Abstract
TNF is a potent cytokine known for its involvement in physiology and pathology. In Rheumatoid Arthritis (RA), persistent TNF signals cause aberrant activation of synovial fibroblasts (SFs), the resident cells crucially involved in the inflammatory and destructive responses of the affected synovial membrane. However, the molecular switches that control the pathogenic activation of SFs remain poorly defined. Cyld is a major component of deubiquitination (DUB) machinery regulating the signaling responses towards survival/inflammation and programmed necrosis that induced by cytokines, growth factors and microbial products. Herein, we follow functional genetic approaches to understand how Cyld affects arthritogenic TNF signaling in SFs. We demonstrate that in spontaneous and induced RA models, SF-Cyld DUB deficiency deteriorates arthritic phenotypes due to increased levels of chemokines, adhesion receptors and bone-degrading enzymes generated by mutant SFs. Mechanistically, Cyld serves to restrict the TNF-induced hyperactivation of SFs by limiting Tak1-mediated signaling, and, therefore, leading to supervised NF-κB and JNK activity. However, Cyld is not critically involved in the regulation of TNF-induced death of SFs. Our results identify SF-Cyld as a regulator of TNF-mediated arthritis and inform the signaling landscape underpinning the SF responses.
Similar content being viewed by others
Introduction
Rheumatoid Arthritis (RA) is a complex inflammatory disease irreversibly affecting synovial joints. The thin synovial membrane is inflamed, gradually reverted into a hyperplastic and invasive tissue mass namely pannus, which causes joint destruction. The synovial fibroblasts (SFs), a major mesenchymal cell type populating synovium, actively participate in the pannus by exhibiting a proinflammatory profile with migratory and tissue-invading properties leading the tissue destruction [1].
We have previously shown that SFs exclusively receive the pathogenic load of TNF to orchestrate the arthritic disease in modeled arthritis, such as the hTNFTg and TNFΔARE models or Collagen Antibody Induced Arthritis (CAIA) [2, 3]. Transcriptomic and epigenomic analysis of the hTNFTg SFs highlighted NF-κB as a major component of inflammatory arthritic process [4]. In concert with this finding, the SF-specific IKK2 targeting in the hTNFTg mouse uncoupled the function of IKK2 in both TNF-induced NF-κB and death responses in vivo, highlighting the complexity of signaling events under chronic inflammatory conditions [2]. Interestingly, accumulating evidence indicates several molecular checkpoints that regulate the formation of Complexes I and II in the TNF signaling pathway leading towards survival/inflammatory signals or cell death respectively [5].
Ubiquitination is a posttranslational modification that is implicated in the regulation of signaling pathways by tagging ubiquitin (Ub) via its C terminus to a target protein directing it either for degradation or becoming scaffolds. Ub chains is formed by constitutive attachment of Ub to either of seven different lysine (K) residues (K6, K11, K27, K29, K33, K48, K63) or the N-terminal methionine (M1, Met1, linear chains) [6]. Remodeling of Ub loads of proteins is performed by specific Ub ligases or deubiquitinases (DUBs). The most well-studied types of Ub chains are the K48, which predispose to proteosomal degradation, the K63, which affects activation status of proteins, and the M1 which mainly contributes to scaffolding properties of proteins [7]. The Ub-editing enzymes A20 (encoded by Tnfaip3), Otulin and Cylindromatosis (Cyld) are critical components with DUB activity modifying cytokine- and growth factor-induced signaling pathways [8]. Multiple studies causally linked mutations in A20 with several inflammatory conditions, including arthritic diseases [9]. More recently, it was shown that mutations of Otulin, a DUB that removes M1 Ub chains, are associated to an autoinflammatory syndrome in humans and lethal poly-inflammatory consequences in mice [10,11,12,13,14]. CYLD has been originally described as a tumor suppressor gene mutated in familial cylindromatosis, predisposing to the development of cancerous lesions in the skin [15,16,17]. The majority of detected CYLD mutations affect either the DUB activity or its expression levels [18]. Remarkably, genome-wide association studies additionally implicated CYLD polymorphisms in inflammatory diseases [19]. Functional genetic approaches revealed that mutated Cyld is associated not only to cancerous conditions but also to immune homeostasis and inflammation [8, 20]. By removing K63 and M1 Ub- chains, CYLD regulates several signaling pathways [16, 17, 21] acting downstream of TNF, TGFbeta, Wnt/β-catenin, Hippo, Notch signals such as c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (p38 MAPK)), nuclear factor-кB (NF-κB) [22,23,24,25,26]. Recently, CYLD had been also exhibited to function as another checkpoint that prevent RIPK1-dependent programmed necrosis induced by TNF [27].
Accumulating evidence strongly suggests that the output of CYLD is stimuli- and cell-context dependent. Even in the absence of mutations or polymorphisms, downregulation of CYLD has been detected in affected tissues of debilitating diseases such as NASH [28] or inflammatory arthritis [29]. In arthritic synovium, low levels of CYLD are inversely correlated with the NF-κB activity in RASFs [29]. However, mechanistic studies on the effects of SF-specific CYLD knockdown or loss of DUB activity in vivo remains underexplored. In this study, we pursued το decouple the CYLD involvement in the inflammatory behavior of SFs, by employing both acute and chronic TNF-dependent modeled arthritis, coupled with genetic targeting and biochemical studies on DUB-deficient Cyld SFs.
