Abstract
Blood–brain barrier (BBB) is a natural protective membrane that prevents central nervous system (CNS) from toxins and pathogens in blood. However, the presence of BBB complicates the pharmacotherapy for CNS disorders as the most chemical drugs and biopharmaceuticals have been impeded to enter the brain. Insufficient drug delivery into the brain leads to low therapeutic efficacy as well as aggravated side effects due to the accumulation in other organs and tissues. Recent breakthrough in materials science and nanotechnology provides a library of advanced materials with customized structure and property serving as a powerful toolkit for targeted drug delivery. In-depth research in the field of anatomical and pathological study on brain and BBB further facilitates the development of brain-targeted strategies for enhanced BBB crossing. In this review, the physiological structure and different cells contributing to this barrier are summarized. Various emerging strategies for permeability regulation and BBB crossing including passive transcytosis, intranasal administration, ligands conjugation, membrane coating, stimuli-triggered BBB disruption, and other strategies to overcome BBB obstacle are highlighted. Versatile drug delivery systems ranging from organic, inorganic, and biologics-derived materials with their synthesis procedures and unique physio-chemical properties are summarized and analyzed. This review aims to provide an up-to-date and comprehensive guideline for researchers in diverse fields, offering perspectives on further development of brain-targeted drug delivery system.
Similar content being viewed by others
Introduction
Blood–brain barrier (BBB) is a semi-permeable barrier encompassing microvasculature of central nervous system (CNS). In the capillaries, the wedged endothelial cells line in the interior vessels forming extensive tight junctions.1,2,3 Together with an ensemble of receptors, transporters, efflux pumps and other cellular components, the barrier takes control of entrance and expulsion of the molecules in vascular compartment to the brain. The intact BBB impedes the influx of most blood-borne substances from entering the brain. But it should be noted that at meantime of brain protection, BBB also excludes more than 98% of small-molecule drugs and all macromolecular therapeutics from access to the brain.4,5 The tight gap allows only passive diffusion of lipid-soluble drugs at a molecular weight lower than 400-600 Da. Increasing lipophilicity of the therapeutic agents is a feasible method to improve the BBB permeability. For example, Crizotinib, an oral selective small-molecular tyrosine kinase inhibitor, is an effective anti-cancer medicine but with poor activity against brain tumor metastases due to its low BBB penetration.6 Structural modification of conjugation of a fluoroethyl moiety increases the lipophilicity of Crizotinib and results in enhanced brain permeability.7 However, increasing lipophilicity is not a universal strategy as it may inhibit the biological activity of drugs of interest. Further, therapeutic drugs with high liposolubility have longer retention and duration of action in non-target peripheral organs, causing considerable side effects. In addition, due to the presence of P-glycoprotein (referred to as multidrug resistance associated membrane protein), drugs could be transported back into the blood by ATP-dependent efflux pumps.8,9 Thus, it is urging to address the issue of brain-targeted therapeutics by developing effective and safe delivery strategies.
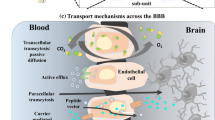
Spurred by recent development of materials science and nanotechnology, various strategies for regulation of BBB permeability were developed as well as a library of brain-targeted drug delivery systems. Transport routes of the drug molecules across the BBB occurs via the pathways including paracellular and transcellular diffusion, receptor-mediated transcytosis, cell-mediated transcytosis, transporter-mediated transcytosis, and adsorptive mediated transcytosis (Fig. 1a).10 Many efforts have been made in response to each process of the drug transportation, which have also been accompanied by reviews of latest progress in this field, but most of literature emphasize the BBB breakdown in specific brain disorders,11,12,13,14 or give a general review of delivery strategies concentrating on the barrier physiology.15,16 On the basis of these strategies, functional materials with small size, tailored architecture and therapeutic motifs that facilitate the targeted drug delivery are widely engineered. They can be constructed by using a wide range of substrates including organic (e.g., liposomes, micelles, hydrogels, etc.),17,18,19 inorganic (e.g., metal/metal oxide particles, silica nanoparticles, quantum dots, etc.),20,21 and biomass-derived materials (e.g., exosomes, cells, bacteria, etc.).22,23,24 Improved performance of brain-targeted drug delivery was proved thanks to these materials with obvious advantages including non-invasive delivery, high drug loading, good biocompatibility, prolonged blood circulation, and importantly brain targeting effect. Over the past few years, we have witnessed the booming advances in exploiting materials science and nanotechnology in brain-targeted drug delivery. Brain disorder therapy utilizing tailored systems has been a benefactor of this emergence, yet a timely and comprehensive review is lacking. In the light of excellent performance of BBB crossing strategies for enhanced drug delivery, we will first summarize the structure and different cells contributing to the integrity of BBB (Fig. 1b). Further, main BBB crossing strategies derived are highlighted with relevant transcytosis mechanisms as well as their interactions at barrier interface (Fig. 1c). Specifically, we addressed the issues of different pathways from passive transcytosis, intranasal administration, ligands conjugation for brain targeting, membrane coating for brain targeting, to stimuli-mediated BBB disruption. Then, the updated progress of customized materials was overviewed and how they were fabricated and utilized for the treatment of brain disorders was summarized (Fig. 1d). The discussion of potential translational trials and the remaining challenging in this field will also be presented. This review will be of great interest to those working in materials science, nanotechnology, and especially to those in biomedical engineering and translational medicine, which will not only serve as an up-to-date compilation of achievements in this area, but also provide a generalized guideline for BBB regulation and rational design of targeted drug delivery systems.
BBB structure and physiology
Anatomical structure of the BBB
The existence of the BBB was first found by Paul Ehrlich and proved by Edwin Goldmann.25,26 BBB is a spacious, multicellular, and dynamic semi-permeable membrane that isolates the foreign substances in the blood from the CNS.27 The presence of BBB avoids the brain from damage by keeping a stable environment.28,29 But it also limits the drugs that enter into the CNS for treating brain diseases such as neurodegenerative diseases and brain cancer.30,31 Capillaries are the major site of the BBB. Because the neural cell is close to a capillary no further than 25 µm, passaging the BBB for drug delivery is a favored route when compared to another by-passed route which is relatively longer.32 This case pushes researchers to develop effective strategies to regulate BBB permeability and targeted delivery systems to overcome the BBB limits. Some reviews have discussed the BBB recognition which is one of the key steps for enhanced brain-targeted delivery.33,34,35 For a deeper understating of the interaction between delivery systems and the brain, how the BBB is constructed should be clarified.
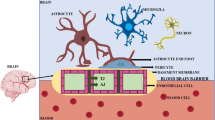
From the physiological point of view, endothelial cells, astroglia, pericytes, and junctional complexes including tight junctions and adherens junctions compose the BBB basically.36,37,38,39 In this section, we focus on the five constituents mentioned above and others are not covered here.
Endothelial cells
Endothelial cells are considered as the BBB’s core anatomical structure for lining the cerebral blood vessels and interacting with different types of cells in the CNS.40,41 The endothelial cells in the BBB differ from peripheral endothelial cells in morphology and function.42,43 The barrier performance is not the innate properties of endothelial cells.44,45 For morphology, the endothelial cells in the BBB are fastened by both tight junctions and adherens junctions, resulting in distinct lumenal and abluminal membrane compartments.46 They further present with no fenestrations, also known as small transcellular pores, which greatly limit free diffusion and the rapid exchange of molecules between brain tissue and blood.47 Besides, the amounts of mitochondria in endothelial cells in the BBB are higher than in peripheral endothelial cells, which means more energy is needed for transport.48,49 For function, first, they display a net negative surface charge, refusing to accept negatively charged compounds, as well as quite low degrees of leukocyte adhesion molecules, hampering the entry of the number of immune cells.50,51,52 Second, they show designated transporters for regulating the inflow and outflow of specific substrates.53 Third, they show a restriction on the number of transcellular vesicles through the vessel wall due to the high transendothelial electrical resistance.54,55 Because of the existence of the local environment, endothelial cells can together form and maintain the BBB.
Astrocytes
Astrocytes, also known as astroglia, are the most numerous glial cells, expressing polarized and complex morphology, which are heterogeneous throughout the brain.56 In tradition, they are divided into two categories, that one is protoplasmic which locates in the well-vascularized gray matter, and the other one is fibrous which locates in the less vascular white matter.57,58,59 Their end feet link them with the basement membrane, via the binding of a set of proteins (aquaporin IV and the dystroglycan-dystrophin complex) with the proteoglycan agrin.60,61 In the CNS, they play a major role in dynamic signaling such as clearing waste, tuning brain blood flow, regulating vascular function, ion hemostasis and balancing neuroimmune responses.62,63,64,65,66 However, the exact role of astrocytes in BBB function is still controversial.67,68,69 Some studies considered astrocytes can arise barrier behaviors in cerebral, other endothelial cells, and related epithelial, while other studies thought the BBB goes first before the appearance of astrocytes. In this regard, there is no doubt that the BBB exists primarily through the coordination between cells, and astrocytes are a type of neural cells that together with pericytes surround blood vessels in the brain, serving as the interface between neurons and endothelial cells.70,71 Moreover, for invertebrates that lack a vascularized circulatory system, astrocytes are the main components of the barrier separating humoral fluids from the CNS.72
Pericytes
Pericytes are mural cells presenting at intervals along the walls of the capillary blood vessels.73 They are embedded in the basement membrane and lie abluminal to the endothelial cells.74 The length that pericytes covering the CNS endothelium approaches 100%.75 It should be noted that pericytes are central to the neurovascular unit function.76,77,78 Because of the physical apposition, pericytes and endothelial cells are in close communicate with each other. For example, the PDGF-B signaling pathway is one such communication.79,80 Endothelial cells secrete the PDGF-B to bind PDGFRβ on pericytes, which recruits pericytes to blood vessels.81,82 In turn, pericytes also can release signaling factors to affect endothelial cells by determining the numbers of tight junctions and polarizing the end feet of astrocytes.83 If the amounts of pericytes reduce, the tight junctions between endothelial cells will also be reduced.84 Except for modulating and maintaining the BBB, pericytes also have functions in adjusting cerebral blood flow, vascular development and maintenance, and neuroinflammation.85,86,87
Tight junctions
In the BBB, tight junctions are the main functional components in sustaining the permeability barrier and controlling tissue homeostasis.88,89,90,91 They are also known as occluding junctions or zonulae occludes which can restrict the cross of hydrophilic molecules and macromolecules.92 They reside between endothelial cells, seal the interendothelial cleft, and work as gates and fences to limit paracellular permeability and the lateral diffusion of integral membrane proteins and lipids, thus maintaining cell polarization.93,94 There are many transmembrane and cytoplasmic proteins involved in forming tight junctions. Claudins and occludins that situate in two-cell contacts are the major tight junction proteins.95,96 Claudins display essential barrier function and occludins ensure the tightness of the tight junctions.97 Tricellulin and the lipolysis-stimulated lipoprotein receptor that reside in three-cell contacts are also the major tight junction proteins.98,99 Besides, more like junction adhesion molecules, calcium/calmodulin-dependent serine protein kinase, monoclonal antibody 7H6, and heterotrimeric G protein also contribute to the constitution of tight junctions.100,101,102,103 Junction adhesion molecules mediate the early attachment in the BBB developmental processes.104,105 Kinase-like proteins modulate the blood–brain barrier permeability.106 Interactions among these proteins provide physical support for the complex of tight junctions.107 Downregulation of tight junction-related proteins will loss of the BBB phenotype.108
Adherens junctions
Adherens junction is extra crucial for the BBB structural integrity and appropriate assembly of proteins of tight junctions.109,110 At cellular interfaces, they build spatially, chemically, and mechanically discrete microdomains.111 In common with tight junctions, adherens junctions are linked to the cytoskeleton and consisted of transmembrane and cytoplasmic plaque proteins.112,113 Vascular endothelial-cadherin, scaffolding proteins catenins, scaffolding proteins p120, plakoprotein are the main components of adherens junctions.114,115 Vascular endothelial-cadherin primarily is associated with cell-to-cell adhesion.116,117 It is a homo-dimeric transmembrane protein whose extracellular domain can connect with the other same molecules of neighboring endothelial cells in the paracellular cleft, and the cytoplasmic domain can interact with the actin filaments via scaffolding proteins.2 In the meantime, catenins, p120, plakoprotein are responsible for supporting physics and regulating junctions by forming a bridge that interacts with zonula occludens-1 (tight junctions proteins) and the actin filaments.118,119 Besides, other proteins, such as platelet and endothelial cell adhesion molecule 1, CD99, and nectin, have been reported that might be related to the adherens junction.120,121,122 Totally, adherens junctions are fundamental for the integrity of BBB, any change of adherens junctions may disrupt inter-endothelial cell connections.123
Physiology of the BBB
The existence of BBB provides a controlled microenvironment by regulating the exchange of ions and molecules between the bloodstream and brain tissue. Numerous studies have revealed the physiological functions of the BBB such as brain protection. In addition to physical restriction of the potentially harmful substances, the BBB also plays multiple roles in maintaining homeostasis,124,125 facilitating transport of the essential molecules,126 regulating inflammation127,128 and so forth. The BBB maintains the brain homeostasis by regulating the specific ions channels and transporters. For example, Na+, K+, Ca2+, Cl− are the major ions in the CNS, which should be kept at an optimal level for neural and synaptic signaling functions. These ions are asymmetrically distributed between luminal and abluminal membranes, of which efflux and influx were mainly dependent on the ion transporters on BBB. For example, the influx of Na+ and the efflux of K+ are regulated by abluminal Na-K-ATPase against the concentration gradient for maintenance of the electrochemical gradient across the cell membrane.129 Meanwhile, cotransporters such as NKCC1 regulate the ion balance by transporting Na+, K+, Cl−.130 The dysfunction of the ion transporters may induce the pathological alterations. Other BBB transporters such as solute carriers and ATP-binding case families control the transport of other essential molecules, metabolites and nutrients, maintaining the brain homeostasis.131 Intriguingly, pathways through the junctional complex or across the cells actively that participate in transport of ions, nutrients and other molecules could also be potential routes for drug delivery, which is discussed in detail in Section 3.1.
