Abstract
Background
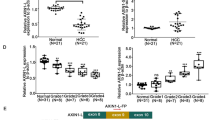
Liver metastasis is the primary cause of colorectal cancer (CRC)-associated death. Aryl-hydrocarbon receptor-interacting protein (AIP), a putative positive intermediary in aryl-hydrocarbon receptor-mediated signalling, is overexpressed in highly metastatic human KM12SM CRC cells and other highly metastatic CRC cells.
Methods
Meta-analysis and immunohistochemistry were used to assess the relevance of AIP. Cellular functions and signalling mechanisms mediated by AIP were assessed by gain-of-function experiments and in vitro and in vivo experiments.
Results
A significant association of high AIP expression with poor CRC patients’ survival was observed. Gain-of-function and quantitative proteomics experiments demonstrated that AIP increased tumorigenic and metastatic properties of isogenic KM12C (poorly metastatic) and KM12SM (highly metastatic to the liver) CRC cells. AIP overexpression dysregulated epithelial-to-mesenchymal (EMT) markers and induced several transcription factors and Cadherin-17 activation. The former induced the signalling activation of AKT, SRC and JNK kinases to increase adhesion, migration and invasion of CRC cells. In vivo, AIP expressing KM12 cells induced tumour growth and liver metastasis. Furthermore, KM12C (poorly metastatic) cells ectopically expressing AIP became metastatic to the liver.
Conclusions
Our data reveal new roles for AIP in regulating proteins associated with cancer and metastasis to induce tumorigenic and metastatic properties in colon cancer cells driving liver metastasis.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files. The Mass Spectrometry data were deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD031119. Other data are available from the corresponding author on reasonable request.
References
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64.
Torres S, Bartolome RA, Mendes M, Barderas R, Fernandez-Acenero MJ, Pelaez-Garcia A, et al. Proteome profiling of cancer-associated fibroblasts identifies novel proinflammatory signatures and prognostic markers for colorectal cancer. Clin Cancer Res. 2013;19:6006–19.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19:1423–37.
Cai Z, Chiu JF, He QY. Application of proteomics in the study of tumor metastasis. Genomics Proteom Bioinforma. 2004;2:152–66.
Aleckovic M, Wei Y, LeRoy G, Sidoli S, Liu DD, Garcia BA, et al. Identification of Nidogen 1 as a lung metastasis protein through secretome analysis. Genes Dev. 2017;31:1439–55.
Song P, Bao H, Yu Y, Xue Y, Yun D, Zhang Y, et al. Comprehensive profiling of metastasis-related proteins in paired hepatocellular carcinoma cells with different metastasis potentials. Proteom Clin Appl. 2009;3:841–52.
Sun B, Zhang S, Zhang D, Li Y, Zhao X, Luo Y, et al. Identification of metastasis-related proteins and their clinical relevance to triple-negative human breast cancer. Clin Cancer Res. 2008;14:7050–9.
Barderas R, Mendes M, Torres S, Bartolome RA, Lopez-Lucendo M, Villar-Vazquez R, et al. In-depth characterization of the secretome of colorectal cancer metastatic cells identifies key proteins in cell adhesion, migration, and invasion. Mol Cell Proteom. 2013;12:1602–20.
Mendes M, Pelaez-Garcia A, Lopez-Lucendo M, Bartolome RA, Calvino E, Barderas R, et al. Mapping the spatial proteome of metastatic cells in colorectal cancer. Proteomics. 2017;17:1700094.
Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988;48:1943–8.
Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–71.
Li A, Varney ML, Singh RK. Constitutive expression of growth regulated oncogene (gro) in human colon carcinoma cells with different metastatic potential and its role in regulating their metastatic phenotype. Clin Exp Metastasis. 2004;21:571–9.