Results
TNF-mediated arthritis is deteriorated by mesenchymal Cyld-DUB deficiency
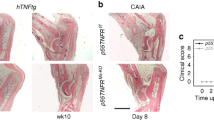
To study the role of Cyld in SFs we generated mice carrying a mesenchymal-specific homozygous deletion of Cyld exon 9, by crossing mice containing floxed exon 9 of Cyld [30] with Col6a1-Cre transgenic mice [3]. Exon 9 deletion results in the expression of a truncated form of Cyld which lacks DUB activity but retains domains interacting with TRAF2 or NEMO [30]. The Col6a1-Cre Cyld f/f mice (from herein referred as CyldM-Δ9/Δ9) were viable and fertile. The mice appeared normal in development with no apparent growth defects even at 12 months of age (data not shown). To evaluate the mesenchymal-specific contribution of Cyld in regulating the TNF-modeled arthritis, we generated mice of the hTNFTg CyldM-Δ9/Δ9 genotype. The mice were viable and born in normal mendelian frequency. However, their growth was evidently retarded as early as 4 weeks of age, compared to non-Cre littermate controls (Fig. 1a). The gross observation of the mice at the age of 6 weeks revealed excessive swelling and redness of ankle joints (Fig. 1b) and deformity of wrinkle joints (Fig. 1c).
a Gross appearance and the corresponding b hindpaws and c forepaws of Cyldf/f, hTNFTg Cyldf/f and hTNFTg CyldM-Δ9/Δ9 mice at 6 weeks of age. d Weight differences among Cyldf/f, hTNFTg Cyldf/f and hTNFTg CyldM-Δ9/Δ9 mice at age of 4 and 8 weeks. e Histological examination of joints to evaluate inflammation (H/E panel), cartilage degradation (Toluidine blue-T/B panel) and bone erosions (Tartrate-resistant acid phosphatase-TRAP panel) in serial sections of 8-week old mice (n = 5–7 mice/group); Scale bar: 500 μm. f mCT-based imaging and histomorhometric indexes for hTNFTg Cyldf/f and hTNFTg CyldM-Δ9/Δ9 mice at age of 4 and 8 weeks (n = 9–13, week 4; n = 4–8, week 8). (BV/TV (%) bone volume fraction, Tb.Th. trabecular thickness, Tb.N trabecular number, Tb.Sp trabecular separation). Data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 by two-tailed Student’s t-test (e, f).
To further evaluate the effects of mesenchymal-specific Cyld inhibition on arthritic features, histological sections of ankle joints from all groups of mice were analyzed in early (week 4) and established hTNFTg disease (8 weeks). Four-week-old control hTNFTg Cyldf/f mice showed moderate inflammatory synovitis and mild cartilage degradation as previously reported [31]. In sharp contrast, the hTNFTg CyldM-Δ9/Δ9 mice developed severe inflammation of the synovium which was accompanied by marked proteoglycan loss of articular cartilage (Fig. 1e). In later stages (8 weeks), the hTNFTg Cyldf/f mice developed full-blown manifestations of synovitis, cartilage destruction and bone erosion (Fig. 1e). At the same age, the histological findings in the ankle joints of hTNFTg CyldM-Δ9/Δ9 mice suggested an extremely severe arthritic phenotype as this exhibited by the complete loss of joint architecture (Fig. 1e). The 3D histomorphometric analysis of calcaneus bone fully supported the histopathological findings, with all bone parameters being heavily affected (Fig. 1f).
To further confirm the role of mesenchymal Cyld-DUB deficiency in the development of arthritic phenotypes, we subjected CyldM-Δ9/Δ9 and littermate Cyldf/f control mice to CAIA [2] and we followed up the disease for 10 days (Fig. 2a). CyldM-Δ9/Δ9 mice exhibited worsened arthritic manifestations both macroscopically and histologically at the peak of disease (day 8) and the remission phase (day 10), compared to Cyld-sufficient littermate controls (Fig. 2b). Collectively, these results clearly show that mesenchymal Cyld-DUB deficiency deteriorates arthritic manifestations in both innate- and autoimmune-based arthritis.
a CAIA was induced in mice by injection of an anti-collagen antibody cocktail on day 0, followed by a single injection of 75 µg of LPS on day 3 (left panel). Semiquantitative arthritis scores of Cyldf/f and CyldM-Δ9/Δ9 mice upon CAIA induction (right panel) (n = 14, two experiments). b Representative photos of ankle joint histology from mice at day 8 and day 10 upon CAIA induction. c Semiquantitative evaluation of inflammation (see b, H/E panel), cartilage degradation (see b, T/B panel) and bone erosions (see b, TRAP panel) in serial sections of mice (n = 7–8 mice/group); Scale bar: 500 μm. Data are presented as the mean ± SEM. *p < 0.05 and **p < 0.01.
Cyld-DUB deficiency predisposes SFs to generate an amplified inflammatory signature
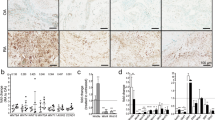
To better understand the effects of Cyld-DUB deficiency in the deterioration of arthritis, we analyzed major cellular composites characterizing arthritic joints during the early phase of hTNFTg arthritis (4 weeks of age). The relative abundance of lining (Thy1-) and sublining (Thy1+) synovial fibroblast types did not significantly differ between the hTNFTg CyldM-Δ9/Δ9 and hTNFTg Cyldf/f genotypes, however, both genotypes showed statistical differences compared to normal SFs (Fig. 3a-left panel, Supplementary Fig. 1). The analysis of the immune content at the same age highlighted higher numbers of neutrophils and Ly-6Clow monocytes in the hTNFTg CyldM-Δ9/Δ9 joints compared to hTNFTg Cyldf/f controls (Fig. 3a-right panel). Interestingly, neutrophils and Ly-6Clow monocytes are crucial for the initiation of arthritic phenotype and their infiltrating level reflects the severity of disease [32, 33]. Therefore, the analysis of cellular content of diseased joints suggests that the enhanced immune infiltrations in hTNFTg CyldM-Δ9/Δ9 joints, rather than overrepresentation of SFs, characterize the accelerated disease onset.
