Abstract
Developing positron emission tomography (PET) radioligands for the detection of endogenous serotonin release will enable the investigation of serotonergic deficits in many neuropsychiatric disorders. The present study investigates how acute challenges that aim to increase or decrease cerebral serotonin levels affect binding of the serotonin 2A receptor (5-HT2AR) agonist radioligand [11C]Cimbi-36. In a randomized, double-blind, placebo-controlled, three-arm design, 23 healthy volunteers were PET scanned twice with [11C]Cimbi-36: at baseline and following double-blind assignment to one of three interventions (1) infusion of the selective serotonin reuptake inhibitor (SSRI) citalopram preceded by oral dosing of the 5-HT1AR antagonist pindolol, (n = 8) (2) acute tryptophan depletion (ATD) (n = 7) and (3) placebo (n = 8). Two-sample t-tests revealed no significant group differences in percent change of neocortical [11C]Cimbi-36 binding from baseline to intervention between placebo and citalopram/pindolol (p = 0.4) or between placebo and ATD (p = 0.5). Notably, there was a significantly larger within-group variation in 5-HT2AR binding after intervention with citalopram/pindolol, as compared with placebo (p = 0.007). These findings suggest that neither ATD nor a combination of citalopram and pindolol elicit acute unidirectional changes in serotonin levels sufficient to be detected with [11C]Cimbi-36 PET in neocortex. We suggest that the large interindividual variation in 5-HT2AR binding after citalopram/pindolol reflects that after an acute SSRI intervention, individuals respond substantially different in terms of their brain serotonin levels. Our observation has a potential impact for the understanding of patient responses to SSRI.
Similar content being viewed by others
Introduction
Serotonergic neurotransmission is implicated in cognitive and emotional processes, and altered serotonin signalling is thought to contribute to a variety of disorders such as depression, addiction, schizophrenia, and anxiety. The development of novel methods to examine the serotonin system in the human brain is important because they can give important insights into the serotonergic mechanisms involved in the pathophysiology of neuropsychiatric disorders.
Positron emission tomography (PET) is a neuroimaging technique with unsurpassed selectivity and sensitivity to quantify neuroreceptors in vivo. PET can be used to measure dynamic changes in neurotransmission that occur in response to endogenous fluctuations in neurotransmitter levels or to experimental challenges1. Appropriate PET radioligands enables the detection of endogenous neurotransmitter release, i.e. radioligand binding is inversely correlated with in vivo concentration of neurotransmitters2. Radioligand agonists are hypothesised to be superior to antagonists for the detection of acute neurotransmitter release because of differential receptor-affinity states2. For the dopamine system, it has been reported that D2/D3 receptor agonist radioligands are more sensitive to endogenous changes in dopamine than antagonist radioligands3. This difference has been explained by agonist radioligands binding to the high affinity state receptor only, inducing competition with endogenous neurotransmitters2. Accordingly, it was recently shown in non-human primates that the serotonin 2A (5-HT2A) receptor agonist radioligand [11C[Cimbi-36 is more sensitive to serotonin release than the antagonist 5-HT2A receptor radioligand [11C]MDL 1009074.
In humans, several PET serotonergic radioligand studies have failed to show convincing radioligand displacement after drug-induced changes in brain serotonin levels2. Two studies with the partial agonist radiotracer for the 5-HT1A receptor ([11C]CUMI) found either no change5 or an unexpected increase in radioligand binding6. Likewise, the binding of the 5-HT1B partial agonist radioligand [11C]AZ10419369 increased after an acute challenge with a selective serotonin reuptake inhibitor (SSRI) in humans7. It was suggested that the unexpected increase in radiotracer binding in postsynaptic regions was caused by stimulation of the 5-HT1A and 5-HT1B autoreceptors in the raphe nuclei, which led to a subsequent inhibition of serotonin release in the terminals6,7.
The aim of this study was to assess the sensitivity of [11C]Cimbi-36 binding to acute changes in serotonin levels in the healthy human brain. In a pseudo-randomized, double-blind, placebo-controlled, 3-arm design, healthy volunteers were scanned with [11C]Cimbi-36 PET at baseline and following intervention with either placebo, the SSRI citalopram combined with a 5-HT1A receptor antagonist pindolol, or acute tryptophan depletion (ATD). We hypothesised that citalopram/pindolol would increase brain serotonin levels and be associated with a decrease in [11C]Cimbi-36 binding and conversely, that ATD would decrease brain serotonin levels and thereby increase [11C]Cimbi-36 binding.