Kuniyasu H, Ohmori H, Sasaki T, Sasahira T, Yoshida K, Kitadai Y, et al. Production of interleukin 15 by human colon cancer cells is associated with induction of mucosal hyperplasia, angiogenesis, and metastasis. Clin Cancer Res. 2003;9:4802–10.
Calon A, Espinet E, Palomo-Ponce S, Tauriello DV, Iglesias M, Cespedes MV, et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell. 2012;22:571–84.
Barderas R, Bartolome RA, Fernandez-Acenero MJ, Torres S, Casal JI. High expression of IL-13 receptor alpha2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72:2780–90.
Hegde P, Qi R, Gaspard R, Abernathy K, Dharap S, Earle-Hughes J, et al. Identification of tumor markers in models of human colorectal cancer using a 19,200-element complementary DNA microarray. Cancer Res. 2001;61:7792–7.
Ellens KW, Christian N, Singh C, Satagopam VP, May P, Linster CL. Confronting the catalytic dark matter encoded by sequenced genomes. Nucleic Acids Res. 2017;45:11495–514.
Perdigao N, Heinrich J, Stolte C, Sabir KS, Buckley MJ, Tabor B, et al. Unexpected features of the dark proteome. Proc Natl Acad Sci USA. 2015;112:15898–903.
Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–W560.
Garranzo-Asensio M, San Segundo-Acosta P, Poves C, Fernandez-Acenero MJ, Martinez-Useros J, Montero-Calle A, et al. Identification of tumor-associated antigens with diagnostic ability of colorectal cancer by in-depth immunomic and seroproteomic analysis. J Proteom. 2020;214:103635.
Garranzo-Asensio M, San Segundo-Acosta P, Martinez-Useros J, Montero-Calle A, Fernandez-Acenero MJ, Haggmark-Manberg A, et al. Identification of prefrontal cortex protein alterations in Alzheimer’s disease. Oncotarget. 2018;9:10847–67.
Barderas R, Villar-Vazquez R, Fernandez-Acenero MJ, Babel I, Pelaez-Garcia A, Torres S, et al. Sporadic colon cancer murine models demonstrate the value of autoantibody detection for preclinical cancer diagnosis. Sci Rep. 2013;3:2938.
Bartolome RA, Barderas R, Torres S, Fernandez-Acenero MJ, Mendes M, Garcia-Foncillas J, et al. Cadherin-17 interacts with alpha2beta1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene. 2014;33:1658–69.
Pelaez-Garcia A, Barderas R, Torres S, Hernandez-Varas P, Teixido J, Bonilla F, et al. FGFR4 role in epithelial-mesenchymal transition and its therapeutic value in colorectal cancer. PLoS ONE. 2013;8:e63695.
Barderas R, Shochat S, Timmerman P, Hollestelle MJ, Martinez-Torrecuadrada JL, Hoppener JW, et al. Designing antibodies for the inhibition of gastrin activity in tumoral cell lines. Int J Cancer. 2008;122:2351–9.
Borowicz S, Van Scoyk M, Avasarala S, Karuppusamy Rathinam MK, Tauler J, Bikkavilli RK, et al. The soft agar colony formation assay. J Vis Exp. 2014;92:e51998.
Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–5.
Exploring data normalization and analysis in large TMT experimental designs [https://pwilmart.github.io/IRS_normalization/understanding_IRS.html].
Kubens BS, Zanker KS. Differences in the migration capacity of primary human colon carcinoma cells (SW480) and their lymph node metastatic derivatives (SW620). Cancer Lett. 1998;131:55–64.
Araki K, Shimura T, Suzuki H, Tsutsumi S, Wada W, Yajima T, et al. E/N-cadherin switch mediates cancer progression via TGF-beta-induced epithelial-to-mesenchymal transition in extrahepatic cholangiocarcinoma. Br J Cancer. 2011;105:1885–93.
Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47:D607–D613.
Griss J, Viteri G, Sidiropoulos K, Nguyen V, Fabregat A, Hermjakob H. ReactomeGSA—efficient Multi-Omics comparative pathway analysis. Mol Cell Proteom. 2020;19:2115–25.