a Flow cytometric analysis of SF subtypes (Τhy1-, Lining SFs; Thy1+, Sublining SFs) and myeloid infiltrates in the ankle joints of hTNFTg Cyldf/f and hTNFTg CyldM-Δ9/Δ9 4-week-old mice compared to WT controls. b Cyld expression in SF cultures derived from indicated genotypes. Extracts from Cyld-deficient MEFs served as control. c Relative gene expression of arthritic mediators Il1b, Il6, Mmp3, Mmp9, Mmp13, and Timp1 (normalized to b2m levels) in SFs of Cyld-proficient and Cyld DUB-deficient SFs upon TNF (20 ng/ml, 24 h) (n = 4). d Relative gene expression of arthritic mediators Il1b, Il6, Mmp3, Mmp13 and Timp1 (normalized to b2m levels) in SFs of hTNFTg Cyld-proficient and Cyld DUB-deficient SFs. e Representative gelatin-based zymography utilizing supernatants of SF cultures derived from hTNFTg Cyld-proficient and Cyld DUB-deficient SFs. f hTNF protein levels in cultures of hTNFTg Cyld-proficient and Cyld DUB-deficient SFs (n = 3–6). g Flow cytometric detection of Vcam-1 and Icam-1 levels in cultured hTNFTg Cyldf/f (black), hTNFTg CyldM-Δ9/Δ9 (gray) (upper panel) and representative FACS plots (n = 8, 2 experiments). Data are presented as the mean ± SEM (or mean ± SD for f) *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001 by two-way ANOVA (Bonferroni correction) (a) one-way ANOVA (Bonferroni correction) (c) or unpaired t test with Welch’s correction (d, g).
To determine how Cyld-DUB-deficient SFs affect the recruitment and the tissue destruction in the arthritic joints, we examined SF responses ex vivo. Reduction of Cyld levels in cultured SFs isolated from ankle joints of both genotypes (Cre and non-Cre littermates) (Fig. 3b) is consistent with the recombination efficacy reported for the Col6a1-Cre transgenic mice [3]. We then analyzed gene expression of known regulators of arthritic disease. TNF-stimulated Cyld-DUB-deficient SFs were highly responsive to TNF signals compared to the Cyld-sufficient SFs, expressing higher levels of cytokines and proteases (Fig. 3c). Interestingly, the levels of Mmp9 mRNA in Cyld-DUB-deficient SFs were higher even in naïve conditions. Similarly, hTNFTg Cyld-DUB-deficient cells showed a particularly high expression profile of IL-6 as well as disbalanced expression ratio of the proteases Mmp3 and Mmp13 (Fig. 3d). Moreover, supernatants of the hTNFTg Cyld-deficient SF cultures showed higher MMP9 activity when analyzed by zymograms (Fig. 3e). Remarkably, the expression levels of hTNF transgene remained unaltered (Fig. 3f). Expression of adhesion molecules VCAM-1 and ICAM-1 were elevated in hTNFTg Cyld-DUB-deficient SFs compared to hTNFTg Cyld-sufficient cells (Fig. 3g). These results suggest that Cyld-DUB regulates gene expression upon inflammatory stimuli in SFs and correlate well with the exacerbated arthritic phenotype of hTNFTg CyldM-Δ9/Δ9 mice.
Cyld-DUB deficiency causes aberrant TNF-mediated signaling responses in SFs
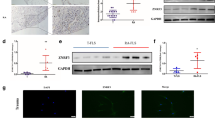
We analyzed signaling events that underline the arthritic gene expression in Cyld-DUB-deficient SFs. As previously reported for Cyld-DUB-deficient MEFs [30], the Cyld DUB-null SFs exhibited apparent aberration in NF-κB and JNK responses upon TNF stimulation, characterized by enhanced and prolonged phosphorylation of Jnk1/2 kinases, as well as IKK2 kinase followed by altered IkB phosphorylation pattern (Fig. 4a, b, Supplementary Fig. 2a, b, d). Interestingly, ERK and p38 activation was less affected (Fig. 4a, Supplementary Fig. 2a). Consistently, the nuclear extracts from Cyld deficient SFs exhibited increased DNA binding activity to NF-κB binding sites compared to Cyld-proficient SFs (Fig. 4c). We also confirmed the aberrant activation of JNK and NF-κB in arthritic Cyld DUB-null SFs (Fig. 4d, Supplementary Fig. 2c). Collectively, the above results demonstrate that, upon TNF stimulation, Cyld DUB inactivation in SFs causes enhanced NF-κB and JNK responses, suggesting that Cyld-DUB deficiency renders SFs hypersensitive to TNF-mediated signals.
a Western blot analysis for the detection of MAPK/SAPK and IKK activation in Cyldf/f and CyldM-Δ9/Δ9 cultured SFs upon TNF stimulation (10 ng/ml) for the indicated time points. (n = 5). b Immunoblot for the detection of IkB phosphorylation and degradation pattern upon TNF stimulation (10 ng/ml) (n = 3). c Band shift assay for the detection of NF-κB binding activity of nuclear extracts derived from Cyldf/f and CyldM-Δ9/Δ9 SF cultures, upon TNF stimulation for the indicated time points (n = 3). (CP cold probe). The same amount of lysates was subjected to Western blot analysis of LaminB1 expression. d Western blot analysis for the detection of JNK, IKKs, and NF-κB activation in hTNFTg Cyldf/f and hTNFTg CyldM-Δ9/Δ9 cultured SFs upon TNF stimulation (10 ng/ml) for the indicated time points (n = 5).
Mesenchymal Cyld targets TAK1/IKK2 axis to regulate proinflammatory SF responses in TNF-mediated arthritis
To determine the molecular basis of aberrant TNF-mediated NF-κB and JNK responses due to Cyld-DUB deficiency, we sought to examine the activity of Tak1. Tak1 is implicated in both TNF-induced NF-κB and JNK pathways, and its kinase activity is regulated by Cyld-mediated deubiquitination of K63-Ub chains [20]. We reasoned that the prolonged ubiquitination of Tak1 due to Cyld-DUB deficiency in SFs, leads to the sustained phosphorylation of IKK2 and Jnk (Fig. 4a, d). The pattern of K63 ubiquitination of Tak1 following TNF stimulation differed between Cyld-sufficient and Cyld-targeted SFs. K63 ubiquitination of Tak1 was evident even in unstimulated conditions in Cyld-targeted SFs and was significantly higher in later time points compared to Cyld-sufficient SFs (Fig. 5a, Supplementary Fig. 2e). Consistently, Cyld-DUB-deficient SFs exhibited sustained Tak1 phosphorylation compared to control SFs (Fig. 5b).