Strategies for BBB regulation and crossing
Brain tumors, cerebrovascular diseases, and neurodegenerative diseases, including Parkinson’s disease, Alzheimer’s disease, and multiple sclerosis, are serious CNS diseases.132 However, therapies for these challenging diseases are limited, because of the lack of effective methods, to enable drugs surpass natural protective hindrances to maintain homeostasis within the brain for preventing the entry of drug molecules to the CNS.133 A number of drugs are routinely administrated by invasive strategies for the management of these diseases and symptom control. For instance, intrathecal drug administration is a typical method for drug delivery into the entire ventricular system without passing through the BBB.134,135 It has been approved to deliver anti-sense oligonucleotides for the treatment of spinal muscular atrophy.136 Other invasive strategies including convection-enhanced delivery,137,138 intracranial implantation,139,140 and deep-brain stimulation were developed for brain drug delivery.141,142 Recent development of microneedle and polymeric wafers with minimal invasiveness provides sustained drug release for CNS disease management which circumvent the BBB.143,144,145,146 But the potential risks from brain exposure and damage of these invasive strategies limited their applications for long-term use. In order to efficiently deliver drugs into the brain with safety and precision, various non-invasive strategies to overcome BBB have been exploited. These strategies include BBB penetration via passive transcytosis, intranasal administration, ligands conjugation, membrane coating, and the BBB disruption using light, focused ultrasound, biochemical reagents, and radiation.
Passive transcytosis
Passive transcytosis, also known as non-specific transfers, has two possible routes across the microvascular endothelial layer, via paracellular and transcellular.147
The paracellular pathway is the principal route blocked by the tight junctions of cell gaps, restricting ions, polar solutes, and most macromolecules.148 But the tight junctions are not perfect, small and soluble substances could sufficiently cross through the paracellular pathway.149 Typically, BBB is destroyed in some brain diseases. One strategy for therapeutics to use the paracellular pathway is by downregulating the expression of tight junction proteins. For example, a modulator called minoxidil sulfate (MS) that anchored the potassium channel, enhances shipping by attenuating the tight junction proteins.150 Zhou et al. developed brain tumor-targeting ligand-modified nanoparticles named CTX-mHph2-III-62%, inside which co-encapsulation of three modulators, minoxidil, lexiscan, and NECA.151 If without further engineering, the ability of terpolymer III-62% to penetrate the BBB is limited. Through co-encapsulation, such nanoparticles could release BBB modulators in the local tumor site, which adjust the permeability of BBB in the paracellular pathway to ensure more nanoparticles in the same area. Besides, Han et al. developed M@H-NPs using hyaluronic acid-based nanoparticles to load minoxidil, which can target brain metastatic tumors.152 Hyaluronic acid can specially target cell surface receptor CD44, which is highly expressed in breast cancer.153 With the help of the CD44 target and MS boost, M@H-NPs could penetrate blood–brain tumor barrier (BTB), internalizing into brain metastatic tumor cells (BMTCs), blunting drug efflux at BMTCs, and producing effective treatment which can extend the median survival time in breast cancer brain metastases models.
The transcellular pathway is the preferable route for carriers to enter and transport therapeutic compounds, when compared to the paracellular pathway. In brief, the molecules can partition into the cellular membranes from the top to the bottom lateral side through carrier-mediated and receptor-mediated transcytosis.154 In particular, lipophilic carriers and some other known carrier systems like cationic amino acids are mainly transported via the transcellular pathway. For instance, Allan et al. showed targeting is possible in intracerebral hemorrhage (ICH) using lipid nanoparticles.155 They fabricated a liposome HSPC:CHOL: DSPE-PEG2000 and injected them into ICH mice. Brain liposome accumulation was measured via radioisotope and optical detection methods, in order to investigate the kinetics of the liposome across the brain. From their results, after injection, the entry of liposome amounts peaked at 3 h and 48 h, which provided evidence that by utilizing transcellular pathways, liposomes can gather at the lesion site through self-diffusion. Gu et al. modified OX26 antibody to the surface of PEGylated cationic lipid nanoparticles to load baicalin, which was known as OX26-PEG-CSLN.156 OX26-PEG-CSLN not only can deliver drugs across BBB but also can adjust the changes of extracellular amino acids. OX26-PEG-CSLN showed a stronger effect than the baicalin solution., which might be attributed to the more enhanced ability to penetrate the BBB and prolong the valid time of the drugs.
The tightness of the BBB precludes the entrance of most pharmaceuticals into the brain via passive transcytosis, except for hydrophilic compounds which have a mass lower than 150 Da and highly hydrophobic compounds which have a mass lower than 400–600 Da.157,158 For this reason, if passive transcytosis is demanded to realize the delivery of the drugs at higher molecular weight, some strategies to enhance the BBB permeability or break BBB temporarily will be desired.
Intranasal administration
Intranasal administration is a non-invasive route, allowing rapid brain targeting from nose to brain, when compared to direct injection by intraventricular and intraparenchymal into brain tissue.159 Except for those cleared drug molecules by mucociliary clearance, the remains of drugs entered the nasal cavity via neuronal pathway and systemic circulation. As depicted in Fig. 2a, the perineural and perivascular regions of the olfactory and trigeminal nerves are major contributors to the process of the target after intranasal administration. There are three nose-to-brain pathways: (1) Olfactory nerve-olfactory bulb-brain; (2) Trigeminal nerve-brain; and (3) Lungs/ Gastrointestinal tract-blood–brain.160 Among them, (1) and (2) are the major routes through neuronal pathways while (3) is the minor route through the systemic circulation. Specifically, (1) is the shortest and most direct route. It has been used advantageously to deliver drugs at high speed. It was possible to complete the delivery within the time window of 1.5-6 h, and even the route via olfactory epithelial cells takes only a few minutes.161 (2) is also a direct route of drug delivery, because the trigeminal nerve locates in not only the respiratory region but also the olfactory region. The trigeminal nerve has three branches, each of them joined to the brain stem and olfactory bulb, mainly liable for sensating pain and temperature.162 (3) is an indirect route connected to the systemic circulation involving the gastrointestinal and respiratory systems. It belongs to systematic circulation, which may cause extensive drug metabolism. To sum up, the amount of indirect delivery of drugs, when compared to direct delivery, is probably less, due to unexpected elimination in the body.
Intranasal administration allows rapid brain targeting from nose to brain. a Schematic illustration of the routes from the nasal cavity to the brain. b Transmission electron microscopy and structure schematic images of LENK nanoparticles. c The change of concentration of LENK in the olfactory bulb and cerebrum after the administration of nanopeptide or as the peptide alone. b, c Reproduced with permission. Copyright 2017, Elsevier. d Dynamics of fluorescence in rat trigeminal nerve. e Fluorescence intensity in the trigeminal nerve. f CSF concentration of Alexa-dextran. g Dynamics of MPEGePCLeTat complex in brain tissue. Reproduced with permission. d–g Reproduced with permission. Copyright 2013, Elsevier
Intranasal drug delivery systems, as one of the important brain-targeted systems, possess the ability to pass through the BBB using above discussed nose-to-brain pathways. Uchegbu et al. constructed a nano-peptitde with a 30–60 nm particle size, encapsulating leucine5-enkephalin hydrochloride (LENK), and proved this nanoparticle was able to transport LENK through intranasal administration (Fig. 2b, c).163 The results presented that after administered, LENK was found in the olfactory bulb, but into the brain it was hard to find them. But using the formulations of nanoparticles, the brain distribution of LENK was facilitated, with no peripheral exposure, and within the thalamus and cortex, nanoparticle localization can be observed. Similarly, in another study, Seta et al. developed nano micelles MPEG-PCL-Tat for intranasal administration using a cell-penetrating peptide (Tat), who is derived from HIV, to modify nano micelles, which were comprised of polyethylene glycol-polycaprolactone polymers (Fig. 2d–g).164 Functionally, this system played an important role to deliver siRNA to the brain. The authors harvested nasal olfactory mucosa or olfactory bulb, and prepared frozen samples of that, demonstrating the pathway of nucleic acid transfer using the system, focusing on the major nose-to-brain pathways involving olfactory nerve and trigeminal nerve.
Due to the unique anatomical relationship between the CNS and the nasal cavity, relatively quick along with easy access for nanodrugs to the brain could be realized via intranasal administration. However, there are also some limitations, including the difference in the shape of respective nasal cavities, the exact dosing of intranasal drugs, the mucociliary elimination, and the drainage to the pharynx or to the lower part.165 Moreover, the health status of the body also needs to think, otherwise, maybe someone occurs conditions such as allergies or colds, which is not suited for intranasal administration. It appears the outcomes of delivery by the nasal route differ widely between the studies.166 Thus, the nasal route to deliver drugs into the brain is rather immature and more high-quality drug delivery systems should note to solve the above-motioned limitations.
Ligands conjugation for brain targeting
Ligands conjugation is an active targeting strategy using ligands that have high specificity toward the receptor expressed on the brain endothelial cells.167 As shown in Fig. 3a, here we take transferrin receptors, insulin receptors, low-density lipoprotein receptors, and folate receptors as examples.
Ligands conjugation is an active targeting strategy using ligands that have high specificity toward the receptor on the endothelial cells of the brain. a Schematic illustration of receptor-mediated drug delivery using ligands conjugated nanoparticles to the brain. b The MRI images of the brain of glioma-bearing mouse. The photo and micrograph image of glioma which was removed from U87-MG glioma nude mice. c Real-time imaging of the U87-MG glioma nude mice. b, c Reproduced with permission. Copyright 2020, Ivyspring International Publisher. d Microscopic visualization of nanoconjugates after permeating the BBB. Scale bar: 20 μm. Reproduced with permission. Copyright 2022, American Chemical Society. e Biodistribution results of NIR-797-labeled NP-5N and NP-5N-FRα-FA in ICR mice. Reproduced with permission. Copyright 2019, American Chemical Society
Transferrin receptor as a glycoprotein, has two subunits of 90 kDa linked by a disulfide bridge, participating in the transcytosis of cellular iron by each subunit is capable of binding to one molecule of transferrin.168 There have been a lot of studies undertaken using transferrin ligands. Rao et al. formulated a di-block polymer of Poly-lactic-co-glycolic acid (PLGA) and hetero bi-functional COOH-PEG-NH2, embedded with an imidazotetrazine alkylating agent (TMZ), and conjugated to a ligand (polysorbate-80/transferrin) and a stem cell targeting moiety (anti-nestin antibody).169 Such nanocomposites having targeting ligands could deliver TMZ to intracerebral glioblastoma xenografts and present favorable pharmacokinetics and anti-cancer potential. Qi et al. used RI7217, a monoclonal antibody from mouse, which shows high selectivity and sensitivity for the transferrin receptor, to modify long-circulating liposomes (Fig. 3b, c).170 In their research, hCMEC/D3 cells and U87-MG glioma cells were used to evaluate the uptake and mechanism of the targeted liposomes, and intracranial U87-MG glioma was used to test the capacity of the targeted liposomes to cross the BBB and anti-tumor. They concluded that RI7217 antibody decoration is a promising strategy to make a drug delivery system towards brains at the end. Xie et al. optimized dual-mediated liposomes with transferrin and cell-penetrating peptide.171 Firstly, in order to construct the rational dual-mediated liposomes, they screened the different PEG molecular weight which is used for connecting transferrin and cell-penetrating peptide (CPP) with liposomes and the densities of ligands. Then, they evaluated the permeability for the BBB of the liposomes to confirm the role of transferrin, and the behaviors of cellular internalization and lysosomal escape to confirm the role of CPP. Meanwhile, nude mice were used as models to trace Tf-CPP-SSL in vivo, demonstrating this drug delivery system performed well in brain targeting and prolonged circulation. However, it should be noted that ligands could not be easily separated from the transferrin receptor and the internalization by endo/lysosomes also compromised the detaching. For this reason, Gao and his group developed a series of nanoplatforms on the basis of acidic cleavable ligand modification.172,173 For example, acid-sensitive imine linker (DAK) was conjugated with D-T7 peptide on the nanoparticle surface, which would break up in acidic environment, facilitating the endo/lysosomal escape.174 The system was demonstrated to have significant BBB transcytosis enhancement and was employed for further use in the treatment of autism spectrum disorder. Such responsive strategy mitigates the defects of transferrin receptor-mediated transcytosis.
