Abstract
Objectives
To compare whitening efficiency and tooth sensitivity (TS) of two different over the counter (OTC) whitening systems in comparison to conventional at-home bleaching using 20% carbamide peroxide.
Materials and methods
A randomised controlled clinical trial was conducted with three parallel groups (n = 13): (A) at-home whitening using 20% carbamide peroxide (20% CP), (B) OTC ready-to-use gel trays and (C) OTC-paint on gel. Clinical colour change values (ΔE) were measured using spectrophotometry at T0: baseline, T1: 7-day and T2: 14-day from whitening start, T3: 2 weeks and T4: 6 months after whitening end. TS was recorded using a visual analogue scale (VAS). ΔE and TS values were statistically analysed. The level of significance for all tests was 5%.
Results
Significant differences in ∆E values were recorded between the experimental groups. ∆E values were significantly higher in the 20% CP conventional at-home whitening group. TS measurements were significantly higher in the 20% CP whitening group (p < 0.05).
Conclusion
Conventional at-home whitening revealed significantly improved colour change when compared to the OTC-paint on gel and OTC ready-to-use gel tray whitening systems. There was a significant colour relapse in OTC systems.
Clinical relevance
The use of tested OTC systems is not recommended as they are not effective in a clear and prolonged improved shade change. Conventional at-home whitening using 20% CP showed higher whitening efficiency and colour stability. This trial was registered with a International Standard Randomised Controlled Trial Number (ISRCTN23096480), Registration date: 12/04/2023.
Similar content being viewed by others
Introduction
Tooth discoloration is one of the most common reasons to seek dental aesthetic procedures [1]. Tooth whitening (bleaching) is an effective, non-invasive therapeutic method to manage tooth discoloration [2]. Various whitening protocols are available, including dentist-supervised-at-home and in-office procedures which utilise different concentrations of carbamide peroxide (CP) and hydrogen peroxide (HP) [3]. Using an oxidation/reduction reaction, hydrogen peroxide breaks down chromogenic molecules in dentine and enamel, resulting in the conversion of pigment ring structures into terminal carboxylic acids. As a result, the tooth’s colour is lightened and the acids are expelled from its surface and structure [4,5,6].
Over-the-counter (OTC) whitening products have been introduced as an alternative approach to dentist-supervised whitening, with a lower cost and ease of availability. OTC whitening products sector have played a significant role in the growth of the global tooth whitening market which is anticipated to reach over $2 billion in 2024 [7, 8]. The ‘DIY ‘nature of OTC products, however, arouses concerns about the risk of misuse, overuse and/or abuse [9, 10]. OTC products are available in shops, pharmacies and online stores and customers can obtain and use them easily without any medical / dental diagnosis or supervision [11, 12]. This approach ignores important clinical interventions including a thorough dental examination, diagnosis and determination of the aetiology of the discoloration, caries susceptibility / management, replacing failed restorations and hypersensitivity management [13].
Different OTC whitening products and types are available in the market including strips, rinses, dentifrices, paint-on films and prefabricated trays including gel [14, 15]. The efficiency/safety evaluation of OTC whitening products revealed conflicting outcomes. The variances observed in previous studies can be attributed to the utilisation of distinct analytical methods and OTC types [16]. In addition, a limited number of carefully planned clinical trials regarding their evaluation have been reported in the dental literature. Therefore, this randomised controlled clinical trial explored the whitening efficiency and tooth sensitivity associated with two popular OTC systems in the Middle East (OTC-paint on gel and OTC-ready to use trays with gel) in comparison with a conventional at-home dental whitening protocol with 20% carbamide peroxide. The tested OTC systems in the present study are available at a convenient price for the majority of people in the Middle East. The null hypothesis investigated was that there were no differences in the efficiency and tooth sensitivity between the three tested whitening systems after a 6-month clinical follow-up.
Materials and methods
Ethics approval and protocol registration
This randomised controlled clinical trial was approved and registered with an International Standard Randomised Controlled Trial Number (ISRCTN23096480), Registration date: 12/04/2023. This study follows the Consolidated Standards of Reporting Trials (CONSORT) statement [17], and was conducted in accordance with the Declaration of Helsinki and was approved by the Damascus University Research Ethics Committee (ref: MS3833). This trial was conducted in the Faculty of Dental Medicine, Damascus University.
Sample size and recruitment
The sample size was calculated using GPower 3.1 (Franz Faul, Kiel University, Kiel, Germany), using the t-student statistical test for two independent samples with a statistical power of 95% and a significance level 5%. In accordance with a previous study, it was necessary to recruit 10 participants per group to detect a significance of a clinical difference of at least a 2.5 increase, assuming a standard deviation of 1.2 [18]. Thirteen participants per group were enroled, taking into consideration the potential loss to follow-up of 62%, with an overall sample size n = 39. The inclusion and exclusion criteria are presented in Table 1. All participants in this trial signed an informed consent after a cooling off period.