a Detection of Tak1 ubiquitination by immunoassay of Cyldf/f and CyldM-Δ9/Δ9 SFs stimulated with TNF (0–60 min), followed by immunoprecipitation of lysates with anti-Tak1 and immunoblot analysis with anti-K63-Ub, and then re-probing with anti-Tak1 and anti-Tab1. (Lower panel) Immunoblot analysis of corresponding whole cell lysates (input) with anti-Cyld, anti-Tak1 and anti-Tab1 (n = 3). b Detection of Tak1 phosphorylation by immunoassay of Cyldf/f and CyldM-Δ9/Δ9 SFs stimulated with TNF (0–30 min), followed by immunoprecipitation of lysates with anti-Tab1 and immunoblot analysis with anti-pTak1. c Histological evaluation of 10-week-old hTNFTg Ikk2f/f Cyldf/f, hTNFTg Ikk2M-HetCyldΜ-Δ9/Δ9 and hTNFTg Ikk2M-KOCyldΜ-Δ9/Δ9 mice; Scale bar: 500 μm. d Survival rates of Cyldf/f and CyldM-Δ9/Δ9 SFs treated either with 50 ng/ml TNF or combinations of TNF with zVAD or Nec1 as indicated (T + Z: TNF 50 ng/ml + zVAD, T + N1s: TNF 50 ng/ml+ Nec1s) in the absence (Black bars) or presence (Gray bars) of ΙΚΚ2 inhibitor ML120b, s (n = 3–5). Data are presented as the mean ± SEM *p < 0.05, **p < 0.01, and ****p < 0.0001 by one-way ANOVA (Bonferroni correction) (c) or two-tailed Student’s t-test (d).
Interestingly, neither Jnk1 nor Jnk2 deficiency was sufficient to modify the hTNFTg disease ([34] and Suppl Fig. 3 respectively). However, mice with mesenchymal -specific Cyld/IKK2 deficiency in the hTNFTg background (hTNFTg Ikk2 M-KOCyldM-Δ9/Δ9) exhibited modified and attenuated arthritic phenotype compared to their littermate Ikk2/Cyld-sufficient controls (Fig. 5c), resembling the phenotype of hTNFTg Ikk2M-KO mice [2]. This finding indicates that dominant IKK2-mediated signaling is responsible for the exaggerated arthritic response in hTNFTg CyldM-Δ9/Δ9 mice.
Taken together, these results argue against a sufficient role of JNK1/2 kinase activation for the development of hTNF-mediated disease and indicate that the prolonged activation of TAK1 due to Cyld-DUB deficiency in SFs promotes a prolonged activation of IKK2 and NF-κB (Fig. 4a, d), leading to exacerbated arthritogenic responses in vivo.
Cyld is insufficient to control the TNF-induced death of SFs
Previous evidence demonstrated that Cyld is implicated in the regulation of TNF-mediated programmed necrosis through the deubiquitination of Ripk1, abrogating the formation of the Ripk1/Ripk3 complex which activates the necroptosis executor kinase Mlkl [27]. In the context of hTNFTg arthritis, complete absence of Ripk3 kinase does not affect development of arthritis, indicating that Ripk3-mediated signals are not directly implicated in the pathology. However, Ripk3 deficiency rescues residual synovial inflammation of hTNFTg Ikk2M-KO mice by restricting inflammatory SF death caused by the absence of SF-IKK2 kinase [2]. Herein, we additionally illustrate that the residual inflammation of hTNFTg Ikk2M-KO mice is fully restored by the deficiency of Mlkl (Supplementary Fig. 4). This finding strongly suggests that the remaining inflammatory activity in hTNFTg Ikk2M-KO mice is caused by necroptosis of IKK2-null SFs. In sharp contrast, SF-Cyld DUB deficiency was not sufficient to abrogate residual disease of hTNFTg Ikk2M-KO mice (Fig. 5c) also compare the age-matched hTNFTg Ikk2M-KO Mlkl-/- phenotype (Supplementary Fig. 4) to hTNFTg Ikk2M-KO CyldM-Δ9/Δ9 and littermate control mice). Consistent with the in vivo phenotype of hTNFTg mesenchymal-specific Cyld/Ikk2 mutants, TNF-induced cytotoxicity in Cyld-proficient and deficient SFs exhibited similar survival outcomes either in the presence or absence of IKK2 activity and caspases. The addition of Nec1s (Ripk1 inhibition), however, was sufficient to rescue lethality by TNF and concomitant IKK2 inhibition in SFs of all examined genotypes [2] (Fig. 5d). This finding indicates that Cyld is redundant for the regulation of the TNF-induced, Ripk1-mediated death of IKK2-null SFs and, consequently, the necroptosis-mediated residual disease in the hTNFTg mesenchymal -specific Ikk2 mutants.
Collectively, our results show that SF-Cyld controls TAK1 kinase to prevent IKK2 and NF-κB hyperactivation but not Ripk1-mediated death, leading to deterioration of arthritic phenotype in TNF-dependent arthritis.
Discussion
Compelling evidence suggests important contributions of Cyld-mediated deubiquitination in signaling pathways involved in development, homeostasis and several pathologies [8], but less is known about the role of CYLD in inflammatory arthritis. While CYLD is significantly downregulated in human-cultured RASFs compared to Osteoarthritic SFs [29], mechanistic insights into the role of the SF-CYLD are still missing. It is well-acknowledged that the persistent inflammation in RA entails a positive feedback whereby activated SFs recruit and retain immune cells to the joint, causing both cell types to shape and respond to the microenvironment in a self-sustaining manner [35]. Here we provide evidence that underlines CYLD in SFs as a robust modulator of inflammatory arthritis that functions to coordinately limit excessive activation of inflammatory and destructive properties of SFs. We show that mesenchymal Cyld contributes to prevent excessive JNK and NF-κB activation in TNF-stimulated naive and arthritic SFs. Accordingly, high transcriptional activity of proteases and cytokines involved in the arthritic process accompanied the deteriorated phenotype of mesenchymal Cyld DUB-deficient mice. These effects were observed both in genetic and induced models of arthritis. In sharp contrast, the elimination of Cyld DUB activity in the mesenchymal tissues (Col6a1-Cre+) is not detrimental for homeostasis.