Insulin receptors are highly expressed in the brain, not only in the hypothalamus, olfactory bulb, hippocampus, and striatum, but also in cerebral cortex, and cerebellum.175 Insulin could bind to the α-subunits of insulin receptors which locate outside of the cells, inducing the β-subunits which locate in the inside of the cells dimerization and autophosphorylation.176 When compared to insulin, insulin-like growth factor 1 (IGF1) showed relative lower binding affinity to insulin receptors.177 Similar intracellular signaling pathways could be initiated by both insulin and IGF1 receptors. Jörg et al. employed human serum albumin (HSA) nanoparticles to couple insulin or an anti-insulin receptor monoclonal antibody covalently.178 They used loperamide as delivered drugs inside HSA and evaluated the potential of loperamide across the BBB. Loperamide-loaded and insulin-modified HSA presented an increase in size because of the existence of insulin agglomeration on the surface, demonstrating the successful preparation of such insulin-targeting nanoparticles using NHS-PEG-MAL5000 crosslinker. After being injected through the tails of ICR (CD-1) mice, the NP showed significant antinociceptive effects which means loperamide was able to be transported across BBB. Frey II et al. investigated the delivery of IGF1 to CNS, confirming they reached CNS target sites of rats by administering a mixture of [125I]-labeled IGF1.179 The results from high-resolution phosphor imaging autoradiography established the specific binding of IGF1 and binding sites. Further, they proved IGF1 could activate different signaling pathways in diverse CNS areas.
Low-density lipoprotein receptor-related protein (LRP) as a transmembrane glycoprotein can mediate the uptake of cholesterol-rich low-density lipoprotein, including cholesterol, tocopherol, and Apos.180 The family of LRP has a lot of members, such as LRP-1, low-density lipoprotein (LDL) receptor-related protein 1B, megalin/LRP-2, apolipoprotein E receptor 2, sortilin-related receptor, LRP-5, and LRP-6.33 Previously, our group has reported an angiopep-2 (a dual-targeting ligand towards LRP-1) modified nanogel for targeted delivery of anti-epileptic drugs.181,182 The brain accumulation of electro-responsive nanogels was significantly improved by the functionalization. Interestingly, although the mechanism remains to be explored, the nanogel was found to distribute at temporal lobe which is the common brain region of epileptic focus. Chung et al. synthesized nanoparticles conjugated with angiopep-2 which have core-shell structure, to target and treat glioma.183 They proved the decoration of angiopep-2 can improve selective glioma targeting for the amounts of cellular uptake of nanoparticles by C6 glioma cells were higher than that by L929 fibroblasts. Besides, when to contrast the control group, intravenous injection of these angiopep-2 decorated nanoparticles could achieve a 10-fold diminution in tumor volume. Holler and co-wokers reported six peptide vectors were attached to a poly(β-l-malic acid)-trileucine polymer (Fig. 3d).184 These peptides could act the specific targeting function, aiming at low-density lipoprotein receptor-related protein-1, transferrin receptor, bee venom-derived ion channel, and Aβ/LRP-1 related transcytosis complex. They studied the ability of nanoconjugates to cross the BBB extensively, including tumor-bearing brains, Alzheimer’s disease-like brains, and healthy brains. What’s more, they conducted molecular mechanisms about regulating cross-talks between transcytosis pathways.
Folate receptors, high-affinity receptors, could mediate the cell uptake of folate or folic acid (FA), anticipating DNA synthesis and nutrient provision.185 They are in relatively common use in the design of drug delivery systems. Zhang et al. demonstrated a grapefruit-derived nanovector (GNV) coated with folic acid to carry miR17 for treating the GL-26 brain tumors which is one kind of the folate receptor-positive tumors.186 They found GNV reduces the toxicity of the polyethylenimine, which was used for the load of RNA, and FA-GNVs show better targeting behavior than GNVs with rapid movement into the brain within 1.5 h. Shu et al. constructed a folate-modified polymeric micellar delivery system using the thin-film hydration method.187 The delivery drug was pterostilbene (Pt) and the main structure of this system was contributed by mPEG-PCL. Compared to free Pt/mPEG-PCL, FA-Pt/mPEG-PCL showed enhanced toxicity toward folate receptors-overexpressing A172 cells, which proves folate anticipated in the condensation of Pt in A172 cells through folate receptor-mediated route. Additionally, in vivo, they analyzed the BBB penetration value and drug targeting index, illustrating the developed delivery system had great potential for brain delivery. Sosnik et al. produced poly(ethylene glycol)-b-poly(ε-caprolactone) block polymers and functioned them in the edge with folate receptor alpha (FRα) and FA (Fig. 3e).188 Modified nanoparticles showed better compatibility and greater internalized extent by primary human choroid plexus epithelial cells. After intravenous administration, the biodistribution of unmodified nanoparticles and FRα-FA-modified nanoparticles was tested. As results showed from the systemic circulation, the introduction of FRα and FA to the surface of nanoparticles promote brain accumulation.
Membrane coating for brain targeting
Recently, cell membranes as a new root of materials have gained wide focus. They have unique characteristics coming from their parent cells, including the natural functionalities and transmitting information networks that could conquer many obstacles confronted in vivo.23 Thanks to the multiple molecular interactions (hydrogen binding, electrostatic interaction, π interaction, etc.) and specific receptor recognition, between the membranes and potential substrates, the membranes of interest could be serve as the outer layer of the delivery systems.189,190,191 For this reason, many drug delivery systems based on different cells’ membrane coating, such as red cell membrane, brain tumor cell membrane, immune cell membrane, and so on, could endow the brain targeting abilities (Fig. 4a).192,193,194
Cell membranes donate drug delivery systems with the brain targeting abilities. a Schematic illustration of examples of cell membrane coating strategies. b The fluorescence images of orthotopic U87-Luc glioblastoma tumor. c In vivo pharmacokinetics. d Quantification in different organs and tumor of (doxorubicin) DOX accumulation. b–d Reproduced with permission. Copyright 2018, Wiley-VCH. e The analysis of PD-1 levels on different phenotypes of macrophages. f The analysis of the PD-1+ cell ratio in different types of macrophages. g The fluorescence images pictured in groups of PLGA/DiR and PD-1-MM@PLGA/DiR. h Immunohistochemistry staining images. Scale bar: left 1 mm; right 50 μm. e–h Reproduced with permission. Copyright 2022, American Chemical Society. i Schematic illustration of the process of NK@AIEdots to inhibit the growth of the brain tumor. Reproduced with permission. Copyright 2020, American Chemical Society
Red blood cells, or erythrocytes, have been exploited as delivery systems due to the advantages of prolonging the life span of drugs in circulation and preventing drugs from immune clearance for many years.195 They are easy to obtain and have uniformed size and shape. CD47 proteins rich on the red cell membrane ensure red cell membrane-coated systems can circulate almost 100–120 days without being cleared by macrophages.196 Lu et al. used a facile method of avidin-biotin chemistry to modify the red blood cell membrane with CDX peptide, which shows high bond towards nicotinic acetylcholine receptors.197 They demonstrated successful preparation of the modified blood cell membrane-coated systems and loading of DOX. And they verified the red cell membrane coating could improve the circulation time of the systems and locate them close to tumor vessels. Both targeting and therapeutic efficiency studies in cells and in animals illustrated that red blood cell membrane-peptide-coated systems not only had the capability to traverse the BBB, displaying exceptional brain targeting effect, but also could release DOX and prolong the survival of mice. Shi’s group developed a biomimetic nanoparticles by modifying angiopep-2 to the surface of red blood cell membranes to camouflage polymer which was pH-sensitive and coload with anti-cancer drug DOX and BBB regulator lexiscan (Fig. 4b–d).198 The system with low immunogenicity and systemic toxicity improved blood cycle time and tumor accumulation in U87MG glioblastoma tumor-bearing nude mice.
Brain tumor cell membrane, originated from brain tumor cells, tend to have homotypic targeting, long-time circulation, and BBB crossing abilities.199 Specific membrane proteins, such as focal adhesion proteins, integrin, focal adhesion kinase, and ras homologous family proteins, contribute to the functions of brain tumor cell membrane-coated systems.200 Liu et al. fabricated lanthanide-doped nanoparticles with the coating of brain tumor cell membrane, which can be used for brain tumor visualization and surgical navigation in the window from 1500 nm to 1700 nm in near-infrared-IIb.201 Because of the existence of the cell membrane from the brain tumor, this nanoparticle could easily home to the tumor site. They compared the particle with indocyanine green which is a clinically approved imaging agent, and discovered this particle had a superior resolution with lower background signals, offering a clear view of the location of the tumor.
Immune cell membranes, like their mother immune cells, are a promising choice for constructing drug delivery systems, showing great biocompatibility and unnoticeable adverse effects to normal cells.202
Macrophages play important part in the physiological microenvironment and the polarization of their phenotypes affects tumor progression and metastasis directly. In response to neuroinflammation, macrophages could be activated into anti-inflammatory state, serving as protectors digesting cell debris and pathogens, as well as releasing anti-inflammatory factors and activating other immune cells. Thus, the macrophage-based strategy for drug delivery harnesses the prolonged circulation time, abundant surface receptors, and active targeting ability of macrophages under specific phenotype.203 Wang et al. enhanced programmed cell death-1 expression on macrophage membranes and coated them onto rapamycin (RAPA)-loaded PLGA core to fabricate a novel nano-platform (PD-1-MM@PLGA/RAPA) (Fig. 4e-h).204 Macrophage membranes help the nano-platform to travel across the BBB in response to multiple chemokines. Programmed cell death-1 expression on macrophage membranes optimized the efficacy of immunotherapy, due to the PD-1/PD-L1 signal axis blockade. RAPA, as model drug, could induce cancer cell death and complement immunotherapy. This novel nano-platform provided an anti-glioblastoma multiforme (GBM) strategy through the combination of chemotherapy and immunotherapy. Their results showed more tumor-infiltrating immune-stimulatory cells, especially CD8+ cytotoxic T-lymphocyte, were recruited and triggered the release of anti-tumor cytokines, magnifying the anti-tumor effect. Sun et al. attached rabies virus glycoprotein and triphenylphosphine cation molecules on the macrophages membrane and coated them to solid lipid nanoparticles (SLNs), constructing RVG/TPP-MASLNs for delivering natural antioxidants Genistein (GS) to neuronal mitochondria which is a new curative target for Alzheimer’s disease.205 The MA membranes provided RVG/TPP-MASLNs with favorable biocompatibility and reticuloendothelial system evasion behaviors. In addition, the combination of MA membranes and functional ligands endowed RVG/TPP-MASLNs with the capabilities for double targeting including neuronal targeting and mitochondria targeting.
Neutrophils are the main type of white blood cells defensing against the pathogens and could migrate from circulation to injured brain region by crossing the BBB. Upon recruitment, the membrane adhesion proteins such as intercellular adhesion molecule-1 on endothelial cells are up-regulated, and the proteins including integrin β2, macrophage-1 antigen, and lymphocyte function-associated antigen 1 are overexpressed on the neutrophil membrane, together facilitating the transmigration.206 Chen et al. proposed a “nanobuffer”, (LA-NM-NP/CBD), that has a clear structure.201 The inner core consists of PLGA nanoparticles and reactive oxygen species (ROS)-scavenging cannabidiol (CBD). The neutrophil membrane serves as the shell to orient to the infarct core while α-lipoic acid (LA) serves as the corona to scavenge ROS. They used LA-NM-NP/CBD to change the adverse environment of the brain and take care of the salvageable penumbra for the therapy of ischemic stroke.
Natural killer (NK) cells are large granular lymphocytes and can naturally undergo immunosurveillance of diseased/stressed cells.207 With the assistance of inhibitory and activating receptors on the cell surface such as LAF-1 and VLA-4, they could efficient target specific cells, providing the potential of brain-targeted delivery. They can directly bind to cancer cells via receptors and kill them without prior sensitization. Tang and co-workers developed nanorobots by coating an aggregation-induced emission-active polymeric endoskeleton with a membrane from NK cells to mimic NK cells (Fig. 4i).208 The mechanistic studies demonstrated that receptors from NK cells to the surface of the nanorobots, play a major duty in BBB traversing and tumor identification. Besides, with help of a laser, the AIE-active conjugated polymer could be a tight junction modulator, helping to disrupt the tight junction and making this nanorobot cross the BBB easier.
