8:52–73. https://doi.org/10.1039/C6PY01082B.
Cui J, Del Campo A. Photo-responsive polymers: properties, synthesis and applications. In: Aguilar MR, Román JS, editors. Smart polymers and their applications. Sawston, Cambridge, UK: Woodhead Publishing; 2014, p. 93–133.
Zhao Y, Ikeda T. Smart light-responsive materials: Azobenzene-containing polymers and liquid crystals. 1st ed. New Jersey: Wiley-Interscience; 2009.
Toyoda T, Nakamura H. Photoacoustic spectroscopy of polyaniline films. Jpn J Appl Phys. 1995;34:2907–10.
Wu C-Y, Benatar A. Microwave welding of high density polyethylene using intrinsically conductive polyaniline. Polym Eng Sci. 1997;37:738–43. https://doi.org/10.1002/pen.11717.
Urban D, Takamura K. Polymer dispersions and their industrial applications. Weinheim: Wiley-VCH; 2002. https://doi.org/10.1002/pi.1257.
Tumarkin E, Park JI, Nie Z, Kumacheva E. Temperature mediated generation of armoured bubbles. Chem Commun. 2011;47:12712–4. https://doi.org/10.1039/c1cc14545b.
Subramaniam AB, Abkarian M, Mahadevan L, Stone HA. Mechanics of interfacial composite materials. Langmuir. 2006;22:10204–8. https://doi.org/10.1021/la061475s.
Fameau A-L, Adrian C, Saint-Jalmes A, Klitzing RV. Responsive aqueous foams. ChemPhysChem. 2015;16:66–75. https://doi.org/10.1002/cphc.201402580.
Fujii S, Nakamura Y. Stimuli-responsive bubbles and foams stabilized with solid particles. Langmuir. 2017;33:7365–79. https://doi.org/10.1021/acs.langmuir.7b01024.
Fujii S, Suzaki M, Nakamura Y, Sakai K, Ishida N, Bigg S. Surface characterization of nanoparticles carrying pH-responsive polymer hair. Polymer. 2010;51:6240–7. https://doi.org/10.1016/j.polymer.2010.10.041.
Kettlewell SL, Schmid A, Fujii S, Dupin D, Armes SP. Is latex surface charge an important parameter for foam stabilization? Langmuir. 2007;23:11381–6. https://doi.org/10.1021/la702099t.
Mathew MD, Manga MS, Hunter TN, Cayre OJ, Biggs S. Behavior of pH-sensitive core shell particles at the air–water interface. Langmuir. 2012;28:5085–92. https://doi.org/10.1021/la2048568.
Fujii S, Mouri E, Akiyama K, Nakayama S, Uda K, Nakamura Y, et al. pH-Sensitive adsorption behavior of polymer particles at the air-water interface. Langmuir. 2017;33:1451–9. https://doi.org/10.1021/acs.langmuir.6b03895.
Cohin Y, Fisson M, Jourde K, Fuller GG, Sanson N, Talini L, et al. Tracking the interfacial dynamics of PNiPAM soft microgels particles adsorbed at the air–water interface and in thin liquid films. Rheol Acta. 2013;52:445–54. https://doi.org/10.1007/s00397-013-0697-3.
McHale G, Shirtcliffe NJ, Newton MI, Pyatt FB, Doerr SH. Self-organization of hydrophobic soil and granular surfaces. Appl Phys Lett. 2007;90:054110. https://doi.org/10.1063/1.2435594.
Pike N, Richard D, Foster W, Mahadevan L. How aphids lose their marbles. P R Soc B Biol Sci. 2002;269:1211–5. https://doi.org/10.1098/rspb.2002.1999.
Kasahara M, Akimoto S, Hariyama T, Takaku Y, Yusa S, Okada S, et al. Liquid marbles in nature: Craft of aphids for survival. Langmuir. 2019;35:6169–78. https://doi.org/10.1021/acs.langmuir.9b00771.