The molecular analysis of Cyld DUB activity in limiting activation of NF-κB and JNK in TNF-treated SFs highlights a common denominator of the two pathways, the Tak1 kinase. Cyld-DUB-deficient SFs exhibit Tak1 hyperactivation, leading to sustained IKK2 and IkB phosphorylation and, eventually, deregulated activation of NF-κB. Indeed, hTNFTg mice with concomitant mesenchymal Cyld/Ikk2 mutation display amelioration of arthritogenic responses, phenocopying the hTNFTg Ikk2M-KO mice [2]. Even though JNK/MAPK pathway is also deregulated in Cyld-deficient SFs, our results and previous studies argue against a dominant pathogenic role of either JNK2 or JNK1 in TNF-mediated arthritis [34], without excluding, however, their synergistic role in disease. Moreover, other mechanisms, including accumulation of reactive oxygen species (ROS), may also contribute to increased JNK phosphorylation [36, 37]. Consistent with our findings on TAK1 hyperactivation, previous pharmacological studies have identified TAK1 as a central signal transducer that mediates the pathogenic activation of SFs ex vivo and the arthritic outcome in vivo [38, 39]. The embryonic lethality of mesenchymal double genetic Cyld/Tak1 mutant mice and the absence of inducible SF-specific Cre lines have not allowed us to further analyze the mesenchymal Cyld/TAK1 crosstalk in arthritis, as previously exhibited for hepatocytes [26, 28].
In the context of the role of Cyld-DUB in cell death responses of SFs, the concomitant mesenchymal Cyld/Ikk2 deficiency under hTNFTg conditions additionally suggested the redundant function of Cyld in regulating the de novo RIPK3/MLKL-dependent necroptosis detected in IΚΚ2-targeted arthritic SFs in vivo and ex vivo [2]. This finding contrasts current concepts on the dominance of Cyld-DUB activity in regulating necroptosis. Limited evidence supports our contradictory findings, as the RIPK3-mediated death observed in the colon of intestinal epithelial NEMO deficiency was not rescued by Cyld DUB deficiency [40], while the repression of Cyld in neuronal cells promoted cell death but did not alter NF-κB activity upon brain injury [41]. Interestingly, several studies corroborate the context-specific effects of complete Cyld protein deficiency in different cell types, such as gingival fibroblasts in periodontal diseases, where TNF and LPS exerted differential NF-κB outcomes [42] or in Cyld-deficient macrophages and T-cells which differentially respond to innate (TNF, LPS) or adaptive (anti-CD3) stimuli respectively [43, 44]. However, we cannot fully dismiss that incomplete Cyld targeting, dominant-negative functions of truncated Cyld [28], other spatiotemporal signaling events [45] or context-specific signaling counterparts of Cyld (Itch1, usp18, USP4, spata2) may account for the insufficiency of Cyld to prohibit necroptosis in our experiments with the IKK2-prohibited SFs.
Likewise, it is also tempting to consider that K63 deubiquinating activity of A20 cannot reconstitute for the Cyld insufficiency in SFs, indicating that the crucial target of Cyld in SFs could be other than the common set of targets such as TRAF2, TRAF6, RIPK1, and NEMO [8]. It was previously suggested that A20 and Cyld differentially act to regulate TNF pathway; A20 expression is induced by inflammatory stimuli and acts to terminate responses [46, 47] while Cyld is constitutively active in order to prohibit uncontrolled NF-κB activation [48]. Alternatively, a prominent role of Cyld-mediated M1 deubiquitination in TNF signaling in SFs remains to be explored [49, 50].
Collectively, by exhibiting the major contribution of Cyld deubiquitinase activity in the TAK1/IKK2 axis leading to pathogenic NF-κB activation, we identify Cyld as a negative regulator of the major inflammatory signaling acting downstream of TNFR1 in SFs. Our study, therefore, contributes to the signaling map underlying SF responses in TNF-mediated arthritis that would inform potential therapeutic - strategies targeting SFs.
Material and methods
Animals and induction of arthritis
Human TNF transgenic (hTNFTg) [31], TgCol6a1-Cre [3], Cyld fxl9/flx9 (referred in the text and figures as Cyldf/f), Milk−/− [51] and Ikbkbf/f [52] (referred in the text and figures as Ikk2f/f) mice have been previously described. TgCol6a1-Cre Cyldflx9/flx9 mice referred as CyldM-Δ9/Δ9. Collagen-Antibody-Induced Arthritis (CAIA) was induced according to the manufacturer’s instructions (MD Biosciences). Mice were maintained on a C57BL/6J genetic background.
Clinical assessment of arthritis
Arthritis in hTNFTg animals was evaluated in ankle joints in a blinded manner using a semiquantitative arthritis score ranging from 0 to 4; 0: no arthritis (normal appearance and grip strength); 1: mild arthritis (joint swelling); 2: moderate joint swelling and digit deformation, reduced grip strength; 3: severe joint swelling and digit deformation, impaired movement, no grip strength; 4: severe joint swelling and digit deformation, impaired movement, no grip strength and obvious weight loss-cachexia. At indicated weeks of age, mice were sacrificed and the hind ankle joints were removed for histology analysis. CAIA clinical evaluation was performed as previously described [53].
Histologic analysis
Formalin-fixed, EDTA-decalcified, paraffin-embedded mouse tissue specimens were sectioned and stained with hematoxylin and eosin (H/E), Toluidine Blue (TB) or TRAP kit [Sigma-Aldrich]. H&E-stained joint sections were semi-quantitatively blindly evaluated for the following parameters: synovial inflammation/ hyperplasia (scale of 0–4), cartilage erosion (scale of 0–4), and bone loss (scale of 0–4) [54].
Microcomputed tomography
Microcomputed tomography (mCT) of excised joints was carried out by a SkyScan 1172 CT scanner (Bruker, Aartselaar, Belgium) following the general guidelines used for assessment of bone microarchitecture in rodents using mCT [55]. Briefly, scanning was conducted at 50 kV, 100 mA using a 0.5-mm aluminum filter, at a resolution of 6 μm/pixel. Reconstruction of sections was achieved using the NRECON software (Bruker) with beam hardening correction set to 40%. The analysis was performed on a spherical volume of interest (VOI) (diameter 0.53 mm) within 62 slices of the trabecular region of calcaneus. Morphometric quantification of trabecular bone indices such as trabecular bone volume fraction (BV/TV; %), trabecular thickness (Tb. Th; mm), trabecular number (Tb. N; 1/mm) and trabecular separation (Tb. Sp; mm) were performed using the CT analyzer program (Bruker).