External stimuli-mediated BBB disruption
To regulate the BBB permeability, external stimuli-mediated BBB disruption based on the energy conversion materials has been widely explored. Such strategies can be manipulated with various external stimulations, such as light, ultrasound, electroacupuncture, etc.
Light has been widely used due to its advantages, such as spatiotemporal precision, minimized scattering and domestic nonlinear absorption.27 As mentioned in the above chapter, light could help to open the BBB, inducing specific changes in the integrity of BBB. This phenomenon is temporary and could recover. In 1990, Eggert et al. investigated the Nd:YAG laser irradiation.209 They found laser irradiation immediately caused BBB breakdown which looked to be associated with structural damaged regions of brain microvessels. Besides, it suggested that laser-induced BBB abnormality or impairment is monophasic. After irradiation, the BBB dysfunction peaked at 2 h and persisted for 24 h approximately. However, the effects on brain from the irradiation is highly dependent on power intensity, irradiation time and distance between laser source and targeted area, which in all contributed to the temperature elevation. Thanks to the development of temperature measurement technology, researchers are able to monitor the temperature in a non-contact manner. Among all, near-infrared light (NIR) at the wavelength from 700 to 1600 nm has attracted great attention in this field due to its deep tissue penetration.210,211,212 In recent studies, NIR irradiation for BBB permeability regulation was applied under infrared thermal monitoring and the head temperature was kept lower than 43 °C. At this power intensity, the irradiation caused negligible brain damage confirmed by histological staining.213,214 The reduced transendothelial electrical resistance of cellular monolayer induced by irradiation could recover within 10 min.215 In 2011, Choi et al. reported without compromising vascular integrity, the ultrashort pulsed laser could induce transient leakage of blood plasma.216 They combined the ultrashort pulsed laser with a systemic injection to deliver target molecules to brain cortex and different other tissues. This strategy allowed invasive local delivery to an extremely small extent. In 2018, Guo and co-workers demonstrated that 2D black phosphorus nanosheets that possess excellent photothermal effects can be a new neuroprotective platform to selectively capture Cu2+ for treating neurodegenerative disorders.214 Under near-infrared irradiation, local hyperthermia for five minutes with the temperature at 41–43 °C increased the generation of tiny mechanical waves thus increasing the BBB permeability. Their results showed that the power density of light could be kept over 37% of the original value and the depth could be ~1.3–2.6 mm for the mouse brain. In addition, they studied the risk of cerebral thrombosis using nuclear magnetic resonance imaging and found no obvious cerebral thrombosis, which means the black phosphorus nanosheets have great potential in future clinical applications. In 2019, Wang and co-workers also designed black phosphorus nanosheets-based drug delivery system for loading with the antidepressant drug, Fluoxetine.217 They conducted the release ability of fluoxetine and proved 90% of the drugs could be released under 30 min light irradiation. For in vivo studies, they reported, with near-infrared irradiation, the local temperature was kept at 41–43 °C for five minutes. Finally, they compared free fluoxetine and black phosphorus nanosheets loaded fluoxetine and got the conclusion that black phosphorus nanosheets loaded fluoxetine shorted the therapy time of depression with the help of light. In 2021, Qin et al. modulated BBB by using light boost of molecular targeted nanoparticles, the synthesized gold nanoparticles, which were conjugated with the antibody BV11.218 Their results showed after light stimulation of BV11 modified gold nanoparticles, tight junctions of BBB ameliorated, allowing particles like macromolecules and virus to cross. Brain microvasculature and parenchyma were also examined. There were no obvious disruptions in vast dynamics or neuronal injury. Recently, an electro-responsive dopamine-pyrrole hybrid system that improved the delivery efficiency of anti-epileptic drugs by improving the cross of BBB via the combination of receptor-mediated transcytosis and photothermal conversion of NIR were reported.215 This system was smart for epilepsy pharmacotherapy, showing enhanced conductivity and sensitivity in various seizure models, including acute seizure, continuous seizure, and spontaneous seizure. The authors realized two hours sustained and 30 s rapid release of phenytoin and reduced drug dosage.
Ultrasound is a technique that can noninvasively focus deep into the body using an ultrasound field.219 They are mechanical or elastic vibrations in a medium of a frequency above the range of human hearing (18–20 kHz).220 Since the 1940s, ultrasound has been noted for non-invasive ablation in the brain.221 For ultrasonic BBB disruption, varied sonication parameters can cause different impacts, including the threshold pressure, the magnitude, and the drug quantity for delivering.222 In recent years, gas-filled microbubble has emerged as a contrast agent in conjunction with ultrasound for opening the BBB in an image-guided and targeted manner, ensuring the local delivery of drugs.223 Qin et al. constructed a microbubble delivery system, fixing quercetin-modified sulfur nanoparticles.224 In combination with ultrasound, this system could accumulate in the brain and promote drug delivery because of the transient opening of the BBB. Moreover, Qc@SNPs-MB effectively treated Alzheimer’s disease by protecting nerve cells and reducing endoplasmic reticulum stress which comes from oxidative stress, inflammatory response, calcium homeostasis imbalance, and neuronal apoptosis. Price’s group utilized gas-filled microbubbles for the selective transfection of endothelial cells of the cerebral vasculature.225 The negative mCherry plasmid was conjugated to cationic microbubbles. Such microbubbles have good stability. Their method through the oscillating of microbubbles under the ultrasound field could implement the transport of the gene product beyond the blood vessels without breaking the tight junctions and disrupting the BBB. To be noted, one highlight of this study is the experiments on cell identification and enrichment of whole-brain tissue samples, demonstrating their strategy achieved around 90% cell specificity of selective transfection with no extra use of a cell-specific promoter.
Electroacupuncture is a widely accepted complementary therapy through stimulating acupoints, although there is limited supporting information in modern anatomical studies.226 Studies found that electroacupuncture stimulation at certain parameters could improve the permeability of the BBB.227 Lin and co-workers found the Baihui and Shuigou acupoints are ideal regions to use for electroacupuncture for 40 min to open BBB.228 However, research on the application of nanomaterials in synergy with electroacupuncture in the brain has not yet been found. We believe there will be improvements in this cross-field in the future.
Other non-invasive strategies
Besides the widely used strategies that have been discussed before, among which active strategies for BBB regulation and crossing are summarized in Table 1, there are more strategies that people used to achieve the intention of brain-targeted drug delivery (Fig. 5a).
Other strategies can achieve the intention of brain-targeted drug delivery. a Schematic illustration of examples of other strategies. b Fluorescence images using in vivo imaging system of isolated brains. c Schematic illustration of the weight drop-induced TBI model. d Time points of physically invaded BBB was studied. e Schematic illustration of the isolation of primary neuronal cells from mouse embryos. b–e Reproduced with permission. Copyright 2020, American Association for the Advancement of Science. f Ex vivo fluorescence images of primary organs of glioma-bearing mice. g Confocal images and corresponding fluorescence intensity of 3D tumor microspheres. f, g Reproduced with permission. Copyright 2022, Nature Publishing Group. h Evaluation of general toxicity, kidney function and liver function over time. Reproduced with permission. Copyright 2022, American Association for the Advancement of Science
Agents, such as metals, polysorbate-80 (PS-80), etc., are used to shrink the endothelial cells of the brain.229 Using this treatment, various drug delivery systems could bypass BBB. However, it has serious disadvantages, for example, compromising the integrity of BBB and further leading unwanted exogenous agents, including blood components, neurotoxic, and xenobiotics, to accumulate in cerebral tissue. For short, it is a non-patient-friendly method that may cause injury to the CNS. Joshi et al. reported a PLGA-based with different surface coatings platforms used for brain delivery of siRNA to treat traumatic brain injury (TBI; Fig. 5b–e).230 They tried a nonionic surfactant PS-80 coating among their formulations and proved drug delivery across BBB could be promoted by interacting with lipoprotein receptors. They euthanized mice after injection and extracted brains for observing the fluorescence signals labeled nanoparticles. PS-80 nanoparticles showed significantly superior fluorescence signals than other formulations. They pointed out in their manuscript that although the joint modulation of surface chemistry and PS-80 density, is a useful tool to adjust BBB penetration, functional or behavior evaluations, for instance, cell death, neuroinflammation, or larger animal models, are lacking in the study. Hence, biosafety and performance in clinical may still have questions.
AAVs, presenting targeting ligands, could interact with specific molecules identified on the luminal surface of the BBB.231 The integrity of BBB could be breached by the intrinsic properties of the pathogens.232 Directed evolution and capsid engineering have been developed to engineer a number of BBB-crossing AAVs.233 Among the AAV family, a natural AAV9 capsid variant which was isolated from human liver tissue, has the ability to bypass BBB, becoming a star capsid for drug delivery into CNS.234 After intravenous injection, AAV9 mainly enriches the brain and liver.235 Bei et al. reported a rational design of two AAV9 variants for brain drug delivery.236 Confirmed by rodent and primate models, the variants prepared by insertion of cell-penetrating peptide enhanced both BBB transcytosis and cellular transduction. The authors claimed that these variants not only displayed increased BBB crossing, but also of greater importance maintained the neurotropism, paving the translational potential of neurological disorders treatment.
Bacteria can go through the BBB and infect phagocytes.237 Researchers have proved the feasibility of adopting bacteria to build drug delivery systems to resist central nervous system diseases. For glioblastoma photothermal immunotherapy, Sun et al. developed a ‘Trojan bacteria’ consisting of two types of bacteria (Fig. 5f, g).238 They demonstrated that intravenously injected Trojan bacteria system could target and penetrate glioblastoma. The confocal images showed that 3D tumor microspheres could be penetrated with a depth of around 260 μm. With the help of a laser, bacterial cells and the adjacent tumor cells could be destructed by the heat from irradiation. The debris from bacteria and tumor cells also can act as antigens to promote cancer immunotherapy.
BBB is also modulated by redox-sensitive systems and cytokine-mediated systems. The former is mainly developed for oxidative stress, which is the similarity between brain disease.239 The latter is mainly developed for the pro-inflammatory state, which is a common state in a number of CNS pathologies.240 Oxidative stress is incited by an imbalance of oxidants.241 These oxidants can further impact a variety of signaling pathways associated with pathological processes, resulting in BBB dysfunction eventually.242 Redox-sensitive systems could be designed in response to the high level of oxidants, for example, Kong et al. reviewed the related developments in nervine.243 They discussed the choice of ROS-responsive functional groups, including sulfides, selenide, ferrocene, amino acrylate, etc. Meanwhile, the pro-inflammatory state, a result of local oxidative stress, stimulates many cytokines, such as IL-1 and TNF-α. Thus, conducting cytokine-mediated systems is an optional method. Veiseh et al. reported a cytokine delivery platform as interleukin-2-producing cytokine factory organized with polymer and ARPE-19 cells which can be clinically translatable (Fig. 5h).244 They conducted this robust platform in peritoneal tumors in ovarian and colorectal mouse models and considered it could be addressed in other cancers including brain cancer. They found no significant deviations from healthy ranges using complete blood count and blood chemistry analysis, showing this system was well tolerated in nonhuman primates.
Engineered brain-targeted drug delivery systems
To fight against the BBB, a growing number of advanced materials and technologies have been developed for enhanced brain-targeted drug delivery in CNS disorder treatment. By using the passive and/or active strategies for BBB regulation and crossing in a controlled and non-invasive manner, various drug delivery systems are engineered to facilitate the brain-targeted delivery of specific therapeutics including small-molecule drugs, proteins, genes, and other biopharmaceutics. Due to the requirement of both delivery efficiency and safety, the majority of drug delivery systems with the ability to cross BBB are designed at a nanoscale level with tailored chemical composition and surface properties (Table 2). Since the approval of DOX in liposomes (Doxil) for anti-cancer treatment nearly three decades ago, increasing numbers of drug delivery systems are studied in clinical trials and some formulations for enhanced drug delivery have entered to clinic.245,246,247
Liposomal formulations
Liposomes are the first generation of drug delivery systems and have been widely used since their discovery in 1965.248 They are composed of one or more lipid bilayers and hollow aqueous compartment, which endow them with loading versatility for both hydrophobic and hydrophilic therapeutic agents.249 Together with high biocompatibility, biodegradability and its intrinsic capability of BBB crossing, liposomes are considered as one of the most successful delivery systems with a great potential in translational medicine. In the past decade, research on liposomes has further substantially increased along with the advance of materials engineering and nanotechnology.250 Various types of functionalization strategies have been involved for the development of liposomal delivery system such as brain/tumor-targeting delivery, controlled drug release, imaging-guided delivery, etc.251 These strategies facilitate the development of liposomal formulations to improve brain-specific delivery.