Materials and methods
Study design
This randomized double-blind, placebo-controlled intervention study was approved by the Ethics committee for the Capital Region of Denmark (Journal number: H-4-2012-105), and the first-in-human use of [11C]Cimbi-36 was approved by the Danish Health and Medicines Authority (EudraCT number: 2012-002056-16). Prior to initiation, the trial was registered for public access at clinicaltrials.gov (Identifier: NCT01778686). Twenty-four healthy volunteers were PET scanned twice with [11C]Cimbi-36 at baseline and following random assignment to one of three groups: eight subjects received the SSRI citalopram in combination with the 5-HT1A receptor antagonist pindolol, eight subjects received ATD and eight subjects received placebo. Sample size was not based on test-retest [11C]Cimbi-36, because this study was part of the first study done in humans with [11C]Cimbi-36. The study took place at the Neurobiology Research Unit (NRU), Department of Radiology and PET and Cyclotron Unit of the Copenhagen University Hospital Rigshospitalet, Copenhagen, Denmark, from January to October 2013.
Subjects
Healthy volunteers were recruited through online advertisements (www.forsogsperson.dk) and paper advertisements on bulletin boards at universities. Interested individuals signed up electronically informing their contact details, age, gender and demographic variables, which are stored in a database accessible for researchers at NRU to enroll possible participants for brain scanning studies. Exclusion criteria were current or lifetime history of psychiatric disorder, medical or neurological disease, severe head trauma, alcohol or substance abuse, as well as severe visual or hearing impairment and contraindications for MRI. No participant had any medical or neurological illness according to history, routine blood biochemistry and physical examination, evaluated on the day of scanning. The participants took no medications, except for seven women who used hormonal contraceptives and one participant who reported taking antihistamines. None of the participants had been exposed to psychotropic medications. All had a negative on urine drug screen (Rapid Response Multi-Drug; BTNX Inc., Toronto, Ontario, Canada), and all female subjects had a negative urine pregnancy test on the day of scanning. The participants showed no signs of psychopathology according to the Symptom Checklist Revised (SCL-92, Global Severity Index score, mean score ± SD: 0.21 ± 0.21)8. All participants provided written informed consent following full description of the procedures and received monetary compensation for their participation. One subject in the ATD group was excluded from the analysis after suffering an allergic reaction during the intervention scan. This subject was referred to an allergist consultant and examinations excluded that [11C]Cimbi-36 was the cause of the allergic reaction. Eight subjects (placebo group) were also included in a previous study evaluating the test-retest reliability of [11C]Cimbi-369.
Serotonergic interventions and procedure
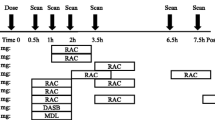
Each subject participated in two PET experiments with [11C]Cimbi-36. The participants were scanned at baseline, and approximately four weeks later they were scanned following intervention or placebo. An overview and timing of the interventions are shown in Table 1. During PET scanning, subjects were monitored with 3-lead electrocardiography continuously and non-invasive measurements of oxygen saturation and blood pressure at regular intervals. In one of the PET scans (baseline scan, placebo group) the scanner failed to start correctly. The time course of the first three minutes of tissue time-activity curves from this scan were interpolated from the subject’s intervention scan using the shape of the uptake curve from the same subject intervention scan while scaling to the radioactive concentration obtained after the initial missing three minutes of the baseline scan.
In order to elevate brain serotonin levels, eight subjects received an intravenous administration of citalopram immediately before the intervention scan (starting 30 minutes before the scan). Prior to this, these subjects received the 5-HT1A/1B receptor antagonist pindolol (Hexapindol®) to block the autoreceptors and thus inhibit the raphe nuclei-mediated response that could decrease serotonergic firing rates in response to SSRI-induced rise in serotonin levels. The subjects were instructed by blinded investigators to take either placebo or pindolol in a dose escalation regime to minimize side effects. They took three daily pindolol doses of 2.5 mg, 5 mg, and 7.5 mg, three days, two days, and one day before the intervention day, respectively. On the intervention day, they took 7.5 mg pindolol in the morning and again approximately one hour before the [11C]Cimbi-36 scanning. The 16 subjects who were not randomized to this group received placebo tablets in a corresponding regime. The randomization and preparation of pindolol/placebo pre-treatment were undertaken by two unblinded research administrators, who were not otherwise involved in the study. Before the subjects left the research facilities after the baseline scan, they received an envelope containing Hexapindol® or placebo tablets along with written instructions of dosing, possible side effects and a contact number to a medical doctor in case of adverse reactions or intolerable side effects. To ensure compliance during this period, reminder text messages were sent to their mobile phones three times daily. Citalopram (Seropram® Lundbeck, Valby, Denmark) was administered intravenously as a constant infusion over one hour where subjects received 40 mg starting 30min before [11C]Cimbi-36 injection. The 16 subjects in the ATD and placebo groups received saline infusion. Infusions were prepared by an unblinded research nurse, but the infusions were blinded both to the participants and to the investigators.