Flow cytometric analysis of joint tissue
The ankle joint tissues were disaggregated by incubation for 40 min at 37 °C in an enzymatic digestion medium consisting of DMEM, 10% heat-inactivated FBS, collagenase type IV (300 units) from Clostridium histolyticum [Sigma] and 0.03 mg ml−1 DNase [Sigma]. The cells were then blocked in 1% BSA in PBS and Fc blocker (anti-CD16/32) for 10 min at 4 °C. For SF subtype determination, the following fluorophore-conjugated antibodies were employed: anti-Pdpn PE/Cyanine7; anti-Thy1 Alexa Fluor 647; anti-CD31 APC/Fire™ 750; anti-CD45 APC/Cyanine7; anti-Ter119 APC/Fire™ 750 (Supplementary table 1). For myeloid infiltrates, we used the following antibodies: anti- Ly-6C FITC; anti-NK-1.1 PE; anti- CD11c PE/Dazzle™ 594; anti- Ly-6G PE/Cyanine7; anti-CD11b APC; anti- CD45 APC/Cyanine7. The analysis was performed with BD FACSCanto II and the BD FACSDiva software and dead cells were excluded by Propidium Iodide staining. Analysis of the results were performed employing FlowJo software (v.10)
Cell culture
Primary mouse SFs were isolated from mice with indicated genotypes and cultured for four passages as previously described [56]. Upon evaluation of purity (less of 5% CD45+ cells in the culture), SFs were used in the assays. Flow cytometric analysis was performed in cells stained with the following antibodies CD90.2, VCAM-1, CD45, ICAM-1, and CD45 using FlowJo analysis software.
In experiments with IKK2 inhibitor, pretreatment of cells with ML120B (Tocris, 10uM) [57] was performed for 3 h before the addition of other reagents. zVAD [Bachem] and Nec1s [BioVision] were used in concentrations 20 and 30 mM, respectively. TNF cytotoxicity assays were performed in 96-well plates, terminated 16–18 h post TNF addition, and evaluated upon crystal violet staining, solubilization in 33% acetic acid solution and determination of optical density at 570 nm as a test filter and 630 nm as a reference filter.
Quantitative PCR
The RNA extraction was performed employing TRIZOL (Thermo). The extracted RNA was further treated with DNAse to eliminate DNA residues (Quigen, RNeasy MinElute cleanup kit). Upon RT reaction using the MMLV Reverse Transcriptase (Promega), according to the manufacturer’s instruction. The cDNA was further subjected to qPCR (ThermoFisher Scientific, Platinum™ SYBR™ Green qPCR SuperMix) for the detection of Il1b, Il6, Mmp3, Mmp9, Mmp13 and Timp1 (oligos provided in Supplementary Table 2) in a CFX96 Touch Real-Time PCR Detection System (Biorad). Quantification was performed with the ddCt method.
Immunoblotting and Immunoprecipitation
For immunoblotting, samples were collected in RIPA buffer, containing 1% Triton X-100, 0.1% SDS, 150 mM NaCl, 10 mM Tris–HCl, pH 7.4, 1 mM EDTA, protease inhibitors [Roche], and phosphatase inhibitors [Sigma-Aldrich], separated by SDS/PAGE (10–12.5%), transferred to nitrocellulose membranes [Millipore], and probed with antibodies (Supplementary Table 1). Chemiluminescent signals were detected by the Bio-Rad Gel Doc EZ imaging system or by conventional methods.
For immunoprecipitation experiments, SFs were stimulated with murine TNF (20 ng/ml) (VIB Protein Service Facility) as indicated. Cells were lysed in Triton X-100 buffer (50 mM Tris–HCl pH 7.5, 1% Triton X-100, 1 mM EDTA pH 8, 1 mM EGTA pH 8, 250 mM Sucrose, protease inhibitors [Roche] and phosphatase inhibitors [Sigma-Aldrich]), and the extracts were incubated either with 2ug anti-Tak1 [Cell Signaling] or 2 μg anti-Tab1 antibody [58] (kindly provided by Philip Cohen/University of Dundee) for 4 h. Protein A/G beads [Santa Cruz Biotechnology] were added for the 3 h of incubation. The beads were then washed three times and the samples were eluted from the beads using Laemni buffer, separated by SDS/PAGE (10%), transferred to nitrocellulose membranes and subjected to immunoblot, as described, utilizing appropriate antibodies (Supplementary table 1)
EMSA
To generate dsDNA with NF-κB binding site, we mixed appropriate oligos with complementary sequences (200 ng each) (sense5′-ATCAGGGACTTTCCGCTGGGGACTTT-3′ and antisense5′CGGAAAGTCCCCAGCGGAAAGTCCCT-3′), heated to 100 °C, and allowed to return slowly to room temperature. Upon 32P-labeleling, the ds DNA fragments with the NF-κB binding site were incubated with nuclear extract (5 μg per reaction) in 25 mM HEPES-KOH at pH 7.9, 250 mM KCl, 25 mM MgCl2, 2.5 mM DTT, 50% glycerol, at room temperature for 20 min. After addition of 20% Ficoll, samples were subjected to electrophoresis on a 6% polyacrylamide gels, run with 50 mM Tris-borate buffer pH 8.3, 1 mM EDTA at 4oC. Dried gels were visualized by phospho-imaging using Personal Molecular Imager FX system (Bio-Rad Laboratories) or films.
Statistical analysis
Data are presented as mean ± SEM. Analyses were performed by employing SigmaPlot and GraphPad software. p values < 0.05 were considered significant.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Firestein G, Budd R, Gabriel S, McInnes I, O’Dell J. Kelley and Firestein’s textbook of rheumatology, 2-volume set. 10th ed. Philadelphia, PA: Elsevier; 2016.