Randomisation and sample allocation
Thirty-nine participants meeting the inclusion criteria were assigned randomly into three experimental groups (n = 13); CP: conventional at-home whitening using 20% CP (Opalescence, Ultradent, South Jordan, USA), OTC-WL: WhiteLightTM tooth whitening set and OTC-DW: Dazzling white (paint on gel) system (Table 2). The randomisation was conducted by a staff member, who was not involved in this study, by choosing random allocation of the group codes.
Intervention
A custom tray was fabricated for each patient using soft vinyl sheets, 0.8 mm (Sof-Tray Classic, Ultradent, South Jordan, UT, USA) and trimmed 1 mm beyond the gingival margin. This tray included a hole in the middle of the buccal surface of the left maxillary central incisor to standardise colour change measurement during the follow-up periods. Another custom tray, spaced around the labial surfaces of the anterior teeth, was made for each patient in the at-home whitening group for whitening procedure. Each patient was provided with oral hygiene instruction with a toothbrush and a non-whitening dentifrice (Colgate Total Colgate-Palmolive Company, New York, USA) in order to standardise the daily oral hygiene protocol for all subjects. The whitening products were applied according to the manufacturer’s recommendations. Table 2 presents the experimental groups and the manufacturer regime of the whitening protocols.
Colour change evaluation
The colour of the teeth was assessed using International Commission on Illumination (CIE) L*, a*, b* parameters. An Easy Shade Advance 4.0 spectrophotometer (VITA Zahnfabrik, Bad Säkingen, Germany) was used on the middle third of the buccal surface of the maxillary right canine and maxillary left central incisor through the holes in the guide tray. Colour measurement was performed at; T0: baseline, T1: 7-day and T2: 14-day from whitening start, T3: 2 weeks and T4: 6 months after whitening end.
The total colour difference ∆E* was calculated by the following equation:
Tooth sensitivity evaluation
Patients were asked to evaluate tooth sensitivity at (1-day, 2-day, 3-day, 4-day, 5-day, 7-day and 14-day) of whitening using a visual analogue scale (VAS). Patients were asked to draw a vertical line cutting a horizontal line (VAS chart) if they experienced any pain or discomfort in their six maxillary interior teeth. The scores for the sensitivity levels were: 0 = no sensitivity; 1–3 = mild sensitivity; 4–6 = moderate sensitivity, 7–8 = severe sensitivity; 9–10 = unbearable sensitivity.
Statistical analysis
Statistical analysis was accomplished using SPSS software (v22; SPSS Inc. Chicago, IL, USA). The normal distribution of the data was evaluated using the Kolmogorov- distribution test. Differences in ∆E and tooth sensitivity measurements were analysed using one-way ANOVA and Kruskal Wallis tests respectively. The level of significance for all tests was 5%.
Results
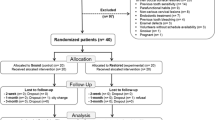
A total of 60 volunteers were screened for eligibility. Thirty-nine of them were recruited in this trial, with an age range between 19 and 30 years (23.6 ± 3.3 mean ± standard deviation SD). Thirty-two were females (Fig. 1). The means ± standard error (SE) of the ΔE values in the three experimental groups are shown in the Table 3. There were statistically significant differences (p < 0.05) in ΔE values between conventional at-home whitening vs. OTC-DW groups at all measurement points and between conventional at-home whitening vs. OTC-WL at T2, T3 and T4 measurement points. No significant differences in ΔE values were detected between OTC-DW vs. OTC-WL at any measurement point (p > 0.05). The mean ± SE of the ΔE values were (9.54 ± 3.86) for the conventional at-home whitening, (4.24 ± 2.97) for OTC-WL and (2.74 ± 2.70) for OTC-DW at 14-days of whitening.
Table 4 presents the means ± SE of tooth sensitivity (TS) values reported by participants according VAS scale. Significantly higher TS values were shown in conventional at-home whitening group at all measurement points (p < 0.05); 1-day (0.69 ± 1.03), 2-day (0.92 ± 1.26), 3-day (1.08 ± 0.86), 4-day (1.34 ± 0.96), 5-day (1.46 ± 1.39), 7-day (1 ± 1) and 14-day (2.15 ± 1.91).
Discussion
The null hypothesis investigated in this trial was rejected as the results demonstrated statistical differences in efficiency and TS observed among the tested whitening systems. Conventional at-home whitening revealed significantly improved colour change when compared to the other OTC systems, in agreement with previous investigations [14, 19,20,21]. The higher concentration of CP (20%) and the extended application time (4 h daily) may explain this superiority. OTC-ready to use trays produced a colour change similar to that obtained using at-home whitening with CP under laboratory conditions [22]. The effect of OTC tray adaptation, the application protocol, food colour, beverages and saliva can explain the diverse outcomes reported in previous laboratory studies.