To achieve a reduction in serotonin levels, eight subjects were randomized to ATD, which is a dietary method to lower plasma tryptophan and thereby reduce the amount available for central serotonin synthesis10,11. We used a previously published approach10, which involves ingestion of either a gelatin-based collagen peptide (CP) mixture (Solugel 5000®, PB Gelatins, Tessenderlo, Belgium) that is naturally lacking tryptophan (CP-Trp), or a CP mixture supplemented with tryptophan (CP+Trp) (Supplementary Figure S1 for full description of content). The CP-Trp drink was prepared by adding 200 ml water to 100 g Solugel 5000®. Fifty mL artificial flavoring and ice was also added to make the mixture palatable. The CP+Trp drink was prepared by adding 1.2 g of L-tryptophan (Sigma-Aldrich, Denmark) to the CP-Trp drink. The randomization and preparation of the CP mixtures were undertaken by unblinded research administrators who were not otherwise involved in the study. The subjects ingested the drink at the research facilities four hours prior to the intervention scan. The participants were instructed to avoid protein-rich food on the day before the intervention scan (Supplementary Methods), as well as to fast from 6 am on the intervention day. The 16 subjects in the citalopram/pindolol and placebo groups also followed the low protein diet, and were given a placebo drink four hours before the intervention scan (CP+Trp). Tryptophan passes the blood-brain-barrier by a specific carrier for which tryptophan competes with all other large neutral amino acids (LNAAs)12. All subjects received a standardized diet throughout the intervention day to ensure low tryptophan levels and low carbohydrate intake (Supplementary Methods), as insulin secretion preferentially stimulates uptake of all LNAAs except for tryptophan into tissue. This leads to depletion of LNAAs in plasma and consequently an increase in the ratio between tryptophan and the other LNAAs in plasma, which can result in elevated levels of tryptophan entering the brain13. That is, a high carbohydrate load can facilitate the entrance of tryptophan into the brain. During the intervention scan day, plasma levels of prolactin, citalopram, tryptophan and LNAAs were measured to verify the effects of the citalopram/pindolol and ATD interventions, respectively. Venous blood samples were analyzed for content of tryptophan and LNAAs using HPLC (Supplementary Methods). Tryptophan load was calculated as the ratio between tryptophan and the sum of the other LNAA (Trp/ΣLNAA ratio).
Magnetic Resonance Imaging
All subjects underwent MRI scans in a 3T Siemens Magnetom Verio scanner (Siemens AG, Erlangen, Germany) using a Siemens 32-channel head coil. Structural T1- and T2-weighted images (T1 protocol: Isotropic 0.9 × 0.9 × 0.9 mm resolution, repetition time (TR) = 1900 ms, echo delay time (TE) = 2.32 ms, inversion time = 900 ms, and flip angle = 9 degrees. T2 protocol: Isotropic 1 × 1 × 1 mm resolution, TR = 3200 ms, TE = 409 ms) were recorded for each subject, and based on these, a segmented MR-image was produced with vbm8 in SPM8 (Welcome Department of Imaging Neuroscience, London, http://www.fil.ion.ucl.ac.uk/spm) to mask grey matter in the subsequent automated extraction of tissue time-activity curves.
PET scanning
The 5-HT2A receptor agonist PET radioligand [11C]Cimbi-36 was produced for human administration, as previously described14. All participants were scanned in a high resolution research tomography (HRRT) PET scanner (CTI/Siemens, Knoxville, TN). Following a ten minute transmission scan a two hour emission scan was started at the time of a [11C]Cimbi-36 bolus injection (mean injected activity: 500 ± 117 MBq; injected cold Cimbi-36 dose: 0.83 ± 0.51 µg). Attentuation correction was performed as previously described15. [11C]Cimbi-36 scanning data were reconstructed into 45 dynamic frames (6 × 10 s, 6 × 20 s, 6 × 60 s, 8 × 120 s, 19 × 300 s). All PET images were motion corrected using the AIR (Automated Image Registration, v. 5.2.5, LONI, UCLA, http://bishopw.loni.ucla.edu/air5/) software. All frames were aligned to the first 5-minute frame, and no partial-volume correction was applied. Tissue time-activity curves were automatically extracted from a set of 45 distinct regions of interest (ROIs) using a data pipeline similar to previously reported16. Briefly, unfiltered PET images were coregistered and aligned to the subjects’ T1-weighted MRI image in SPM. Coregistration and ROI placement were visually inspected for each subject by overlaying ROIs on PET and MRI images. A global neocortical region was defined as volume-weighted means of the cortical ROIs: orbitofrontal cortex, superior, middle and inferior frontal gyrus; superior, middle and inferior temporal gyrus, sensorimotor cortex, parietal cortex and occipital cortex. The striatum was defined as volume-weighted means of putamen and caudate.