Armaka M, Ospelt C, Pasparakis M, Kollias G. The p55TNFR-IKK2-Ripk3 axis orchestrates arthritis by regulating death and inflammatory pathways in synovial fibroblasts. Nat Commun. 2018;9:618.
Armaka M, Apostolaki M, Jacques P, Kontoyiannis DL, Elewaut D, Kollias G. Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med. 2008;205:331–7.
Armaka M, Konstantopoulos D, Tzaferis C, Lavigne MD, Sakkou M, Liakos A, et al. Single-cell multimodal analysis identifies common regulatory programs in synovial fibroblasts of rheumatoid arthritis patients and modeled TNF-driven arthritis. Genome Med. 2022;14:78.
van Loo G, Bertrand MJM. Death by TNF: a road to inflammation. Nat Rev Immunol. 2022;23:289–303
Pickart CM. Mechanisms underlying ubiquitination. Annu Rev Biochem. 2001;70:503–33.
Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–29.
Lork M, Verhelst K, Beyaert R. CYLD, A20 and OTULIN deubiquitinases in NF-kappaB signaling and cell death: so similar, yet so different. Cell Death Differ. 2017;24:1172–83.
Martens A, van Loo G. A20 at the crossroads of cell death, inflammation, and autoimmunity. Cold Spring Harb Perspect Biol. 2020;12:a036418.
Zhou Q, Yu X, Demirkaya E, Deuitch N, Stone D, Tsai WL, et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc Natl Acad Sci USA. 2016;113:10127–32.
Nabavi M, Shahrooei M, Rokni-Zadeh H, Vrancken J, Changi-Ashtiani M, Darabi K, et al. Auto-inflammation in a patient with a novel homozygous OTULIN mutation. J Clin Immunol. 2019;39:138–41.
Damgaard RB, Walker JA, Marco-Casanova P, Morgan NV, Titheradge HL, Elliott PR, et al. The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell. 2016;166:1215–30.e20.
Damgaard RB, Elliott PR, Swatek KN, Maher ER, Stepensky P, Elpeleg O, et al. OTULIN deficiency in ORAS causes cell type-specific LUBAC degradation, dysregulated TNF signalling and cell death. EMBO Mol Med. 2019;11:e9324.
Heger K, Wickliffe KE, Ndoja A, Zhang J, Murthy A, Dugger DL, et al. OTULIN limits cell death and inflammation by deubiquitinating LUBAC. Nature. 2018;559:120–4.
Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–5.
Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–6.
Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801.
Massoumi R. CYLD: a deubiquitination enzyme with multiple roles in cancer. Future Oncol. 2011;7:285–97.
Cleynen I, Vazeille E, Artieda M, Verspaget HW, Szczypiorska M, Bringer MA, et al. Genetic and microbial factors modulating the ubiquitin proteasome system in inflammatory bowel disease. Gut. 2014;63:1265–74.
Marín-Rubio JL, Raote I, Inns J, Dobson-Stone C, Rajan N. CYLD in health and disease. Dis Model Mech. 2023;16:dmm050093.
Kovalenko A, Chable-Bessia C, Cantarella G, Israël A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–5.
Chen Y, Wang Z, Wang P, Li D, Zhou J, Wu S. CYLD negatively regulates Hippo signaling by limiting Hpo phosphorylation in Drosophila. Biochem Biophys Res Commun. 2014;452:808–12.
Lim JH, Jono H, Komatsu K, Woo CH, Lee J, Miyata M, et al. CYLD negatively regulates transforming growth factor-β-signalling via deubiquitinating Akt. Nat Commun. 2012;3:771.
Wickström SA, Masoumi KC, Khochbin S, Fässler R, Massoumi R. CYLD negatively regulates cell-cycle progression by inactivating HDAC6 and increasing the levels of acetylated tubulin. EMBO J. 2010;29:131–44.
Rajan N, Elliott RJ, Smith A, Sinclair N, Swift S, Lord CJ, et al. The cylindromatosis gene product, CYLD, interacts with MIB2 to regulate notch signalling. Oncotarget. 2014;5:12126–40.
Nikolaou K, Tsagaratou A, Eftychi C, Kollias G, Mosialos G, Talianidis I. Inactivation of the deubiquitinase CYLD in hepatocytes causes apoptosis, inflammation, fibrosis, and cancer. Cancer Cell. 2012;21:738–50.
O’Donnell MA, Perez-Jimenez E, Oberst A, Ng A, Massoumi R, Xavier R, et al. Caspase 8 inhibits programmed necrosis by processing CYLD. Nat Cell Biol. 2011;13:1437–42.
Ji YX, Huang Z, Yang X, Wang X, Zhao LP, Wang PX, et al. The deubiquitinating enzyme cylindromatosis mitigates nonalcoholic steatohepatitis. Nat Med. 2018;24:213–23.
Zhang LM, Zhou JJ, Luo CL. CYLD suppression enhances the pro-inflammatory effects and hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes by enhancing NF-κB activation. Arthritis Res Ther. 2018;20:219.
Trompouki E, Tsagaratou A, Kosmidis SK, Dollé P, Qian J, Kontoyiannis DL, et al. Truncation of the catalytic domain of the cylindromatosis tumor suppressor impairs lung maturation. Neoplasia. 2009;11:469–76.
Keffer J, Probert L, Cazlaris H, Georgopoulos S, Kaslaris E, Kioussis D, et al. Transgenic mice expressing human tumour necrosis factor: a predictive genetic model of arthritis. EMBO J. 1991;10:4025–31.
Misharin AV, Cuda CM, Saber R, Turner JD, Gierut AK, Haines GK, et al. Nonclassical Ly6C(-) monocytes drive the development of inflammatory arthritis in mice. Cell Rep. 2014;9:591–604.
Jacobs JP, Ortiz-Lopez A, Campbell JJ, Gerard CJ, Mathis D, Benoist C. Deficiency of CXCR2, but not other chemokine receptors, attenuates autoantibody-mediated arthritis in a murine model. Arthritis Rheum. 2010;62:1921–32.