In this trial, the colour change values in OTC-paint on gel group did not exceed 2.74 at any measurement point implying that it is clinically difficult to detect any whitening effect. Human eye cannot see ∆E values below 1, and it barely distinguishes ∆E values between 2 and 3. However, ∆E values below 3.3 were suggested to be clinically insignificant [21]. Previous in-vitro and in-vivo studies showed that the colour change using OTC paint-on gel products caused a colour difference below the clinically detectable threshold [21, 23]. This might be attributed to the design of OTC-paint on gel products where there is no physical barrier to protect the active materials from oral environment and enhance the contact with the tooth surface [24]. In addition, the reduced CP concentration in these products decreases the whitening efficiency [25]. This may encourage patients to overuse these products to get the expected aesthetic outcome.
Colour relapse in the OTC-paint on gel and OTC-ready to use tray groups was detected at 2 weeks and 6 months measurement points. The reduced efficiency of OTC products may encourage patients to overuse these products to get the expected aesthetic outcomes which, in turn, causes morphological and chemical alterations in dental hard tissues [26] It has been shown that whitening products with reduced pH caused more enamel erosion and mineral loss [26]. The acidic pH values of OTC products increase enamel surface roughness, which could cause an increased extrinsic stain accumulation that led to faster shade relapse [19, 27]. In contrast, conventional at-home whitening revealed colour stability after 6 months of whitening, in accordance with previous investigations [28].
Participants in the at-home whitening group reported higher TS when compared to OTC-paint on gel and OTC-ready to use trays groups. This finding has been observed in a previous study that compared at-home bleaching with OTC strips and pre-filled trays [29]. The peroxide penetration into the pulp in conjunction with the increase of enamel/dentine permeability may initiate TS [27]. The CP application period in at-home bleaching was considerably longer than that in OTC groups. The CP concentration in both tested OTC products was not declared by manufacturers. Thus, it can be hypothesised that the low efficiency and reduced TS in OTC groups may be linked to sub-optimal concentration of active agent [20].
Conclusion
Conventional at-home whitening revealed significantly improved shade change when compared to the OTC-paint on gel and OTC-ready to use tray whitening systems. The shade change in OTC-paint on gel group did not exceed the clinically detectable threshold at any measurement point. Participants in OTC whitening groups reported less TS. There was a significant colour relapse in OTC groups after 2 weeks and 6 months of whitening. Conventional at-home whitening using 20% CP showed higher colour stability.
Data availability
The data that support the findings of this study are available on request from the corresponding author.
References
Barbosa JG, Benetti F, de Oliveira Gallinari M, Carminatti M, Da Silva ABD, Lopes INI, et al. Bleaching gel mixed with MI Paste Plus reduces penetration of H2O2 and damage to pulp tissue and maintains bleaching effectiveness. Clin Oral Investig. 2020;24:1299–309.
Pereira R, Silveira J, Dias S, Cardoso A, Mata A, Marques D. Bleaching efficacy and quality of life of different bleaching techniques—randomized controlled trial. Clin Oral Investig. 2022;26:7167–77.
Kim HJ, Jang JH, Choi D, Kim J, Shim JH, Kim DS. Bleaching toothpaste with two different concentrations of hydrogen peroxide: a randomized double-blinded clinical trial. J Dent. 2020;103:103508.
Banerjee A, Millar BJ. Minimally invasive esthetics. Elsevier, Amsterdam, (2015).
Müller-Heupt LK, Wiesmann-Imilowski N, Kaya S, Schumann S, Steiger M, Bjelopavlovic M, et al. Effectiveness and safety of over-the-counter tooth-whitening agents compared to hydrogen peroxide in vitro. Int J Mol Sci. 2023;24:1956.
Torres CR, Zanatta RF, Silva TJ, Borges AB. Effect of calcium and fluoride addition to hydrogen peroxide bleaching gel on tooth diffusion, color, and microhardness. Oper Dent. 2019;44:424–32.
Kwon SR, Cortez E, Wang M, Jagwani M, Oyoyo U, Li Y. Systematic review of in vitro studies evaluating tooth bleaching efficacy. Am J Dent. 2020;33:17–24.
Alkahtani R, Stone S, German M, Waterhouse P. A review on dental whitening. J Dent. 2020;100:103423.
Bruzell EM, Pallesen U, Thoresen NR, Wallman C, Dahl JE. Side effects of external tooth bleaching: a multi-centre practice-based prospective study. Br Dent J. 2013;215:E17.