Brain-targeted delivery of the liposomes could be enhanced by modification of targeting ligands including polymers, peptides, antibodies, and aptamers.252 For example, Zhan et al. functionalized liposomal surface with amyloid-β-derived peptide which has a highly specific binding affinity towards plasm apolipoproteins, giving a protein corona-modified liposomal system. Significant enhancement of the brain distribution of DOX-loaded liposomes as well as higher anti-tumor efficacy were found.253 Quan et al. developed transferrin receptor aptamer-functionalized liposomes to deliver acetylcholinesterase reactivator in brain (Fig. 6a).254 Compared with non-targeting liposomes, this functionalized system has higher BBB penetration efficiency confirmed by both in vitro BBB model and in vivo biodistribution study. Conjugation of brain-targeting peptide is also proved to be an effective method to improve the brain accumulation. Zhang et al. used RVG29, a 29 amino-acid peptide derived from rabies virus glycoprotein, as a targeting ligand in the liposome-based delivery system for treatment of Parkinson’s disease.254 But it begs for the question that how can targeting ligand-modified liposomes bypass through the BBB via receptor-mediated transcytosis. To give a vivid description, Lauritzen et al. characterized all steps of the liposome delivery routes into brain by using transferrin receptor-targeted liposomal nanoparticles as a model system.255 They revealed that post-capillary venules is the key site for transcytosis-mediated brain entry of nanoparticles.
Liposomal formulations and polymeric materials for brain-targeted drug delivery. a Schematic illustration of synthesis of Apt-LP-LuH-6 liposomes and the structure characterization. Reproduced with permission. Copyright 2021, Elsevier. b Small-molecule-loaded ultrasound-controlled liposomal nanocarrier. Reproduced with permission. Copyright 2020, Nature Publishing Group. c Schematic illustration of synthesis of micelles by nanoprecipitation. Reproduced with permission. Copyright 2019, American Association for the Advancement of Science. d Schematic illustration of nanoprecipitation of peptide-modified PLGA and GALC CLEAs for the generation of enzyme delivery system. Reproduced with permission. Copyright 2021, Nature Publishing Group
For brain tumor treatment in particular, the systems are required to have a capability of both BBB crossing and tumor targeting.256 Thus, dual targeting strategy in liposome functionalization becomes a possible solution for drug delivery in brain tumor therapy. For instance, a GBM-specific cell-penetrating peptide and an anti-GBM antibody were simultaneously anchored onto the liposome surface, giving the ability of BBB penetration.257 By incorporation of superparamagnetic iron oxide nanoparticles (SPIONs) and DOX, the liposomes displayed a thermo-responsive drug release in an alternating magnetic field. Another example of dual functionalized liposomes was achieved by Singh’s group.258 Surface modification of transferrin and a cell-penetrating peptide was performed. Their liposome system thus showed around 12 and 3.3-fold increases of two chemotherapeutics (DOX and erlotinib) delivery, respectively compared to the free drugs. These strategies confirmed that ligand modification for BBB and tumor targeting could largely improve the drug delivery for brain tumor treatment. Interestingly, the liposome without any targeting ligands conjugation could also serve as an external stimuli-controlled nanosystem for brain-targeted drug delivery. As shown in Fig. 6b, Yanik et al. prepared drug-loaded liposomes tethered to lipid microbubbles containing perfluorobutane gas core.259 By applying two-component aggregation and uncaging focused ultrasound sequences at different stages, the drug-loaded liposomes could aggregate locally first and then uncage the cargo responsively to achieve high target specificity. The released drugs can cross the intact BBB without compromising the integrity.
Recently, some new types of liposomes with unique structures were reported for enhanced therapy of CNS diseases. Gomes et al. proposed a concept of exosome-like liposomes to overcome the limitations of large size and low productivity. A lipid film including DODAP (1,2-dioleoyl-3-dimethylammonium-propane) and DPPC (dipalmitoylphosphatidylcholine) was introduced in the preparation of the liposomes, giving the bilayer similar to the outer membrane of exosome.260 More liposome-based biomimetic nanomaterials for drug delivery will be emphasized in section 4.7.
Polymeric drug delivery systems
Polymeric materials for brain-targeted delivery refer an extensive number of drug delivery systems.261 They have shown potentials in pre-clinical study in different animal models of CNS diseases demonstrating attractive properties for drug delivery including controlled drug release, cellular targeting and uptake, and the ability to avoid phagocytosis of reticuloendothelial system. PLGA is a typical Food and Drug Administration (FDA)-approved polymer used for the formulation of delivery systems in biomedicine.262 PLGA-based delivery system is qualified to encapsulate a wide range of therapeutic cargos including small-molecule drugs, genes, proteins, vaccines, ensuring high bioavailability by protecting them from degradation. The PLGA assembles without any functionalization have a high distribution up to 16.4% in the brain and can keep at the level for at least seven days.263 Moreover, the possibility of surface decoration of targeting ligands could further promote their penetration of biological barriers. A typical example was reported by Cecchini et al., demonstrating that PLGA polymers conjugated with different targeting peptides could be used for enzyme therapy of brain disorders such as Krabbe disease (Fig. 6c).264
Amphiphilic polymers are known for their advantage of self-assembly into micelles which has a hydrophobic core and a hydrophilic shell at a size of 2–300 nm. Such architecture thus enables high loading capacity and prolonged blood circulation of therapeutic agents, and its efficient accumulation in brain lesions. We recently proposed a micelle-based drug delivery nanosystem for febrile seizure control.265 The designed copolymer of poly(acrylamide coacrylonitrile)-methoxy polyethylene glycolsuccinimidyl carbonate could self-assemble into defined micelles. Because of the incomplete development of BBB in mice pups, the micelles could quickly accumulate in neonatal brain as early as five minutes. Anti-inflammatory small-molecular inhibitor CZL-80 was chosen as a model drug. After micelle encapsulation, therapeutic window of the drugs was significantly increased to at least 4 hours, whereas 20 min for injection of CZL-80 alone. Importantly, the nano-engineered micelle is gifted for an upper critical solution temperature of 39 °C, showing a thermos-sensitive drug release mechanism for on-demand therapy. To combat against drug-resistant gliomas, Saltzman et al. prepared a reduction-responsive polymeric nanoparticle for co-delivery of oxaliplatin (the third-generation platinum anti-cancer drug) and 56MESS (a cationic platinum DNA intercalator) (Fig. 6d).266 Oxaliplatin and 56MESS were encapsulated inside the nanoparticles through hydrophobic interaction and electrostatic complexation, respectively. Higher level of glutathione (GSH) in tumor reductive microenvironment could trigger the drug release due to the rupture of disulfide bonds in polymer backbones. The authors claimed that by using convection-enhanced delivery, the drugs could traverse the BBB and accumulate at the glioma region. Another case of micelle-based drug delivery system for CNS disease treatment without any brain-targeted functionalization was reported by Jiang’s group.267 They developed an microthrombus-targeted micelle-based system for ischemia stroke therapy. Due to the pathologically damaged BBB, microthrombus-targeted micelle could penetrate BBB efficiently and promoted RAPA delivery via hydrophobic interactions. These micelles are assembled by ROS-responsive and fibrin-binding polymer, giving extended drug retention and ROS-triggered drug release for neuroinflammation regulation. Micelle-based delivery systems is also effective in the treatment of those diseases with intact BBB structure. A brain and microglia dual targeting nanosystem was constructed by an targeting peptide derived from β-amyloid protein and ROS-responsive amphiphilic polymer.268 By mimicking the unregulated Aβ transportation, the micelles could target the Alzheimer’s disease microenvironment and release the model drug curcumin in response to excessive ROS generation in Alzheimer’s disease. Similar micelle-based strategies with surface modification of targeting ligands were performed for the synergistic chemotherapy of glioma,269 gene therapy of Alzheimer’s disease,270 and traumatic brain injury.230
Nanogel is formulated by three-dimensional crosslink of functional polymers and has a network capable of efficient drug encapsulation.271 Tunable properties such as deformability, nanoscale size and high hydrophilicity make nanogel a candidate for nose-to-brain delivery. Di Carlo et al. manufactured a poly(N-vinyl pyrrolidone)-based nanogel by e-beam irradiation and evidenced this nanogel-based system could provide nose-to-brain delivery of insulin.272 Compared with free insulin administration, the drug levels in the anterior and cerebellar regions were significantly increased within 60 minutes. The authors indicated this improvement could be resulted from intranasal delivery via olfactory and trigeminal nerve pathways. Another polymeric nanogel delivery system of teriflunomide-loaded lipid-based carbopol-gellan gum in situ gel was reported by Kokare and co-wokers.273 By balancing the interactions of gelling agents, mucoadhesive agents, the nanogel was formulated with lipophilic medicine and achieved two-fold enhancement of drug permeability. The same group further developed paliperidone palmitate poloxamer-guar gum nanogel for schizophrenia treatment by nasal delivery directly to the brain bypassing the BBB.274 Wang et al. fabricated calcium ions-triggered harmine in situ nanogel through coupling homogenization and spray-drying technology.275 The bioavailability of harmine administrated by nanogel was found to be 25-fold higher than that by oral administration. At the meantime, incorporation of brain targeting motifs allows nanogel transcytosis across the BBB for brain-targeted drug delivery. Morgenroth reported nanogel-based carrier for the intracellular delivery of radiophamaceuticals to brain tumor cells by functionalization of ligands of diphtheria toxin receptor overexpressed in abnormal cerebral blood vessels.276 The crosslinked network also contained matrix metalloproteinase substrate, permitting protease responsive drug release. Cell membrane-mimicked nanogel is another typical nanocarrier for BBB crossing. For example, Yu and his group utilized phosphorylcholine nanogels for enhanced GBM chemotherapy.277 The phosphorylcholine polymer exhibited long-lasting circulation and achieved higher accumulation in brain tumor tissue, serving as an effective tool for BBB crossing.
There are other polymers-enabled delivery platforms that have been proved for successful brain-targeted delivery including semiconducting polymers, polysaccharides, gelatin and other synthetic or natural polymers.278,279,280,281,282 Further investigations on bioavailability, degradability, and bio-interactions of polymers in vivo will facilitate the development in this field.
Gold nanomaterials
Gold nanomaterials have a number of distinctive advantages over drug delivery for brain diseases.283,284 Inert surface chemistry of gold allows limited interactions at nano-bio interface and consequently leads to high cellular and tissue compatibility. They could enter the brain by crossing the brain endothelium in a size-dependent manner. In addition, it is comparatively simple to synthesize the gold nanomaterials with tailored structure and defined size. Although only smaller size (<10 nm) gold nanoparticles after intravenous injection was observed to be distributed in the brain slice.285 Sub-100-nm gold nanoparticles can extravasate through the damaged BBB under some pathological circumstance such as brain tumor, cerebral stroke, brain injury and epilepsy. Moreover, gold nanomaterials could be traced by using computed tomography imaging for imaging-guided therapy, evaluating their biodistribution and accumulation in the brain.286,287 A variety of applications based on gold nanoparticles have developed for brain disorders treatment thanks to their excellent properties. For example, some gold nanoparticles that have higher absorbance at near infrared biowindow are potential nanogents for photothermal therapy of brain tumor.288 In this section, we will focus on the discussion of the design and synthesis of gold nanomaterials-based delivery systems.
Functional ligands or drugs can be stably anchored onto the gold surface via gold-thiol bonding for enhanced drug delivery.289 Popovtzer developed insulin-coated gold nanoparticles as endogenous BBB transport system for delivering therapeutics into the brain regions, rich in insulin receptors.290 Prior to the modification, thiol-mPEG-COOH copolymer was attached onto the gold nanoparticles via gold-thiol interaction, leaving carboxylic groups for further EDC/NHS conjugation with insulin. They also proved the 20 nm of gold nanoparticles showed highest accumulation in the brain compared with 50 nm and 70 nm nanoparticles. Another example of maze tetrapeptide-anchored gold nanoparticles was confirmed by Guo’s group.291 The thiolated biomolecules were anchored onto the nanoparticle surface, achieving brain-targeted delivery and neuroprotection to prevent Alzheimer’s disease. As mentioned, the gold-thiol interaction between gold nanoparticles and functional ligand enabled a simple and effective strategy for nanoparticles functionalization.
More recently, chiral gold nanoparticles have received attention in both nanotechnology and biomedicine.292,293 Tang and co-workers firstly revealed that the BBB penetration of gold nanoparticles is clearly affected by their chirality (Fig. 7a, b).294 It is known that GSH transporters at high level in the brain promoted the BBB permeability of GSH-capped nanoparticles. In this work, the authors indicated that the D-GSH-stabilized gold nanoparticles at 3.3 nm (D3.3) possessed higher brain distribution compared to its enantiomer (L-GSH-stabilized nanoparticles, L3.3). Together with a larger binding affinity towards amyloid-β of D3.3, the introduction of chiral ligands endowed the nanoparticles with improved rescue of behavioral impairments in Alzheimer’s disease. This study undoubtfully provides a deeper understanding of nanotechnology-enabled BBB crossing by ligand functionalization.