Eshtiaghi N, Hapgood KP. A quantitative framework for the formation of liquid marbles and hollow granules from hydrophobic powders. Powder Technol. 2012;223:65–76. https://doi.org/10.1016/j.powtec.2011.05.007.
Ireland PM, Noda M, Jarrett ED, Fujii S, Nakamura Y, Wanless EJ, et al. Electrostatic formation of liquid marbles - influence of drop and particle size. Powder Technol. 2016;303:55–58. https://doi.org/10.1016/j.powtec.2016.08.036.
Ireland PM, Webber GB, Fujii S, Thomas CA, Lobel BT, Wanless EJ. An electrostatic method for manufacturing liquid marbles and particle-stabilized aggregates. Front Chem. 2018;6:280. https://doi.org/10.3389/fchem.2018.00280.
Thomas CA, Kido K, Kawashima H, Fujii S, Ireland PM, Webber GB, et al. Electrostatic formation of polymer particle stabilised liquid marbles and metastable droplets - Effect of latex shell conductivity. J Colloid Interf Sci. 2018;529:486–95. https://doi.org/10.1016/j.jcis.2018.04.044.
Ireland PM, Kido K, Webber GB, Fujii S, Wanless EJ. pH-Responsive particle-liquid aggregates - electrostatic formation kinetics. Front Chem. 2018;6:215. https://doi.org/10.3389/fchem.2018.00215.
Fujii S, Yusa S, Nakamura Y. Stimuli-responsive liquid marbles: Controlling structure, shape, stability, and motion. Adv Funct Mater. 2016;26:7206–23. https://doi.org/10.1002/adfm.201603223.
Sun G, Sheng Y, Wu J, Ma G, Ngai T. Liquid marbles stabilized by charged polymer latexes: How does the drying of the latex particles affect the properties of liquid marbles? Langmuir. 2014;30:12503–8. https://doi.org/10.1021/la503105c.
Sekido T, Kappl M, Butt H-J, Yusa S, Nakamura Y, Fujii S. Effects of pH on structure and mechanical properties of dried pH-responsive latex particles. Soft Matter. 2017;13:7562–70. https://doi.org/10.1039/C7SM01625E.
Sekido T, Wooh S, Fuchs R, Kappl M, Nakamura Y, Butt H-J, et al. Controlling the structure of supraballs by pH-responsive particle assembly. Langmuir. 2017;33:1995–2002. https://doi.org/10.1021/acs.langmuir.6b04648.
Saitoh T, Ohyama T, Sakurai T, Kaise T, Takamura K, Suzuki Y, et al. Polymer-mediated extraction of 8-quinolinolato metal chelates for the determination of metal ions in water with graphite furnace atomic absorption spectrometry. Talanta. 1998;46:541–50. https://doi.org/10.1016/S0039-9140(97)00316-0.
Kawata Y, Thomas CA, Asaumi Y, Hanochi H, Ireland PM, Fujii S, et al. Electrostatic formation of liquid marbles using thermo-responsive polymer-coated particles. Chem Lett. 2019;48:578–81. https://doi.org/10.1246/cl.190105.
Cortez-Lemus NA, Licea-Claverie A. Poly(N-vinylcaprolactam), a comprehensive review on a thermoresponsive polymer becoming popular. Prog Polym Sci. 2016;53:1–51. https://doi.org/10.1016/j.progpolymsci.2015.08.001.
Yannic B, Schuetz YB, Gurny R, Jordan O. A novel thermoresponsive hydrogel based on chitosan. Eur J Pharm Biopharm. 2008;68:19–25. https://doi.org/10.1016/j.ejpb.2007.06.020.
Seuring J, Agarwal S. Non-ionic homo- and copolymers with H-donor and H-acceptor units with an UCST in water. Macromol Chem Physic. 2010;211:2109. https://doi.org/10.1002/macp.201000147.