Radiometabolite analyses were performed using high-performance liquid chromatography (HPLC) in order estimate the concentrations of parent compound in arterial plasma, which were used as input functions in subsequent kinetic modeling. During the first 10 minutes after injection, radioactivity in whole blood was continuously measured in 2-second intervals using an Allogg ABSS autosampler (Allogg Technology, Mariefred, Sweden) counting coincidences in a lead-shielded detector (flow: 8 mL/min). Also, blood samples were drawn manually 2.5, 5, 10, 15, 20, 30, 40, 50, 70, 90, 105, and 120 minutes after injection. Plasma was obtained by centrifugation (1,500 × g for 7 minutes at 4 °C) of arterial whole blood. Radioactivity in whole blood and plasma was measured using a well counter (Cobra 5003; Packard Instruments, Meriden, CT, USA), which was cross-calibrated to the high-resolution research tomography scanner and to the autosampler. All samples were decay corrected to the time of radioligand injection.
Quantification of [11C]Cimbi-36 binding
Kinetic modeling was performed in PMOD version 3.0 (PMOD Technologies Inc.). The investigators were blinded to which intervention group the participants were allocated to, until data analysis was complete. The regional binding potential (BPND) of [11C]Cimbi-36 was calculated using the simplified reference tissue model (SRTM)14, and used as the primary outcome measure for the main statistical analyses. Secondary outcome measures included regional BPNDs that were calculated from the total distribution volumes (VT) derived from arterial input measurements and 2-tissue compartment modeling (2-TCM) as (VTtarget−VTcerebellum)/VTcerebellum. Results using 2-TCM are presented in supplemental information. We did not find BPP suitable as an outcome measure due to changes in free radioligand fraction in plasma (fP) as described in the results section and Supplementary Figure S8.
Statistical analyses
From [11C]Cimbi-36 BPNDs, the percentage difference between baseline and intervention scans was calculated as (BPNDintervention – BPNDbaseline)/BPNDbaseline for each subject. We used neocortex as our primary region of interest based on our hypothesis that the interventions would have global effects on serotonin levels. Also, neocortex is a high binding region of 5-HT2A receptors. Differences in percent change of neocortical [11C]Cimbi-36 BPND between groups (citalopram/pindolol vs. placebo, and ATD vs. placebo) were assessed with unpaired t-tests (two-tailed, unequal variances). Normality of the data evaluated in main analyses was assessed with Shapiro-Wilk normality tests, which did not indicate substantial violation of assumptions (all p > 0.2). Homogeneity of variance was evaluated using Levene’s test. Post-hoc we analyzed group differences in cortical and hippocampal regional [11C]Cimbi-36 BPND. We also evaluated at thalamus and striatum but did not find these regions suitable for evaluating group differences because we observed a spurious increase in BPND from baseline to intervention in the placebo group (of 20% and 15%, respectively9).
The effects of the interventions on prolactin and tryptophan concentrations were evaluated using a between-group two-way analysis of variance (ANOVA) of concentrations with time and treatment as main effects. Differences between groups at each timepoint were assessed with Bonferroni-corrected t-tests. The mean absolute difference in neocortical [11C]Cimbi-36 BPND between baseline and placebo intervention was previously reported to be 9% and 4% with 2-TCM and SRTM, respectively9.
Results
Demographics and [11C]Cimbi-36 radioligand injection parameters are presented in Table 2. The mean percent differences (±SD) in neocortical [11C]Cimbi-36 BPND from baseline to intervention were 7.4 ± 16.0% after citalopram/pindolol and 0.6 ± 7.2% after ATD (Fig. 1). Unpaired t-tests revealed no statistically significant differences in percent change of neocortical [11C]Cimbi-36 BPND between placebo and citalopram/pindolol (t(9.7) = 0.7, p = 0.4), or between placebo and ATD (t(12.9) = 1.6, p = 0.5). Similar results were found when 2-TCM was used to estimate [11C]Cimbi-36 BPND (Supplementary Figure S2). As seen in Fig. 1, there was large within-subject variation of [11C]Cimbi-36 binding in the citalopram/pindolol group compared with placebo (Levene’s test F(1) = 9.9, p = 0.007). The change in neocortical BPND from baseline to intervention in each subject are shown in Supplementary Figures S3–S5.
Post hoc regional analyses revealed a significant difference in percent change of hippocampal [11C]Cimbi-36 BPND between placebo and Citalopram/Pindolol (t(13.7) = 2.4, p = 0.03, Supplementary Figure S6–S7), but not between placebo and ATD (t(5.8) = -0.7, p = 0.5). The mean percent difference in hippocampal BPND from baseline to intervention in the Citalopram/Pindolol group was −25.5 ± 25.2%, indicating increased serotonin levels in this region. We found no significant group differences in percent change of [11C]Cimbi-36 BPND in any of the cortical regions of interest (frontal, temporal and parietal cortex, anterior and posterior cingulate).