Demarco FF, Meireles SS, Masotti AS. Over-the-counter whitening agents: a concise review. Braz Oral Res. 2009;23:64–70.
Naidu A, Bennani V, Brunton JM, Brunton P. Over-the-counter tooth whitening agents: a review of literature. Braz Dent J 2020;31:221–35.
Donly KJ, Segura A, Henson T, Barker ML, Gerlach RW. Randomized controlled trial of professional at-home tooth whitening in teenagers. Gen Dent. 2007;55:669–74.
Kugel G. Over-the-counter tooth-whitening systems. Compend Contin Educ Dent. 2003;24:376–82.
Aka B, Celik EU. Evaluation of the efficacy and color stability of two different at‐home bleaching systems on teeth of different shades: a randomized controlled clinical trial. J Esthet Restor Dent. 2017;29:325–38.
Kishta-Derani M, Neiva G, Yaman P, Dennison D. In vitro evaluation of tooth-color change using four paint-on tooth whiteners. Oper Dent. 2007;32:394–8.
Grillon M, Di Bella E, Krejci I, Ardu S. In vitro evaluation of tooth-whitening potential of peroxide-free OTC dental bleaching agents. Dent J. 2023;11:89.
Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–32.21.
Hyland BW, McDonald A, Lewis N, Tredwin C, Petrie A, Hall S, et al. A new three-component formulation for the efficient whitening of teeth (Carbamide Plus). Clin Oral Investig. 2015;19:1395–404.
Omar F, Ab-Ghani Z, Abd Rahman N, Halim MS. Nonprescription bleaching versus home bleaching with professional prescriptions: which one is safer? A comprehensive review of color changes and their side effects on human enamel. Eur J Dent. 2019;13:589–98.
Ribeiro EP, Emídio AG, Zanin GT, Melo E Silva VFF, Lopes MB, Guiraldo RD, et al. Dental aesthetic perception of patients submitted to activated charcoal-based bleaching agents: a randomized clinical trial. J Dent. 2023;139:104744.
Kim YM, Ha AN, Kim JW, Kim SJ. Double-blind randomized study to evaluate the safety and efficacy of over-the-counter tooth-whitening agents containing 2.9% hydrogen peroxide. Oper Dent. 2018;43:272–81.
Singh JKM, Sengut M, Halim MS, Ab-Ghani Z, Abd Rahman N. Safety comparison of over the counter bleaching with professionally prescribed home bleaching agent. Eur J Gen Dent. 2018;7:35–40.
Alshehri A, Almutairi B, Jurado CA, Afrashtehfar KI, Albarrak S, Alharbi A, et al. Biomimetic whitening effect of polyphosphate-bleaching agents on dental enamel. Biomimetics 2022;7:183.
Farrell S, Barker ML, Sagel PA, Gerlach RW. Use of a physical barrier to improve efficacy of a paint-on whitening gel: a seven-day randomized clinical trial. J Clin Dent. 2006;17:117–21.
de Freitas MR, de Carvalho MM, Liporoni PCS, Fort ACB, Moura RDME, Zanatta RF. Effectiveness and adverse effects of over-the-counter whitening products on dental tissues. Front Dent Med. 2021;2:687507.
Pascolutti M, de Oliveira D. A radical-free approach to teeth whitening. Dent J 2021;9:148.
Greenwall-Cohen J, Francois P, Silikas N, Greenwall L, Le Goff S, Attal JP. The safety and efficacy of ‘over the counter’ bleaching products in the UK. Br Dent J. 2019;226:271–6.
Swift EJ Jr, May KN, Wilder AD Jr, Heymann HO, Bayne SC. Two‐year clinical evaluation of tooth whitening using an at‐home bleaching system. J Esthet Restor Dent. 1999;11:36–42.
Cordeiro D, Toda C, Hanan S, Arnhold LP, Reis A, Loguercio AD, et al. Clinical evaluation of different delivery methods of at-home bleaching gels composed of 10% hydrogen peroxide. Oper Dent. 2019;44:13–23.
Funding
This research was funded by Damascus University (No. 501100020595).
Author information
Authors and Affiliations
Contributions
HM and AB participated in designing the study. EK and HM conducted the clinical study, collected the data and did the statistical analysis. All authors read and approved the final manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved for human subjects by the Ethical Committee of Damascus University (permit number MS3833, 23/12/2020). Written informed consent was obtained from all study participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krayem, E., Banerjee, A. & Milly, H. Evaluating the efficiency of two different over-the-counter tooth whitening systems: a randomised controlled clinical trial. BDJ Open 10, 41 (2024). https://doi.org/10.1038/s41405-024-00227-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41405-024-00227-2
- Springer Nature Limited