Glatzel S, Laschewsky A, Lutz J-F. Well-defined uncharged polymers with a sharp UCST in water and in physiological milieu. Macromolecules. 2011;44:413–5. https://doi.org/10.1021/ma102677k.
Shimada N, Ino H, Maie K, Nakayama M, Kano A, Maruyama A. Ureido-derivatized polymers based on both poly(allylurea) and poly(L-citrulline) exhibit UCST-type phase transition behavior under physiologically relevant conditions. Biomacromolecules. 2011;12:3418–22. https://doi.org/10.1021/bm2010752.
Bormashenko E, Bormashenko Y, Grynyov R, Aharoni H, Whyman G, Binks BP. Self-propulsion of liquid marbles: Leidenfrost-like levitation driven by Marangoni flow. J Phys Chem C. 2015;119:9910–5. https://doi.org/10.1021/acs.jpcc.5b01307.
Ooi CH, Nguyen Av, Evans GM, Gendelman O, Bormashenko E, Nguyen N-T. A floating self-propelling liquid marble containing aqueous ethanol solutions. RSC Adv. 2015;5:101006–12. https://doi.org/10.1039/C5RA23946J.
Paven M, Mayama H, Sekido T, Butt HJ, Nakamura Y, Fujii S. Light-driven delivery and release of materials using liquid marbles. Adv Funct Mater. 2016;26:3199–206. https://doi.org/10.1002/adfm.201600034.
Kawashima H, Mayama H, Nakamura Y, Fujii S. Hydrophobic polypyrroles synthesized by aqueous chemical oxidative polymerization and their use as light-responsive liquid marble stabilizers. Polym Chem. 2017;8:2609–18. https://doi.org/10.1039/C7PY00158D.
Kawashima H, Nakamura Y, Fujii S, Paven M, Butt HJ, Mayama H. Transfer of materials from water to solid surfaces using liquid marbles. ACS Appl Mater Interfaces. 2017;9:33351–9. https://doi.org/10.1021/acsami.7b11375.
Kawashima H, Okatani R, Mayama H, Nakamura Y, Fujii S. Synthesis of hydrophobic polyanilines as a light-responsive liquid marble stabilizer. Polymer. 2018;148:217–27. https://doi.org/10.1016/j.polymer.2018.06.039.
Shimogama N, Uda M, Oyama K, Hanochi H, Hirai T, Nakamura Y, et al. Hydrophobic poly(3,4-ethylenedioxythiophene) particles synthesized by aqueous oxidative coupling polymerization and their use as near-infrared-responsive liquid marble stabilizer. Polym J. (2019) https://doi.org/10.1038/s41428-019-0189-0.
Inoue H, Hirai T, Hanochi H, Oyama K, Mayama H, Nakamura Y, et al. Poly(3-hexylthiophene) grains synthesized by solvent-free oxidative coupling polymerization and their use as light-responsive liquid marble stabilizer. Macromolecules. 2019;52:708–17. https://doi.org/10.1021/acs.macromol.8b02426.
Fujii S, Mayama H. Delivery and release of materials based on liquid marble engineering. Acc Mater Surf Res. 2019;4:61–8.
Xu L, Cheng L, Wang C, Peng R, Liu Z. Conjugated polymers for photothermal therapy of cancer. Polym Chem. 2014;5:1573–80. https://doi.org/10.1039/C3PY01196H.
Sun H, Lv F, Liu L, Gu Q, Wang S. Conjugated polymer materials for photothermal therapy. Adv Ther. 2018;1:1800057. https://doi.org/10.1002/adtp.201800057.
Inoue H, Shimogama N, Mukai S, Higashimoto S, Hirai T, Nakamura Y, et al. Poly(3,4-ethylenedioxythiophene) grains synthesized by solvent-free chemical oxidative polymerization. Chem Lett. 2019. https://doi.org/10.1246/cl.190350.
Kawashima H, Shioi A, Archer RJ, Ebbens SJ, Nakamura Y, Fujii S. Light-driven locomotion of a centimeter-sized object at the air-water interface: effect of fluid resistance. RSC Adv. 2019;9:8333–9. https://doi.org/10.1039/C9RA01417A.