Gold nanomaterials and carbon materials for brain-targeted drug delivery. a Simulated structures of Aβ17-36 with L- and b D-GSH-coated Au (111) surface obtained from molecular docking simulation. Reproduced with permission. Copyright 2020, Nature Publishing Group. c Schematic illustration of synthesis and d TEM images of mazindol-B6 peptide-PCB-S-curcumin-siRNA (MBPCS) and intermediate products. Reproduced with permission. Copyright 2019, Royal Society of Chemistry. e Structures and reactivity of the various nuclearities of Ru-CO clusters on GO, and f FTIR spectra of 1Ru-and 11Ru-CO. Reproduced with permission. Copyright 2018, Wiley-VCH. g Schematic illustration of synthesis of drug-loaded graphene quantum dots (GQD-D) and Cetuximab-labeled red blood cell membrane-coated GQD-D anchored inside the nanosponge (Ct-RBC@GQD-D/NS) and h their TEM images. Reproduced with permission. Copyright 2019, American Chemical Society
Gold nanoparticle-based drug delivery systems in unconventional structure other than sphere particle have been designed for enhanced brain-targeted delivery. For example, a switchable nanoplatform for co-delivery of gene and chemical drugs was nano-fabricated by Zhang and his team (Fig. 7c, d).295 The modification of nanoparticles with B6 peptide, mazindol, β-thiother ester bonded-copolymer endowed the nanosystem with brain targeting ability, high affinity towards dopaminergic neurons, and ROS-response drug release, respectively. It significantly improved the targeted delivery of curcumin and small interfering RNA to accomplish a synergistic delivery overcoming the issue of Parkinson’s disease. Interestingly, the levodopa attached on the gold surface provides a Fe3+-enabled assembly in neurons for enhanced computed tomography. Microenvironment-mediated switching between self-assembly and disassembly by gold nanoparticles was developed for brain tumor treatment. The endogenous factors in brain tumor such as low pH and higher GSH could triggered the transition of assembly–disassembly state to improve theranostic efficacy.296,297 Moreover, hybrid nanoparticles that are composed of gold and other materials inherited the intrinsic properties of each component. Bimetallic nanoparticles (gold and palladium) modified with quercetin could serve as a potential inducer for the therapy of Alzheimer’s disease.298
Currently, some gold nanomaterials-based systems are undergoing pre-clinical and clinical trials. The limitations and strengths of these systems should be further investigated and summarized, provide a guidebook for the community. Comparative studies are required to explore the effects of shape, size, surface charge, and other factors on nano-bio interactions. In addition to the BBB crossing, research on the biodistribution and elimination of gold nanomaterials in the brain should be continued.
Carbon materials
Carbon materials including carbon dots (CDs), carbon nanotubes (CNTs), graphene, and graphene oxide have been considered as promising agents for biomedical applications.299,300,301 They are evolving as functional materials not only for drug delivery but also, in parallel, for imaging, diagnosis and other treatments while proving negligible side effects.302,303,304,305
CDs have been exploited as biocompatible nanocarriers for brain-targeted drug delivery due to their tunable properties, photostability, small size, and facial synthesis.306 Leblanc et al. successively reported the development of two CDs-based nanocarriers to treat glioblastoma brain tumor and Alzheimer’s disease.307,308 The CDs for brain tumor treatment were conjugated with transferrin, epirubicin, temozolomide via EDC/NHS reactions with carboxylic groups on CDs surface. And the CDs for Alzheimer’s disease treatment were fabricated by an ultrasonication-mediated strategy, producing amphiphilic yellow-emissive particles at a size of 3 nm. Such CDs with amphiphilicity could cross the BBB through passive fusion.
Graphene oxide (GO) and reduced GO (rGO) are two representative graphene-based materials.309 They were well known for their electro-mobility, and photothermal properties, high specific surface area, giving them great potentials in targeted drug delivery. Drug molecules could be conveniently loaded into graphene-based delivery system via non-covalent interactions (π-π stacking, hydrophobic interactions, hydrogen bonding, and electrostatic interactions).310 Further, the -OH and -COOH groups of GO and rGO provides a feasibility to conjugate with targeted molecules and material matrix. Interestingly, the graphene-based materials themselves have the potential therapeutic effects in treatment of CNS disorders. They have been used to inhibit misfolding and aggregation of amyloid-β protein. By loading dauricine, a dibenzyl tetrahydroisoquinoline alkaloid that alleviates brain inflammation, GO sheets were verified to possess the therapeutic effects for Alzheimer’s disease.311 In addition to small-molecule drugs, clusters such as RuII(CO)2 species could be absorbed onto GO (Fig. 7e, f).312 Under photothermal activation, carbon dioxide was released for the treatment of vascular diseases, like stroke remediation. Obviously, conjugation of functional ligands has been explored to facilitate the BBB transcytosis of graphene-based materials, such as lactoferrin, porphyrin, and transferrin.313,314,315,316 Physico-chemical properties such as size and surface charge also have impact on BBB penetration efficiency of GO and rGO.309 Graphene quantum dots at a size range from 2 to 4 nm have higher BBB permeability than GO at a size from 5 to 20 nm, indicating the crucial role of particle size in brain drug delivery.317 Due to the small size, graphene quantum dots could be encapsulated onto mesoporous nanostructure and mediate the theranostic penetrative delivery of drug and photolytics (Fig. 7g, h).318 her engineering of cell membrane onto the hybrid particles prolongated the blood circulation, leading to enhanced accumulation at brain tumors.
CNTs can been divided into two types, namely single-walled CNTs (SWCNTs) and multi-walled CNTs (MWCNTs) depending on the inner graphene layers. Unique surface chemistry and walled structure of CNTs permit drug loading and polymer conjugation for targeted delivery to brains.319 The CNTs for drug delivery were usually modified with functional groups such as -NH2 or -COOH, providing reactive sites. Raza developed PEGylated CNTs after -NH2 modification for delivery of mangiferin, a potential anti-cancer drug as a nanomedicine for brain tumor.320 Carboxylated SWCNTs were utilized for brain delivery of levodopa for the treatment of Parkinson’s disease.321 Chen et al. combined a mixture of brain targeting peptide, glioma targeting and polyethyleneimine with carboxylated MWCNTs to form nanomedicine for orthotopic brain tumors.322 The therapeutic agents could be loaded within the systems via either covalent conjugation or non-covalent bonding. However, it remains public concern for human health as exposure of CNTs may cause cytotoxicity in CNS,323,324 and more toxicological profiles are needed before they can be applied in clinical trials.
Iron oxide nanoparticles
Iron oxide nanoparticles (IONPs) are a class of widely used nanomaterials for biomedical applications including drug delivery, bioimaging, hyperthermia therapy, and magnetic separation.325,326,327 Magnetite (Fe3O4), hematite (α-Fe2O3) and maghemite (γ-Fe2O3) are three common IONPs, in which Fe3O4 nanoparticles received great interests. Of importance, customized IONPs with different size, shape, morphology, and surface chemistry could be synthesized in a controlled manner. Typically, administration of unmodified IONPs does not permit brain-targeted delivery. Their accumulation pattern in organs of rodent models was revealed as follows: spleen > blood > liver > kidney > lungs > heart > testis > brain.328 Covalent conjugation of targeting ligands, and/or polymeric materials such as PEG chains, carboxymethyl cellulose, dextran could distinctively facilitate the transport of IONPs across the BBB.329,330,331
Spherical IONPs or SPIONs could serve as carriers for brain-targeted delivery. For instance, IONPs synthesized by a mixture of L-aspartic acid, FeCl3·6H2O, and FeCl2·4H2O could be functionalized with carboxylic groups, paclitaxel, PEG polymer chain, and GSH step by step.332 The as-synthesized particles were proved to pass through the BBB with shuttle peptide and deliver the drug molecules into brain. Ni and co-authors fabricated brain-targeted IONPs for co-delivery of GSH peroxidase 4 and cisplatin for gene-chemotherapy.333 The small interfering RNA and the chemical drugs were loaded via EDC/NHS reaction and direct absorption, respectively. Small size IONPs were sometimes used as therapeutic agents other than the carriers. Guo developed an ultrasound-responsive nanoparticle for thrombolysis, by assembly of PLGA and targeting peptide dual-modified nanoparticles onto perfluorohexane nanodroplet (Fig. 8a–d).334 In another report, SPIONs, quantum dots, cilengitide were integrated into one nanoplatform, giving liposomal formulation for imaging-guided therapy. The hydrophilic cilengitide was encapsulated in the aqueous core, whereas SPIONs and quantum dots were concealed inside the lipid membrane. The SPIONs and the quantum dots used in the formulation have a size of ~20 nm and ~8 nm, respectively, and the hydrodynamic diameter of the final liposome was determined to be ~100 nm. The authors claimed the size change could promote the passive targeting via leaking vasculature (7–150 nm) and meanwhile prevent homogenous leakage of SPIONs into normal tissues.335
Iron oxide nanoparticles, silica nanomaterials, biomimetic nanomaterials and Cas9/RNA nanoparticles for brain-targeted drug delivery. a Schematic illustration of synthesis of Fe3O4 nanoparticles-PLGA-perfluorohexane (PFH) nanoparticles with CREKA peptides and b, c representative TEM images of Fe3O4-PLGA-PFH-CREKA and PLGA-PFH-CREKA nanoparticles, and d their elemental mapping results. Reproduced with permission. Copyright 2019, American Chemical Society. e Schematic illustration of synthesis of HA-MMSN-1F12 nanoparticles and f particle size and g TEM images of HA-MMSN and HA-MMSN-1F12 nanoparticles. Reproduced with permission. Copyright 2022, Ivyspring International Publisher. h Schematic illustration of synthesis of cell membrane-coated nanostructures and its targeted synergistic therapy of glioblastoma. Reproduced with permission. Copyright 2022, Nature Publishing Group. i Schematic illustration of synthesis of CRISPR-Cas9 nanocapsules and j their TEM images and k size distribution. Reproduced with permission. Copyright 2022, American Association for the Advancement of Science
Recently, IONPs with tailored structures or multi-component IONPs-based particles were developed for brain diseases. Zhou et al. provided gallic acid-coated magnetic nanoclovers for targeted delivery of nanomedicine to brain tumors.336 The clover-shaped nanoparticle was synthesized by a mixture of CoFe-oleate, oleic acid, and oleyl alcohol after an elongated reaction time. The authors indicated that these nanoclovers have greater heat induction efficiency than common IONPs, leading to enhanced magnetic hyperthermia-chemotherapy combination for brain tumor treatment. Paulmurugan and co-workers synthesized polyfunctional gold-iron oxide nanostars for microRNA delivery to combat against glioblastoma.337 The gold-iron oxide nanostars could be obtained by consecutive seed and growth steps, giving a uniform size distribution of around 34 nm. Further coating of hybrid polymer (β-cyclodextrin-chitosan) and decoration of PEG-T7 peptide enabled efficient cargo loading and brain tumor targeting.
IONPs are widely used in the clinic for diagnostic and/or prognostic applications. The development of IONPs-based drug delivery system enabled trackable delivery and imaging-guided therapy. Recent advances of magnetotherapy offer a new opportunity for combined therapy. Thus, future work on the construction of multi-functional nanoplatform, as well as the study on how to balance the interactions between each component for optimized efficacy should be conducted.
Silica nanomaterials
Silica nanoparticles are a type of stable, low toxic, and nanostructured bioceramics contributing their superlative properties and uses to biomedicine. Mesoporous silica nanoparticles (MSNs) characterized by ordered distribution of mesopores at a pore size between 2 and 50 nm with high pore volume and surface area are ideal candidates of starting biomaterials, particularly as drug carrier.338,339 The presence of silanol groups at particle surface enables the production of multifunctional derivatives of MSNs system for targeted drug delivery in the treatment of brain diseases.
There are mainly two strategies to fabricate MSNs-based delivery system for BBB crossing. First, MSNs can be employed as a drug delivery system by direct drug absorption and surface ligand functionalization. Chen et al. conjugated cRGD peptide with the MSNs and simultaneously load antineoplastic DOX by mixing.340 They found that such system exhibited strong permeability across the BBB and then could induce cancer cells apoptosis by releasing drugs. The transport capability of MSNs at the sizes of 20, 40, and 80 nm were evaluated by the authors and were calculated to be 44.0%, 59.2%, and 38.6%, respectively, which were all higher than that of free drugs (32.8%), indicating a size-dependent penetration mechanism. Gómez-Ruiz and co-workers prepared a MSNs-based nanoplatform for delivery of a cocktail of agents (leptin and pioglitazone) to fight against amyotrophic lateral sclerosis.341 Leptin, a polypeptide hormone possessing neuroprotective effects, was conjugated with -NH2 of MSNs through EDC/NHS coupling, and pioglitazone, an anti-inflammatory agent, was loaded through adsorption. The surface area and the pore volume before and after the drug loading were determined to be 853 m2/g and 0.73 cm3/g, 512 m2/g and 0.47 cm3/g, respectively. These results demonstrated successful incorporation of both agents. Other nanoplatforms using iRGD peptide,342 lactoferrin,343 lipoic acid,344 Angiopep-2,345 lipid bilayer,346 as targeting ligands also demonstrated efficient drug loading and BBB crossing of MSNs-based delivery systems.