The effects of citalopram/pindolol were confirmed by significant elevation of plasma prolactin measured one hour after SSRI infusion (p < 0.01, Fig. 2a), and serum citalopram measured 50 and 120min after radioligand injection (citalopram infusion started 30 min prior to radioligand injection and lasted for one hour) (Fig. 2b). Likewise, the effects of ATD were confirmed by significantly decreased concentrations of plasma tryptophan compared with subjects receiving the placebo drink containing all amino acids (p < 0.001, Fig. 2c). The mean percent change in the ratio of tryptophan to the other LNAAs (tryptophan load) from before to ~4 h after ingestion of the drink was −72.2 ± 7.2% in the ATD group, 40.4 ± 21.5% in the placebo group and 35.4 ± 7.2% in the citalopram/pindolol group.
Time on the x-axis is referenced to the time of [11C]Cimbi-36 injection. a Plasma prolactin levels relative to time of [11C]Cimbi-36 injection. **p < 0.01 indicates Bonferroni-corrected post-test between groups (citalopram/pindolol versus ATD as well as placebo groups) in a 2-way ANOVA of prolactin concentration with time and treatment as main effects. Normal interval for plasma prolactin: 70-440mIU/L. b Serum citalopram concentrations relative to time of [11C]Cimbi-36 injection. Citalopram was given intravenously for one hour starting 30 minutes before time of injection. c. Plasma tryptophan levels relative to time of [11C]Cimbi-36 injection. ***p < 0.001 indicate Bonferroni-corrected post-test between groups (ATD versus citalopram/pindolol as well as placebo groups) in a 2-way ANOVA of tryptophan concentrations with time and treatment as main effects
Free radioligand fraction in plasma (fP) was significantly higher at intervention compared with baseline in the two groups receiving placebo amino acid drinks (Supplementary Figure S8). This change in fP was not associated with changes in BPND from baseline to intervention across groups (slope estimate: −0.002, 95% confidence interval: [−0.28; 0.28], p = 0.99, Fig. 3), nor in each group analyzed separately (placebo group: p = 0.09, ATD group: p = 0.99, citalopram/pindolol group: p = 0.12).
Discussion
This study examined the sensitivity of the 5-HT2A receptor agonist radioligand [11C]Cimbi-36 to changes in endogenous serotonin concentrations in the human brain. Contrary to our hypothesis, neither ATD nor a combination of citalopram and pindolol elicited unidirectional changes in serotonin levels that were detectable with [11C]Cimbi-36 PET, at least not with the current sample size. Interestingly, we observed large within-group variation in [11C]Cimbi-36 binding after intervention with citalopram and pindolol as compared to placebo and ATD.
Studies in pigs and nonhuman primates show reductions in [11C]Cimbi-36 binding after interventions that increase serotonin levels4,17. Using microdialysis and [11C]Cimbi-36 PET in pigs, the potent serotonin releaser fenfluramine produced an increase in extracellular serotonin levels of 1123% relative to baseline, whereas citalopram in combination with pindolol led to an increase of 441% of serotonin baseline level, corresponding to a 5-HT2A receptor occupancy of 44% with fenfluramine and 28% with citalopram and pindolol measured with [11C]Cimbi-3617. This correlation between pharmacologically induced changes in extracellular serotonin levels and changes in [11C]Cimbi-36 PET signal indicates that at least in pigs, [11C]Cimbi-36 is sensitive to changes in endogenous serotonin levels. In nonhuman primates, fenfluramine also decreased [11C]Cimbi-36 binding by 26–62%4. Given these observations in animals, we expected that [11C]Cimbi-36 binding would be reduced following intervention with citalopram/pindolol also in humans and we suspect that when given acutely, and in the doses used here, citalopram has variable effects on serotonin levels in the brain. Other human PET studies evaluating the effects of acute intervention with SSRI support this interpretation; two recent studies reported increased [11C]CUMI-101 (5-HT1A receptor radioligand) and [11C]AZ10419369 (5-HT1B receptor partial agonist) binding in projection areas after acute SSRI intervention (10 mg citalopram intravenously and a single oral dose of 20 mg escitalopram, respectively) suggesting a reduction of serotonin levels6,7. The authors suggested that the increase in cortical 5-HT1A receptor binding after citalopram is driven by an action in the dorsal raphe nuclei, i.e., acute SSRI produces increases in serotonin levels in the raphe nuclei which then decreases firing rates in postsynaptic regions6. Microdialysis and neurophysiological studies in rodents have shown an increase in serotonin concentration in the raphe nuclei after administration of an SSRI, leading to attenuated release of serotonin in projection areas18,19 interpreted as caused by 5-HT1A autoreceptor regulation. One speculation is that the autoregulation of serotonergic firing may be particularly strong in the human brain, which was also the reason for our decision to inhibit the raphe nuclei-mediated response to SSRI by blocking the 5-HT1A autoreceptors with pindolol. However, the chosen dose of pindolol may only have been sufficient in some of the participants, which would be consistent with the larger within-subject variation of [11C]Cimbi-36 binding compared with placebo. Alternatively, we did manage to block the autoreceptors sufficiently, but there was a large interindividual variability in brain serotonin levels in response to SSRIs. In the latter case, our observation has a potential impact for the understanding of patient responses to SSRI. For example, SSRI treatment ameliorates depressive symptoms in some patients but not all20,21. The current sample size does not permit a meaningful analysis of potential causes of the larger variation in the citalopram/pindolol group, but may involve genetic variants, differences in pharmacodynamics, or other factors.