Balmer JA, Schmid A, Armes SP. Colloidal nanocomposite particles: quo vadis? J Mater Chem. 2008;18:5722–30. https://doi.org/10.1039/b805764h.
Chujo Y, Tanaka K. New polymeric materials based on element-blocks. B Chem Soc Jpn. 2015;88:633–43. https://doi.org/10.1246/bcsj.20150081.
BelBruno JJ. Molecularly imprinted polymers. Chem Rev. 2019;1191:94–119. https://doi.org/10.1021/acs.chemrev.8b00171.
Soetanto K, Watarai H. Development of magnetic microbubbles for drug delivery system (DDS). Jpn J Appl Phys. 2000;39:3230–2. https://doi.org/10.1143/JJAP.39.3230.
Campbell AL, Stoyanov SD, Paunov VN. Fabrication of functional anisotropic food-grade micro-rods with micro-particle inclusions with potential application for enhanced stability of food foams. Soft Matter. 2009;5:1019–23. https://doi.org/10.1039/B812706A.
Rodrigues JA, Rio E, Bobroff J, Langevin D, Drenckhan W. Generation and manipulation of bubbles and foams stabilised by magnetic nanoparticles. Colloid Surf A. 2011;384:408–16. https://doi.org/10.1016/j.colsurfa.2011.04.029.
Fujii S, Sawada S, Nakayama S, Kappl M, Ueno K, Shitajima K, et al. Pressure-sensitive adhesive powder. Mater Horiz. 2016;3:47–52. https://doi.org/10.1039/C5MH00203F.
Salehabad SM, Azizian S, Fujii S. Shape-designable liquid marbles stabilized by gel layer. Langmuir. 2019;35:8950–60. https://doi.org/10.1021/acs.langmuir.9b01473.
Ohshio M, Iimura K, Fujii S, Nakamura Y & Yusa S. Oxidation-responsive liquid marbles. Chem Lett. 2019. https://doi.org/10.1246/cl.190148.
Nakai K, Fujii S, Nakamura Y, Yusa S. Ultraviolet-light-responsive liquid marbles. Chem Lett. 2013;42:586–8. https://doi.org/10.1246/cl.130119.
Kozuka S, Banno T, Fujii S, Nakamura Y, Yusa S. Disruption of liquid marbles induced by host-guest interaction. Chem Lett. 2019. https://doi.org/10.1246/cl.190232.
Ohno S, Tsuda Y, Nakai K, Fujii S, Nakamura Y, Yusa S. pH-responsive liquid marbles prepared using fluorinated fatty acid. Chem Lett. 2016;45:547–9. https://doi.org/10.1246/cl.160056.
Nakai K, Fujii S, Nakamura Y, Yusa S. Thermoresponsive liquid marbles prepared with low melting point powder. Chem Lett. 2015;44:1077–9. https://doi.org/10.1246/cl.150381.
Nakai K, Nakagawa H, Kuroda K, Fujii S, Nakamura Y, Yusa S. Near-infrared-responsive liquid marbles stabilized with carbon nanotubes. Chem Lett. 2013;42:719–21. https://doi.org/10.1246/cl.130240.
Fujii S, Ryan AJ, Armes SP. Long-range structural order, moiré patterns, and iridescence in latex-stabilized foams. J Am Chem Soc. 2006;128:7882–6. https://doi.org/10.1021/ja060640n.
Fujii S, Iddon PD, Ryan AJ, Armes SP. Aqueous particulate foams stabilized solely with polymer latex particles. Langmuir. 2006;22:7512–20. https://doi.org/10.1021/la060812u.
Fukuoka K, Tomikawa A, Nakamura Y, Fujii S. Aqueous foams stabilized with several tens of micrometer-sized polymer particles: effects of surface hydrophilic-hydrophobic balance on foamability and foam stability. Chem Lett. 2016;45:667–9. https://doi.org/10.1246/cl.160182.