Moreover, MSNs were used as core substrates for further materials growth, or as the carrier shell coating on other functional nanostructures including those particles mentioned above. For example, a core-shell particle system was constructed by polymerization of tetraethyl orthosilicate grafting on magnetic Fe3O4 nanoparticles for both drug delivery and magnetic resonance imaging. Afterwards, the drug-loaded Fe3O4@MSNs particles were wrapped up by activated neutrophils, producing a biomimetic theranostic platform. The authors claimed the neutrophils not only have the native ability of BBB crossing, but also could act as “living” delivery system targeting inflammatory regions for maximizing the drug bioavailability.347 Similar magnetic core-shell particles were developed by Luo and co-workers (Fig. 8e–g).348 The Fe3O4@MSNs particles were modified with both amyloid-β antibody (1F12) and CD44-targeting ligand (hyaluronic acid). Thus, after BBB penetration enabled by hyaluronic acid, the particles could specifically remove amyloid-β oligomers. In their work, the particles size had a hydrodynamic size ranging from 413 to 478 nm which is not an ideal particle size for BBB crossing. However, the authors successfully settled the problem by conjugation of CD44-targeting ligand with MSNs. Karathanasis demonstrated the BBB permeability of Fe3O4@MSNs modified with NH2-PEG polymer and fibronectin-targeting peptide CREKA. Thanks to the magnetic cores, external low-power radiofrequency field could facilitate deep penetration of drugs across blood–brain tumor interface.349
Unique properties of silica nanomaterials have made them ideal candidates to be used as drug carriers. In particular, the superior surface chemistry and structural feature of the materials distinctively improve the drug loading capacity. Thus, efficient and standardized production protocols of silica materials should be developed to achieve reproducibility.
Biomimetic drug delivery systems
Engineered materials could exert their therapeutic benefits in drug delivery as an enormous quantity of nanoparticles with customized physio-chemical properties according to the requirement in disease treatment. In the past decade, camouflaged drug delivery systems that using biomimetic materials were developed to traverse BBB for brain-targeted drug delivery.350,351,352 The earliest work can be traced back to twelve years ago that Zhang’s group demonstrate an erythrocyte membrane-camouflaged polymeric nanoparticle for drug delivery.353 Since then, in addition to membrane-enabled technology, alternative strategies including biomacromolecule-enabled strategy,354 exosome-mediated carrier,355 virus-inspired synthesis,356 extracellular vesicles bionic method,357 and bacteria bionic strategy358 have been adopted in preparation of custom-build nanocarriers. In terms of the synthesis process, the biomimetic nanomaterials for brain delivery can generally be categorized into two strategies, namely bottom-up and top-down strategies.
The bottom-up strategy for particle construction mainly includes the use of a massive number of targeting ligands derived from living systems. From small molecules, short peptides, nuclei acids, to various functional proteins, researchers have created a toolbox of targeting ligands for nanoparticles decoration. In addition to direct conjugation to nanoparticles that have been mentioned in previous sections, targeting ligands are also able to assemble into the nanostructures in assistance with polymers to accomplish the goals of targeted drug delivery. A synthetic protein nanoparticle assembled by HSA, cell-penetrating peptide (iRGD), reactive macromer (OEG) and siRNA against STAT3, a key factor related to tumor progression, was engineered by Lahann.359 Under the treatment of electrohydrodynamic jetting, the OEG macromer polymerized and covalently interacted with the lysine residues from albumin, giving nanoparticles at an average size of 115 nm. In vitro and in vivo study evidenced the BBB crossing and tumor accumulation of the protein-mediated nanoparticles. To improve the BBB permeability of cytotoxic T-lymphocyte associated antigen 4 (a-CTLA-4) and programmed cell death-1 (a-PD-1) for immunotherapy of brain glioma, Ljubimova and co-workers described a nanoscale immunoconjugate system.360 The immunoconjugate was obtained by covalently attaching antibodies (a-CTLA-4 IgG2b, or a-PD-1 IgG) to poly(β-L-malic acid) polymers, with pre-conjugation of anti-mouse transferrin receptor antibody, and trileucine. Some natural cell membranes such as exosomes without any functionalization have ability to cross the BBB.335,361 The delivery efficiency is mainly dependent on the lipid membrane and the active proteins which mediate the internalization between exosomes and target cells. In addition, some exosomes improved the drug delivery efficiency by increasing the BBB membrane fluidity. Interestingly, some natural exosomes not only could penetrate BBB, but also accumulate at brain tumor site362 or inflammatory region,363 serving as dual targeted delivery systems.
Biomimetic nanomaterials developed with top-down cell-engineered approach inherited complexity of their source cells, bypassing cumbersome synthesis process for particle construction. Cellular internalization and cell membrane coating as typical top-down strategies endow the nanoparticles with bionic communications with biological entities including membrane fusion, cell tropism, immune evasion, cell-cell recognition, which in all contributes to targeted drug delivery. Researchers have reported a variety of biomimetic nanomedicines by engineering whole cells for synthesis of whole-cell-based systems, and parts of cells for semi-biological biomimetic systems. Zhang developed a neutrophil-mediated drug delivery system by anti-cancer drug encapsulation into cationic liposomes, followed by internalization by tumor-associated neutrophils.364 The camouflaged delivery system maintained the physiological features, which could efficiently traverse BBB and migrate to inflammatory sites such as tumor parenchyma. Interestingly, the internalization by leukocytes could complete in vivo. Qin et al. prepared cRGD-modified liposomes and demonstrated their enhanced uptake by monocytes and neutrophils in vivo due to the high and specific binding affinity.365 As known, leukocytes are promptly recruited when neuroinflammation occurs in brain disorders. This cell-mediated delivery strategy could thus facilitate the migration of drug-loaded liposomes across BBB in vivo. Thanks to the homotypic recognition of membrane components, cell membrane coating is an alternative method to fabricate biomimetic delivery system. For example, cell membrane harvested from glioma U251 cells was used as a chaperon to increase brain tumor targeting of delivery system (Fig. 8h).366 The self-assembly nanoparticles composed of hemoglobin, lactate oxidase, chlorin e6, and chemiluminescence reagent were co-extruded with tumor cell membranes to produce membrane-coated nanoparticles. Such system displayed efficient BBB penetration and tumor-targeting capability. In some cases, due to similar amphiphilicity between cell membrane and liposome, the functional proteins from the membranes could be inserted inside the liposomal bilayer after protein fusion, generating proteolipid nanoparticles. Zheng et al. prepared biomimetic proteolipid nanoparticles by embedding glioma cell membrane proteins into indocyanine green-loaded liposomes.367 Unlike membrane-coated polymeric “hard” nanoparticles such as PLGA nanoparticles, these membrane-camouflaged nanoparticles were proved to be “soft” and the active proteins were easy to be inserted. Further study on platelet-camouflaged delivery system also confirmed the enhanced drug delivery in multiple tumor models including glioma, which was enabled by platelet membrane proteins.368 Such delivery systems largely improved the drug accumulation in brain tumor via homologous binding mechanism. To further optimize the membrane-mediated delivery with region-specific targeting, biomimetic systems could be labeled by targeting ligands via covalent or non-covalent conjugations. Ren and co-authors modified the exosome-based system with LDL peptide by simply co-incubation.369 The high binding affinity of the peptide and LDL receptor promoted the BBB penetration, glioma distribution and cellular uptake. Similar strategy was performed in a delivery system for brain tumor treatment by using conjugation of T7 peptide.339
Other materials
In addition to the aforementioned nanomaterials, development of biotechnology and materials engineering offers other opportunities for BBB crossing. Black phosphorus (BP) is two-dimensional layered semiconducting material with high drug loading capacity, efficient photothermal conversion, and satisfactory biocompatibility.370 Chen prepared a BP-based delivery system consisted of brain targeting peptide lactoferrin and Paeoniflorin-loaded BP nanosheets.371 Elevated BBB permeability of the system was observed due to a combination of lactoferrin-mediated BBB transcytosis and photothermal effects. Some examples of metal or metal oxide-based materials (e.g., MnO2, MgO, TiO2) have been exploiting in carrier construction by taking advantages of their catalytic effects and imaging functions.372,373,374,375 More recently, variants of the AAV have been investigated for its potential use for BBB penetration. Nanocapsules containing single CRISPR-Cas9 and GSH-sensitive polymeric shell for glioblastoma gene therapy were designed by Shi and co-workers (Fig. 8i–k).376 The particle core of Cas9/sgRNA complexes were decorated with positively charged polymer via electrostatic interactions, as the particle size increased from 17 nm for naked Cas9/sgRNA to 31 nm.
Conclusion and perspectives
BBB is a natural barrier protecting the brain from the entrance of toxins and pathogens. However, intact BBB consisted of endothelial cells and tight junctions impedes the brain permeability of therapeutic agents, which largely comprises their therapeutic efficacy of CNS diseases. Along with the rapid development of materials science and nanotechnology, various strategies for BBB regulation and crossing have been developed and engineered delivery systems with unique physio-chemical properties and multifunctional motifs were prepared for enhanced drug delivery via different BBB crossing pathways. This review introduced the basic information of BBB structure and physiology and discussed the different strategies to enhance BBB crossing in detail as well as the bypassing routes and mechanisms. Recent progress of various types of drug delivery systems and their distinguishing attributes for BBB crossing were summarized. The engineered drug delivery systems with appropriate physio-chemical properties, multi-functional modules, and good biocompatibility guaranteed excellent performance of BBB penetration for enhanced drug delivery.
Although extensive achievements have been made in this community with several FDA-approved trials, there is still a long way to go from clinical popularization and translational application. More efforts on both development of materials engineering and biomedicine are needed to bridge the gap. We list some pointel aspects for future research as following: (1) More appropriate in vitro and in vivo models for evaluation of BBB permeability. Currently, cell monolayer model (e.g., Transwell model) was widely used to verify the BBB crossing and calculate the penetration efficiency. Yet, a compliant three-dimensional BBB model is more preferred to investigate the role of blood flow and drug transport in BBB maintenance. Recent advances in 3D printing technology may provide an alternative approach to in vitro BBB study;377,378 (2) Targeted delivery with better spatial and temporal precision. Although abovementioned strategies provide means to improve brain targeting, none of them could complete brain region-specific delivery, which is crucial for treatment of brain disorders. Strategy of targeting ligand functionalization significantly relies on the receptor expression, and stimuli-triggered BBB disruption has limited resolution. Drug release in a time-controlled manner would also be highly favored. Treatment of paroxysmal disorders such as epilepsy required timely drug release. (3) Safety issues should be addressed before clinical translation. In addition to conventional acute and chronic toxicity evaluation in cellular, organ, tissue, and system levels, studies on distribution and metabolic fate of nanomaterials in a long-term period should be conducted. Even though most literature claimed satisfactory biocompatibility in their reports, toxicological studies on organic and inorganic drug delivery systems revealed neurotoxicity and inflammatory damages in the brain.379,380,381 Researches on integrity and function of BBB at molecular level after non-invasive regulation should also be performed to verify the biosafety. (4) The interactions between drug delivery systems, cargo drug molecules, and cells should be identified. A deeper understanding of complex interactions and computational modeling, including machine learning algorithms,382 could provide complementary insight on empirical design of drug delivery system. (5) Scalability and reproducibility should be guaranteed. New methodologies for carrier construction and drug loading will facilitate their practical uses. (6) In addition to drug delivery, a combinational delivery of other agents such as imaging probes and biosensors is anticipated to improve the therapeutic efficacy by fabricating theranostic nanoplatforms. Targeted drug delivery is a multi-disciplinary study involving biotechnology, chemistry, material science and medicine. Considered the promising results that shown in this field, we envision that further continuous interdisciplinary cooperation will build a broader platform for enhanced BBB crossing for the treatment of brain diseases in the future.
References
Abbott, N. J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 36, 437–449 (2013).
Abbott, N. J., Patabendige, A. A. K., Dolman, D. E. M., Yusof, S. R. & Begley, D. J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 37, 13–25 (2010).
Ballabh, P., Braun, A. & Nedergaard, M. The blood–brain barrier: an overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 16, 1–13 (2004).
Pandit, R., Chen, L. & Götz, J. The blood-brain barrier: physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 165-166, 1–14 (2020).
Banks, W. A. From blood–brain barrier to blood–brain interface: new opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 15, 275–292 (2016).
Shaw, A. T., Yasothan, U. & Kirkpatrick, P. Crizotinib. Nat. Rev. Drug Discov. 10, 897–898 (2011).