We used a global neocortical region in which [11C]Cimbi-36 binds selectively to 5-HT2A receptors. In our post hoc regional analyses, we found a significant decrease in hippocampal BPND after Citalopram/Pindolol. This suggests that in hippocampus, Citalopram/Pindolol is associated with an increase in serotonin that is subject to less interindividual variation. Interestingly, hippocampal volume in depressed patients increased after treatment with citalopram22. Alternatively, the decline in hippocampal BPND could be caused by its high density of 5-HT2C receptors. Recent studies have found that [11C]Cimbi-36 (in addition to 5-HT2A receptors) also binds to 5-HT2C receptors in hippocampus in the monkey23 and human brain9.
ATD is an established method for manipulating brain serotonin function, with effects on cognition and behavior24. In rodents, acute and chronic tryptophan depletion reduce central serotonin levels25,26,27,28. Using this technique, we compared [11C]Cimbi-36 BPND following ATD and a placebo condition, hypothesizing that if [11C]Cimbi-36 binding is susceptible to competition with endogenous serotonin, we would observe an increase in [11C]Cimbi-36 BPND after ATD. We found no significant changes in BPND, suggesting that the reduction in endogenous serotonin levels elicited by ATD was not sufficient to produce a measurable increase in the binding of [11C]Cimbi-36. Indeed, the effect of ATD on central serotonin levels has been debated29,30, and the extent by which ATD alters serotonin levels in humans may vary across individuals and contexts30. Previous PET studies investigating the effects of ATD on components of the serotonin system have produced mixed results. Studies in healthy controls report no ATD induced changes in 5-HT2A receptor binding ([11C]MDL100907)31, or serotonin transporter binding ([11C]DASB)32,33, whereas prefrontal monoamine oxidase A density was decreased following ATD34. In a study of patients with major depressive disorder, regional 5-HT1A receptor binding ([18F]-MPPF) was not altered after ATD, although six of the eight patients had a transient relapse in depressive symptoms35. Studies examining patients with remitted major depressive disorder found that ATD induced a transient return of depressive symptoms36,37, along with decreased 5-HT2 receptor binding of [18F]Setoperone in patients36, and augmented regional cerebral glucose utilization in patients but not in controls37. We did not observe any effects of ATD on mood in our healthy participants (reported in Stenbæk et al.10). One interpretation of the current data is that these healthy participants, who showed no signs of psychopathology, may be resilient to ATD and are able to maintain stable serotonin levels and mood. Alternatively, ATD did not elicit a sufficient decrease in serotonin levels to be detected with our sample size.
On intervention days, all participants regardless of treatment group received a drink with a high load of amino acids. In the two groups receiving placebo drinks (containing tryptophan) radioligand plasma protein binding was lower on intervention days compared with baseline. Tryptophan in plasma is bound to albumin, with approximately 5% being left free and available for transport into the brain13. We suggest that the high load of amino acids, in particular tryptophan, was responsible for the increase in fP. A possible explanation is that competition between tryptophan and [11C]Cimbi-36 binding with plasma binding proteins (e.g. albumin) occurs, resulting in a larger proportion of free [11C]Cimbi-36 in plasma. Few other PET studies have reported effects of tryptophan on fP. Talbot et al.31 report that the fP of [11C]MDL100907 did not differ between placebo and ATD31. In another study investigating binding of the serotonin transporter tracer [11C]DASB after intervention with ATD, there was no difference in fP of [11C]DASB between the two conditions, but there was a small decrease in both VT and BP from baseline to intervention that was related to a concomitant difference in fP of similar magnitude32. In the latter study, the change in BP and VT became non-significant when corrected for fP, suggesting that the effect may have been driven by changes in fP32. We used BPND as the outcome measure; this parameter is independent of changes in fP, and as such, we consider the findings of this study to reflect changes in 5-HT2A receptor availability.
A few limitations of this study should be mentioned. The study includes a limited sample size, but the low test-retest variability and investigation of the same individuals before and after the intervention is a strong design. Even though we cannot exclude that the variability observed in the citalopram/pindolol group can be attributed to measurement error we find this unlikely, given the low variability in the placebo group. Another limitation is that the increase in both the tryptophan concentration and the tryptophan load (Trp/ΣLNAA ratio) in the groups receiving placebo amino acid drinks may have affected the cerebral serotonin levels in these participants, potentially interfering with the effects of SSRI treatment. Likewise, pindolol treatment may also have affected the cerebral serotonin levels, putatively interfering the effect of SSRI.