Geyer F, Asaumi Y, Vollmer D, Butt H-J, Nakamura Y, Fujii S. Polyhedral liquid marbles. Adv Funct Mater. 2019;29:1808826. https://doi.org/10.1002/adfm.201808826.
Azizian S, Fujii S, Kasahara M, Butt H-J, Kappl M. Effect of particle morphology on mechanical properties of liquid marbles. Adv Powder Technol. 2019;30:330–5. https://doi.org/10.1016/j.apt.2018.11.010.
Warren NJ, Armes SP. Polymerization-induced self-assembly of block copolymer nano-objects via RAFT aqueous dispersion polymerization. J Am Chem Soc. 2014;136:10174–85. https://doi.org/10.1021/ja502843f.
Sacanna S, Korpics M, Rodriguez K, Colón-Meléndez L, Kim S-H, Pine DJ, et al. Shaping colloids for self-assembly. Nat Commun. 2013;4:1688. https://doi.org/10.1038/ncomms2694.
Fujibayashi T, Okubo M. Preparation and thermodynamic stability of micron-sized, monodisperse composite polymer particles of disc-like shapes by seeded dispersion polymerization. Langmuir. 2007;23:7958–62. https://doi.org/10.1021/la7007842.
Zhou W, Cao J, Liu W, Stoyanov S. How rigid rods self-assemble at curved surfaces. Angew Chem Int Ed. 2009;48:378–81. https://doi.org/10.1002/anie.200804194.
Fujii S, Yokoyama Y, Nakayama S, Ito M, Yusa S, Nakamura Y. Gas bubbles stabilized by Janus particles with varying hydrophilic-hydrophobic surface characteristics. Langmuir. 2018;34:933–42. https://doi.org/10.1021/acs.langmuir.7b02670.
Mather PT, Luo X, Rousseau IA. Shape memory polymer research. Annu Rev Mater Res. 2009;39:445–71. https://doi.org/10.1146/annurev-matsci-082908-145419.
Nakayama S, Fukuhara K, Nakamura Y, Fujii S. Hollow microspheres fabricated from aqueous bubbles stabilized with latex particles. Chem Lett. 2015;44:773–5. https://doi.org/10.1246/cl.150161.
Matsukuma D, Watanabe H, Minn M, Fujimoto A, Shinohara T, Jinnai H, et al. Preparation of poly(lactic-acid)-particle stabilized liquid marble and the improvement of its stability by uniform shell formation through solvent vapor exposure. RSC Adv. 2013;3:7862–6. https://doi.org/10.1039/C3RA40693H.
Acknowledgements
I thank all the current and former students in my research group and many collaborators, both in academia and in industry. This work was supported by JSPS-DAAD (Germany) and JSPS-OP (Australia) Bilateral Joint Research Projects, and by Grant-in-Aid for Scientific Research (B) (JSPS KAKENHI Grant Number JP16H04207) and Scientific Research on Innovative Areas “Engineering Neo-Biomimetics (No. 4402)” (JSPS KAKENHI Grant Numbers JP15H01602 and JP25120511), “New Polymeric Materials Based on Element-Blocks (No. 2401)” (JSPS KAKENHI Grant Numbers JP15H00767 and JP25102542), and “Molecular Soft Interface Science (No. 2005)” (JSPS KAKENHI Grant Number 23106720).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fujii, S. Stimulus-responsive soft dispersed systems developed based on functional polymer particles: bubbles and liquid marbles. Polym J 51, 1081–1101 (2019). https://doi.org/10.1038/s41428-019-0233-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41428-019-0233-0
- Springer Nature Limited
This article is cited by
-
Morphological and chemical stabilities of polypyrrole in aqueous media for 1 year
Polymer Journal (2022)
-
Dodecyl sulfate-doped polypyrrole derivative grains as a light-responsive liquid marble stabilizer
Polymer Journal (2020)