Köller M, Hayer S, Redlich K, Ricci R, David JP, Steiner G, et al. JNK1 is not essential for TNF-mediated joint disease. Arthritis Res Ther. 2005;7:R166–73.
Nygaard G, Firestein GS. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat Rev Rheumatol. 2020;16:316–33.
Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–61.
He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–97.
Jones DS, Jenney AP, Swantek JL, Burke JM, Lauffenburger DA, Sorger PK. Profiling drugs for rheumatoid arthritis that inhibit synovial fibroblast activation. Nat Chem Biol. 2017;13:38–45.
Scarneo SA, Eibschutz LS, Bendele PJ, Yang KW, Totzke J, Hughes P, et al. Pharmacological inhibition of TAK1, with the selective inhibitor takinib, alleviates clinical manifestation of arthritis in CIA mice. Arthritis Res Ther. 2019;21:292.
Welz PS, Wullaert A, Vlantis K, Kondylis V, Fernández-Majada V, Ermolaeva M, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–4.
Ganjam GK, Terpolilli NA, Diemert S, Eisenbach I, Hoffmann L, Reuther C, et al. Cylindromatosis mediates neuronal cell death in vitro and in vivo. Cell Death Differ. 2018;25:1394–407.
Fu YW, Li L, Wang XQ, Zhou Y, Zhu LF, Mei YM, et al. The inhibitory effect of the deubiquitinase cylindromatosis (CYLD) on inflammatory responses in human gingival fibroblasts. Oral Dis. 2021;27:1487–97.
Zhang J, Stirling B, Temmerman ST, Ma CA, Fuss IJ, Derry JM, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Investig. 2006;116:3042–9.
Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–85.
Moquin DM, McQuade T, Chan FK. CYLD deubiquitinates RIP1 in the TNFα-induced necrosome to facilitate kinase activation and programmed necrosis. PLoS ONE. 2013;8:e76841.
Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–4.
Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, et al. The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol. 2004;5:1052–60.
Reiley W, Zhang M, Wu X, Granger E, Sun SC. Regulation of the deubiquitinating enzyme CYLD by IkappaB kinase gamma-dependent phosphorylation. Mol Cell Biol. 2005;25:3886–95.
Hrdinka M, Fiil BK, Zucca M, Leske D, Bagola K, Yabal M, et al. CYLD limits Lys63- and Met1-linked ubiquitin at receptor complexes to regulate innate immune signaling. Cell Rep. 2016;14:2846–58.
Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling Complexes. Cell Rep. 2015;13:2258–72.
Polykratis A, Martens A, Eren RO, Shirasaki Y, Yamagishi M, Yamaguchi Y, et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat Cell Biol. 2019;21:731–42.
Pasparakis M, Courtois G, Hafner M, Schmidt-Supprian M, Nenci A, Toksoy A, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–6.
Khachigian LM. Collagen antibody-induced arthritis. Nat Protoc. 2006;1:2512–6.
Hayer S, Vervoordeldonk MJ, Denis MC, Armaka M, Hoffmann M, Bäcklund J, et al. SMASH’ recommendations for standardised microscopic arthritis scoring of histological sections from inflammatory arthritis animal models. Ann Rheum Dis. 2021;80:714–26.
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–86.
Armaka M, Gkretsi V, Kontoyiannis D, Kollias G. A standardized protocol for the isolation and culture of normal and arthritogenic murine synovial fibroblasts. Protocol Exchange. 2009. https://doi.org/10.1038/nprot.2009.102.
Wen D, Nong Y, Morgan JG, Gangurde P, Bielecki A, Dasilva J, et al. A selective small molecule IkappaB Kinase beta inhibitor blocks nuclear factor kappaB-mediated inflammatory responses in human fibroblast-like synoviocytes, chondrocytes, and mast cells. J Pharmacol Exp Ther. 2006;317:989–1001.
Cheung PC, Campbell DG, Nebreda AR, Cohen P. Feedback control of the protein kinase TAK1 by SAPK2a/p38alpha. EMBO J. 2003;22:5793–805.
Acknowledgements
The authors thank Panos Athanasakis, Spyros Lalos and Anna Katevaini for excellent technical assistance. The authors also thank George Kollias for critical discussions and for providing reagents and the hTNFTg model, Manolis Pasparakis (University of Cologne, Germany) for kindly providing the Ikk2fl/fl and Mlkl−/− mice, Claude Libert (VIB, Ghent, Belgium) for the recombinant TNF and Dimitris Kontoyiannis (Aristotle University of Thessaloniki, Greece) for critical reading of the manuscript.
Funding
This work has been supported by the grant from the Stavros Niarchos Foundation to the Biomedical Sciences Research Center “Alexander Fleming” (startup fund to MA), as part of the Foundation’s initiative to support the Greek research center ecosystem, and FOREUM grant (StroPHe, #061 to MA). The authors also wish to acknowledge the support from the InfrafrontierGR infrastructure (co-financed by the ERDF and NSRF 2007-2013) for providing excellent mouse hosting, transgenesis, flow cytometry, histology and microCT facilities.
Author information
Authors and Affiliations
Contributions
VR, KI, LP, and TM performed experiments and analyzed data. GM provided reagents and suggestions for project design and edited the manuscript. MA designed and supervised the study, performed experiments, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Experiments were performed in the conventional unit of the animal facilities in Biomedical Sciences Research Center (BSRC) “Alexander Fleming” under specific pathogen-free conditions, in accordance with the guidance of the Institutional Animal Care and Use Committee of BSRC “Alexander Fleming” and in conjunction with the Veterinary Service Management of the Hellenic Republic Prefecture of Attika/Greece (License Νο: 4405-5.7.2016). Experiments were monitored and reviewed throughout their duration by the respective Animal Welfare Body for compliance with the permission granted.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Edited by Gerry Melino
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rinotas, V., Iliaki, K., Pavlidi, L. et al. Cyld restrains the hyperactivation of synovial fibroblasts in inflammatory arthritis by regulating the TAK1/IKK2 signaling axis. Cell Death Dis 15, 584 (2024). https://doi.org/10.1038/s41419-024-06966-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41419-024-06966-2
- Springer Nature Limited