In conclusion, the data presented in this study suggest that neither ATD nor a combination of citalopram and pindolol elicit acute unidirectional and global changes in extracellular serotonin levels that are sufficient to be detected by [11C]Cimbi-36 PET. Recent data suggest that [11C]Cimbi-36 is sensitive to changes in endogenous serotonin levels only when serotonin release is sufficiently high, e.g. amphetamine challenge38. The large variability in [11C]Cimbi-36 binding after citalopram/pindolol could reflect interindividual differences in brain serotonin levels after an acute SSRI intervention. This observation may have a potential impact for the understanding of patient responses to SSRIs, which should be explored in future studies.
References
Laruelle, M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J. Cereb. Blood. Flow. Metab. 20, 423–451 (2000).
Paterson, L. M., Tyacke, R. J., Nutt, D. J. & Knudsen, G. M. Measuring endogenous 5-HT release by emission tomography: promises and pitfalls. J. Cereb. Blood. Flow. Metab. 30, 1682–1706 (2010).
Narendran, R. et al. In vivo vulnerability to competition by endogenous dopamine: comparison of the D2 receptor agonist radiotracer (-)-N-[11C]propyl-norapomorphine ([11C]NPA) with the D2 receptor antagonist radiotracer [11C]-raclopride. Synapse 52, 188–208 (2004).
Yang, K. C. et al. Fenfluramine reduces [11C]Cimbi-36 binding to the 5-HT2A receptor in the nonhuman primate brain. Int. J. Neuropsychopharmacol. 20, 683–691 (2017).
Pinborg, L. H. et al. No change in [(1)(1)C]CUMI-101 binding to 5-HT(1A) receptors after intravenous citalopram in human. Synapse 66, 880–884 (2012).
Selvaraj, S. et al. Measuring endogenous changes in serotonergic neurotransmission in humans: a [11C]CUMI-101 PET challenge study. Mol. Psychiatry 17, 1254–1260 (2012).
Nord, M., Finnema, S. J., Halldin, C. & Farde, L. Effect of a single dose of escitalopram on serotonin concentration in the non-human and human primate brain. Int. J. Neuropsychopharmacol. 16, 1577–1586 (2013).
Olsen, L. R., Mortensen, E. L. & Bech, P. The SCL-90 and SCL-90R versions validated by item response models in a Danish community sample. Acta Psychiatr. Scand. 110, 225–229 (2004).
Ettrup, A. et al. Serotonin 2A receptor agonist binding in the human brain with [(11)C]Cimbi-36: Test-retest reproducibility and head-to-head comparison with the antagonist [(18)F]altanserin. Neuroimage 130, 167–174 (2016).
Stenbaek, D. S. et al. Evaluation of acute tryptophan depletion and sham depletion with a gelatin-based collagen peptide protein mixture. Eur. Neuropsychopharmacol. 26, 147–149 (2016).
Mendelsohn, D., Riedel, W. J. & Sambeth, A. Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci. Biobehav. Rev. 33, 926–952 (2009).
Oldendorf, W. H. & Szabo, J. Amino acid assignment to one of three blood-brain barrier amino acid carriers. Am. J. Physiol. 230, 94–98 (1976).
Hood, S. D., Bell, C. J. & Nutt, D. J. Acute tryptophan depletion. Part I: rationale and methodology. Aust. N. Z. J. Psychiatry 39, 558–564 (2005).
Ettrup, A. et al. Serotonin 2A receptor agonist binding in the human brain with [(1)(1)C]Cimbi-36. J. Cereb. Blood. Flow. Metab. 34, 1188–1196 (2014).
Keller, S. H., Svarer, C. & Sibomana, M. Attenuation correction for the HRRT PET-scanner using transmission scatter correction and total variation regularization. IEEE Trans. Med. Imaging 32, 1611–1621 (2013).
Svarer, C. et al. MR-based automatic delineation of volumes of interest in human brain PET images using probability maps. Neuroimage 24, 969–979 (2005).
Jorgensen, L. M. et al. Cerebral 5-HT release correlates with [(11)C]Cimbi36 PET measures of 5-HT2A receptor occupancy in the pig brain. J. Cereb. Blood. Flow. Metab. 37, 425–434 (2017).
Invernizzi, R., Belli, S. & Samanin, R. Citalopram’s ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug’s effect in the frontal cortex. Brain Res. 584, 322–324 (1992).
Hervas, I. & Artigas, F. Effect of fluoxetine on extracellular 5-hydroxytryptamine in rat brain. Role of 5-HT autoreceptors. Eur. J. Pharmacol. 358, 9–18 (1998).
Nakajima, S. et al. Is switching antidepressants following early nonresponse more beneficial in acute-phase treatment of depression?: a randomized open-label trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 1983–1989 (2011).