Tian X, Yang C, Yang L, Sun Q, Liu N. PTPRF as a novel tumor suppressor through deactivation of ERK1/2 signaling in gastric adenocarcinoma. Onco Targets Ther. 2018;11:7795–803.
Fang WK, Liao LD, Li LY, Xie YM, Xu XE, Zhao WJ, et al. Down-regulated desmocollin-2 promotes cell aggressiveness through redistributing adherens junctions and activating beta-catenin signalling in oesophageal squamous cell carcinoma. J Pathol. 2013;231:257–70.
Bujko M, Kober P, Mikula M, Ligaj M, Ostrowski J, Siedlecki JA. Expression changes of cell-cell adhesion-related genes in colorectal tumors. Oncol Lett. 2015;9:2463–70.
Bajpai R, Nagaraju GP. Specificity protein 1: its role in colorectal cancer progression and metastasis. Crit Rev Oncol Hematol. 2017;113:1–7.
Wang H, Li K, Mei Y, Huang X, Li Z, Yang Q, et al. Sp1 suppresses miR-3178 to promote the metastasis invasion cascade via upregulation of TRIOBP. Mol Ther Nucleic Acids. 2018;12:1–11.
Khodarev NN, Roach P, Pitroda SP, Golden DW, Bhayani M, Shao MY, et al. STAT1 pathway mediates amplification of metastatic potential and resistance to therapy. PLoS ONE. 2009;4:e5821.
Basak O, van de Born M, Korving J, Beumer J, van der Elst S, van Es JH, et al. Mapping early fate determination in Lgr5+ crypt stem cells using a novel Ki67-RFP allele. EMBO J 2014;33:2057–68.
Coopmans EC, Muhammad A, Daly AF, de Herder WW, van Kemenade FJ, Beckers A, et al. The role of AIP variants in pituitary adenomas and concomitant thyroid carcinomas in the Netherlands: a nationwide pathology registry (PALGA) study. Endocrine. 2020;68:640–9.
Georgitsi M, Karhu A, Winqvist R, Visakorpi T, Waltering K, Vahteristo P, et al. Mutation analysis of aryl hydrocarbon receptor interacting protein (AIP) gene in colorectal, breast, and prostate cancers. Br J Cancer. 2007;96:352–6.
Diaz Del Arco C, Estrada Munoz L, Barderas Manchado R, Pelaez Garcia A, Ortega Medina L, Molina Roldan E, et al. Prognostic role of Aryl hydrocarbon receptor interacting protein (AIP) immunohistochemical expression in patients with resected gastric carcinomas. Pathol Oncol Res. 2020;26:2641–50.
Fernández-Aceñero MJ, Barderas R, Peláez-García A, Martínez-Useros J, Díez-Valladares L, Ortega-Medina L, et al. Aryl hydrocarbon receptor interacting protein (AIP) significantly influences prognosis of pancreatic carcinoma. Annal Diagnostic Pathol. 2021;53:151742.
Barry S, Carlsen E, Marques P, Stiles CE, Gadaleta E, Berney DM, et al. Tumor microenvironment defines the invasive phenotype of AIP-mutation-positive pituitary tumors. Oncogene. 2019;38:5381–95.
Vasilev V, Daly AF, Trivellin G, Stratakis CA, Zacharieva S, Beckers A. Hereditary endocrine tumours: current state-of-the-art and research opportunities: the roles of AIP and GPR101 in familial isolated pituitary adenomas (FIPA). Endocr Relat Cancer. 2020;27:T77–T86.
Leontiou CA, Gueorguiev M, van der Spuy J, Quinton R, Lolli F, Hassan S, et al. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J Clin Endocrinol Metab. 2008;93:2390–401.
Trivellin G, Korbonits M. AIP and its interacting partners. J Endocrinol. 2011;210:137–55.