Rush, A. J. et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry 163, 1905–1917 (2006).
Arnone, D. et al. State-dependent changes in hippocampal grey matter in depression. Mol. Psychiatry 18, 1265–1272 (2013).
Finnema, S. J. et al. Characterization of [(11)C]Cimbi-36 as an agonist PET radioligand for the 5-HT(2A) and 5-HT(2C) receptors in the nonhuman primate brain. Neuroimage 84, 342–353 (2014).
Cools, R., Roberts, A. C. & Robbins, T. W. Serotoninergic regulation of emotional and behavioural control processes. Trends. Cogn. Sci. 12, 31–40 (2008).
Lieben, C. K., Blokland, A., Westerink, B. & Deutz, N. E. Acute tryptophan and serotonin depletion using an optimized tryptophan-free protein-carbohydrate mixture in the adult rat. Neurochem. Int. 44, 9–16 (2004).
Brown, C. M., Fletcher, P. J. & Coscina, D. V. Acute amino acid loads that deplete brain serotonin fail to alter behavior. Pharmacol. Biochem. Behav. 59, 115–121 (1998).
Fadda, F., Cocco, S. & Stancampiano, R. A physiological method to selectively decrease brain serotonin release. Brain. Res. Brain. Res. Protoc. 5, 219–222 (2000).
Merchan, A. et al. Tryptophan depletion affects compulsive behaviour in rats: strain dependent effects and associated neuromechanisms. Psychopharmacology 234, 1223–1236 (2017).
van Donkelaar, E. L. et al. Mechanism of acute tryptophan depletion: is it only serotonin? Mol. Psychiatry 16, 695–713 (2011).
Crockett, M. J. et al. Converging evidence for central 5-HT effects in acute tryptophan depletion. Mol. Psychiatry 17, 121–123 (2012).
Talbot, P. S. et al. Extended characterisation of the serotonin 2A (5-HT2A) receptor-selective PET radiotracer 11C-MDL100907 in humans: quantitative analysis, test-retest reproducibility, and vulnerability to endogenous 5-HT tone. Neuroimage 59, 271–285 (2012).
Talbot, P. S. et al. Effects of reduced endogenous 5-HT on the in vivo binding of the serotonin transporter radioligand 11C-DASB in healthy humans. Synapse 55, 164–175 (2005).
Praschak-Rieder, N. et al. Effects of tryptophan depletion on the serotonin transporter in healthy humans. Biol. Psychiatry 58, 825–830 (2005).
Sacher, J. et al. Dynamic, adaptive changes in MAO-A binding after alterations in substrate availability: an in vivo [(11)C]-harmine positron emission tomography study. J. Cereb. Blood. Flow. Metab. 32, 443–446 (2012).
Praschak-Rieder, N. et al. Tryptophan depletion and serotonin loss in selective serotonin reuptake inhibitor-treated depression: an [(18)F] MPPF positron emission tomography study. Biol. Psychiatry 56, 587–591 (2004).
Yatham, L. N. et al. Positron emission tomography study of the effects of tryptophan depletion on brain serotonin(2) receptors in subjects recently remitted from major depression. Arch. Gen. Psychiatry 69, 601–609 (2012).
Neumeister, A. et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch. Gen. Psychiatry 61, 765–773 (2004).
Erritzoe D. C. A., et al Serotonin release measured in the human brain: A PET study with [C-11]Cimbi-36 and d-amphetamine challenge. 28th International Symposium on Cerebral Blood Flow, Metabolism and Function/13th International Conference on Quantification of Brain Function with PET. SAGE PUBLICATIONS INC2017, pp 73–73.
Acknowledgements
We thank all the volunteers for kindly participating in this study. Lone Ibsgaard Freyr, Bente Dall, Gerda Thomsen, Martin Korsbak Madsen, Svitlana Olsen, and Mikkel Lohmann Schiøtt are gratefully acknowledged for technical assistance. This study was financially supported by the Lundbeck Foundation (Cimbi grant), the Arvid Nilssons Foundation, the Aase and Ejnar Danielsen foundation and The Danish Council for Independent Research Fund. The funding sources were not involved in the study design or in the collection, analysis, writing or publication of data. We gratefully acknowledge the John and Birthe Meyer Foundation for donation of the HRRT scanner and the Toyota Foundation for donation of the HPLC equipment.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
da Cunha-Bang, S., Ettrup, A., Mc Mahon, B. et al. Measuring endogenous changes in serotonergic neurotransmission with [11C]Cimbi-36 positron emission tomography in humans. Transl Psychiatry 9, 134 (2019). https://doi.org/10.1038/s41398-019-0468-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41398-019-0468-8
- Springer Nature Limited
This article is cited by
-
Serotonin release measured in the human brain: a PET study with [11C]CIMBI-36 and d-amphetamine challenge
Neuropsychopharmacology (2020)