Rothhammer V, Quintana FJ. The aryl hydrocarbon receptor: an environmental sensor integrating immune responses in health and disease. Nat Rev Immunol. 2019;19:184–97.
Meyer BK, Pray-Grant MG, Vanden Heuvel JP, Perdew GH. Hepatitis B virus X-associated protein 2 is a subunit of the unliganded aryl hydrocarbon receptor core complex and exhibits transcriptional enhancer activity. Mol Cell Biol. 1998;18:978–88.
Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3–20.
Markman B, Javier Ramos F, Capdevila J, Tabernero J. EGFR and KRAS in colorectal cancer. Adv Clin Chem. 2010;51:71–119.
Sigismund S, Argenzio E, Tosoni D, Cavallaro E, Polo S, Di Fiore PP. Clathrin-mediated internalization is essential for sustained EGFR signaling but dispensable for degradation. Dev Cell. 2008;15:209–19.
Funding
This work was supported by the financial support of the PI17CIII/00045 and PI20CIII/00019 grants from the AES-ISCIII program to RB. J Hendrix acknowledges funding by UH-BOF (BOF20TT06). J Hofkens acknowledges financial support from the Research Foundation-Flanders (FWO, Grant No. ZW15_09-G0H6316N), the Flemish government through long-term structural funding Methusalem (CASAS2, Meth/15/04) and the MPI as MPI fellow. S.R. acknowledges the financial support of the KU Leuven through the internal C1 funding (KU Leuven (C14/16/053)). GSF is the recipient of a predoctoral contract (grant number 1193818 N) supported by The Flanders Research Foundation (FWO). The FPU predoctoral contract to AMC is supported by the Spanish Ministerio de Educación, Cultura y Deporte.
Author information
Authors and Affiliations
Contributions
GSF: data curation; formal analysis, investigation, writing—original draft and writing—review and editing. AMC: data curation, investigation, writing—original draft and writing—review and editing. MSM: data curation and investigation. APG: formal analysis, investigation, methodology, supervision and writing—review and editing. MJFA: formal analysis, methodology, supervision and writing—review and editing. PP: investigation and writing—review. MAN: investigation and writing—review. MM: methodology, supervision and writing—review. J Hendrix: supervision and writing—review. DH: methodology, supervision and writing—review. RAB: conceptualisation, investigation, supervision and writing—review & editing. J Hofkens: conceptualisation, investigation, supervision, writing—review & editing and funding. SR: conceptualisation, formal analysis, investigation, writing—original draft, writing—review and editing and funding. RB: conceptualisation, formal analysis, investigation, writing—original draft, writing—review and editing and funding.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All animal experiments were performed under the guidelines of the Instituto de Salud Carlos III Institutional Animal Care Committee. All experimental protocols were approved by the Committee (Proex 285/19). This article does not contain any studies with human participants by any of the authors out of public databases. However, immunohistochemical analyses of human tumour specimens were in compliance with ethical standards and approved by the Hospital Clínico San Carlos Ethics Board; and informed consent was obtained from all individual participants included in the study.
Consent to publish
All authors of this article gave consent for the publication of the manuscript.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solís-Fernández, G., Montero-Calle, A., Sánchez-Martínez, M. et al. Aryl-hydrocarbon receptor-interacting protein regulates tumorigenic and metastatic properties of colorectal cancer cells driving liver metastasis. Br J Cancer 126, 1604–1615 (2022). https://doi.org/10.1038/s41416-022-01762-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-022-01762-1
- Springer Nature Limited
This article is cited by
-
In-depth quantitative proteomics analysis revealed C1GALT1 depletion in ECC-1 cells mimics an aggressive endometrial cancer phenotype observed in cancer patients with low C1GALT1 expression
Cellular Oncology (2023)
-
AIP: A double agent? The tissue-specific role of AIP as a tumour suppressor or as an oncogene
British Journal of Cancer (2022)