Alauddin, M. M., Radaram, B., Rao, Y., Yang, P. & Piwnica-Worms, D. Novel derivatives of anaplastic lymphoma kinase inhibitors: synthesis, radiolabeling and preliminary biological studies on crizotinib and alectinib analogues. Cancer Res. 79, 10–10 (2019).
Fromm, M. F. Importance of P-glycoprotein at blood–tissue barriers. Trends Pharmacol. Sci. 25, 423–429 (2004).
Endicott, J. A. & Ling, V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu. Rev. Biochem. 58, 137–171 (1989).
Choudhari, M. et al. Evolving new-age strategies to transport therapeutics across the blood-brain-barrier. Int. J. Pharm. 599, 120351 (2021).
Sweeney, M. D., Sagare, A. P. & Zlokovic, B. V. Blood–brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 14, 133–150 (2018).
Arvanitis, C. D., Ferraro, G. B. & Jain, R. K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 20, 26–41 (2020).
Tang, W. et al. Emerging blood–brain-barrier-crossing nanotechnology for brain cancer theranostics. Chem. Soc. Rev. 48, 2967–3014 (2019).
D’Souza, A., Dave, K. M., Stetler, R. A. & S. Manickam, D. Targeting the blood-brain barrier for the delivery of stroke therapies. Adv. Drug Deliv. Rev. 171, 332–351 (2021).
Terstappen, G. C., Meyer, A. H., Bell, R. D. & Zhang, W. Strategies for delivering therapeutics across the blood–brain barrier. Nat. Rev. Drug Discov. 20, 362–383 (2021).
Zhao, Z., Ukidve, A., Kim, J. & Mitragotri, S. Targeting strategies for tissue-specific drug delivery. Cell 181, 151–167 (2020).
Liu, Y., Castro Bravo, K. M. & Liu, J. Targeted liposomal drug delivery: a nanoscience and biophysical perspective. Nanoscale Horiz. 6, 78–94 (2021).
Hwang, D., Ramsey, J. D. & Kabanov, A. V. Polymeric micelles for the delivery of poorly soluble drugs: From nanoformulation to clinical approval. Adv. Drug Deliv. Rev. 156, 80–118 (2020).
Wang, H., Chen, Q. & Zhou, S. Carbon-based hybrid nanogels: a synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 47, 4198–4232 (2018).
Mitchell, M. J. et al. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 20, 101–124 (2021).
Zou, Y., Huang, B., Cao, L., Deng, Y. & Su, J. Tailored mesoporous inorganic biomaterials: assembly, functionalization, and drug delivery engineering. Adv. Mater. 33, 2005215 (2021).
Wu, F. & Liu, J. Decorated bacteria and the application in drug delivery. Adv. Drug Deliv. Rev. 188, 114443 (2022).
Le, Q. V., Lee, J., Lee, H., Shim, G. & Oh, Y. K. Cell membrane-derived vesicles for delivery of therapeutic agents. Acta Pharm. Sin. B 11, 2096–2113 (2021).
Liang, Y., Duan, L., Lu, J. & Xia, J. Engineering exosomes for targeted drug delivery. Theranostics 11, 3183–3195 (2021).
Bentivoglio, M. & Kristensson, K. Tryps and trips: cell trafficking across the 100-year-old blood–brain barrier. Trends Neurosci. 37, 325–333 (2014).
Liebner, S., Czupalla, C. J. & Wolburg, H. Current concepts of blood-brain barrier development. Int. J. Dev. Biol. 55, 467–476 (2011).
Upton, D. H. et al. Challenges and opportunities to penetrate the blood-brain barrier for brain cancer therapy. Theranostics 12, 4734–4752 (2022).
Harati, R., Villégier, A.-S., Banks, W. A. & Mabondzo, A. Susceptibility of juvenile and adult blood–brain barrier to endothelin-1: regulation of P-glycoprotein and breast cancer resistance protein expression and transport activity. J. Neuroinflammation 9, 273 (2012).
Cai, Z. et al. Role of blood-brain barrier in Alzheimer’s disease. J. Alzheimers Dis. 63, 1223–1234 (2018).
Eichler, A. F. et al. The biology of brain metastases-translation to new therapies. Nat. Rev. Clin. Oncol. 8, 344–356 (2011).
Saraiva, C. et al. Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 235, 34–47 (2016).
Greene, C. & Campbell, M. Tight junction modulation of the blood brain barrier: CNS delivery of small molecules. Tissue Barriers 4, e1138017 (2016).
Ruan, S., Zhou, Y., Jiang, X. & Gao, H. Rethinking CRITID procedure of brain targeting drug delivery: circulation, blood brain barrier recognition, intracellular transport, diseased cell targeting, internalization, and drug release. Adv. Sci. 8, 2004025 (2021).
Zheng, M. et al. Tuning the elasticity of polymersomes for brain tumor targeting. Adv. Sci. 8, e2102001 (2021).
Song, Y., Hu, C., Fu, Y. & Gao, H. Modulating the blood–brain tumor barrier for improving drug delivery efficiency and efficacy. VIEW 3, 20200129 (2022).
Begley, D. J. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol. Ther. 104, 29–45 (2004).
Zhou, Y., Peng, Z., Seven, E. S. & Leblanc, R. M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 270, 290–303 (2018).
Kadry, H., Noorani, B. & Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17, 69 (2020).
Xie, J., Shen, Z., Anraku, Y., Kataoka, K. & Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 224, 119491 (2019).
Daneman, R. & Prat, A. The blood-brain barrier. CSH Perspect. Biol. 7, a020412 (2015).
Zhang, T., Li, W., Meng, G., Wang, P. & Liao, W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater. Sci. 4, 219–229 (2016).
Reese, T. S. & Karnovsky, M. J. Fine structural localization of a blood-brain barrier to exogenous peroxidase. J. Cell Biol. 34, 207–217 (1967).
Westergaard, E. & Brightman, M. W. Transport of proteins across normal cerebral arterioles. J. Comp. Neurol. 152, 17–44 (1973).
Langen, U. H., Ayloo, S. & Gu, C. Development and cell biology of the blood-brain barrier. Annu. Rev. Cell Dev. Bi. 35, 591–613 (2019).
Lippmann, E. S. et al. Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nat. Biotechnol. 30, 783–791 (2012).
Betz, A. L., Firth, J. A. & Goldstein, G. W. Polarity of the blood-brain barrier: distribution of enzymes between the luminal and antiluminal membranes of brain capillary endothelial cells. Brain Res. 192, 17–28 (1980).
Alhadidi, Q., Bin Sayeed, M. S. & Shah, Z. A. The interplay between cofilin and phospho-cofilin: Its role in maintaining blood brain barrier integrity. CNS Neurol. Disord. -Drug Targets 16, 279–290 (2017).
Oldendorf, W. H., Cornford, M. E. & Brown, W. J. The large apparent work capability of the blood-brain barrier: a study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1, 409–417 (1977).
Li, X. et al. Cerium oxide nanoparticles with antioxidative neurorestoration for ischemic stroke. Biomaterials 291, 121904 (2022).
Rössler, K. et al. Expression of leucocyte adhesion molecules at the human blood-brain barrier (BBB). J. Neurosci. Res. 31, 365–374 (1992).
Santa-Maria, A. R. et al. Flow induces barrier and glycocalyx-related genes and negative surface charge in a lab-on-a-chip human blood-brain barrier model. J. Cereb. Blood Flow Metab. 41, 2201–2215 (2021).
Zhao, Y. et al. The role of ferroptosis in blood-brain barrier injury. Cell. Mol. Neurobiol. 43, 223–236 (2023).
Chu, C., Li, J., Boado, R. J. & Pardridge, W. M. Blood-brain barrier genomics and cloning of a novel organic anion transporter. J. Cereb. Blood Flow Metab. 28, 291–301 (2008).
Tietz, S. & Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 209, 493–506 (2015).
Wilhelm, I., Fazakas, C. & Krizbai, I. A. In vitro models of the blood-brain barrier. Acta Neurobiol. Exp. (Warsz.) 71, 113–128 (2011).
Yao, S. et al. Astrocytic lactate dehydrogenase A regulates neuronal excitability and depressive-like behaviors through lactate homeostasis in mice. Nat. Commun. 14, 729 (2023).
Sofroniew, M. V. & Vinters, H. V. Astrocytes: biology and pathology. Acta Neuropathol. 119, 7–35 (2010).
Mazumder, A. G., Julé, A. M., Cullen, P. F. & Sun, D. Astrocyte heterogeneity within white matter tracts and a unique subpopulation of optic nerve head astrocytes. iScience 25, 105568 (2022).
Mayo, F. et al. Aquaporin-4 expression switches from white to gray matter regions during postnatal development of the central nervous system. Int. J. Mol. Sci. 24, 3048 (2023).
Nagelhus, E. A. & Ottersen, O. P. Physiological roles of aquaporin-4 in brain. Physiol. Rev. 93, 1543–1562 (2013).
Kim, D. Y. et al. CU06-1004 (endothelial dysfunction blocker) ameliorates astrocyte end-feet swelling by stabilizing endothelial cell junctions in cerebral ischemia/reperfusion injury. J. Mol. Med. 98, 875–886 (2020).
Zhou, X. et al. Glymphatic system in the central nervous system, a novel therapeutic direction against brain edema after stroke. Front. Aging Neurosci. 13, 698036 (2021).
Ahmadpour, N., Kantroo, M. & Stobart, J. L. Extracellular calcium influx pathways in astrocyte calcium microdomain physiology. Biomolecules 11, 1467 (2021).
Abbott, N. J., Rönnbäck, L. & Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 7, 41–53 (2006).
McConnell, H. L. & Mishra, A. Cells of the blood-brain barrier: an overview of the neurovascular unit in health and disease. Methods Mol. Biol. 2492, 3–24 (2022).
Zhao, J. et al. Activated astrocytes attenuate neocortical seizures in rodent models through driving Na+-K+-ATPase. Nat. Commun. 13, 7136 (2022).
Savidge, T. C. et al. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 132, 1344–1358 (2007).
Saunders, N. R., Ek, C. J., Habgood, M. D. & Dziegielewska, K. M. Barriers in the brain: a renaissance? Trends Neurosci. 31, 279–286 (2008).
Araya, R. et al. BMP signaling through BMPRIA in astrocytes is essential for proper cerebral angiogenesis and formation of the blood-brain-barrier. Mol. Cell. Neurosci. 38, 417–430 (2008).
Zhao, Z., Nelson, A. R., Betsholtz, C. & Zlokovic, B. V. Establishment and dysfunction of the blood-brain barrier. Cell 163, 1064–1078 (2015).
Schurhoff, N. & Toborek, M. Circadian rhythms in the blood-brain barrier: impact on neurological disorders and stress responses. Mol. Brain 16, 5 (2023).
Bhasiin, K., Heintz, O. & Colodner, K. J. Optimization and technical considerations for the dye-exclusion protocol used to assess blood-brain barrier integrity in adult drosophila melanogaster. Int. J. Mol. Sci. 24, 1886 (2023).
Daneman, R., Zhou, L., Kebede, A. A. & Barres, B. A. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 468, 562–566 (2010).
Frank, R. N., Turczyn, T. J. & Das, A. Pericyte coverage of retinal and cerebral capillaries. Invest. Ophthalmol. Vis. Sci. 31, 999–1007 (1990).
Armulik, A., Mäe, M. & Betsholtz, C. Pericytes and the blood-brain barrier: recent advances and implications for the delivery of CNS therapy. Ther. Deliv. 2, 419–422 (2011).
Brown, L. S. et al. Pericytes and neurovascular function in the healthy and diseased brain. Front. Cell. Neurosci. 13, 282 (2019).
Liao, K., Niu, F., Hu, G. & Buch, S. Morphine-mediated release of astrocyte-derived extracellular vesicle miR-23a induces loss of pericyte coverage at the blood-brain barrier: Implications for neuroinflammation. Front. Cell Dev. Biol. 10, 984375 (2022).
Goncalves, A. & Antonetti, D. A. Transgenic animal models to explore and modulate the blood brain and blood retinal barriers of the CNS. Fluids Barriers CNS 19, 86 (2022).
Jeske, R., Albo, J., Marzano, M., Bejoy, J. & Li, Y. Engineering brain-specific pericytes from human pluripotent stem cells. Tissue Eng. Part B-Rev. 26, 367–382 (2020).
Hellström, M., Kalén, M., Lindahl, P., Abramsson, A. & Betsholtz, C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development 126, 3047–3055 (1999).
Tillie, R. et al. A switch from cell-associated to soluble PDGF-B protects against atherosclerosis, despite driving extramedullary hematopoiesis. Cells 10, 1746 (2021).
Lindahl, P., Johansson, B. R., Levéen, P. & Betsholtz, C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science 277, 242–245 (1997).
Armulik, A. et al. Pericytes regulate the blood-brain barrier. Nature 468, 557–561 (2010).
Sengillo, J. D. et al. Deficiency in mural vascular cells coincides with blood-brain barrier disruption in Alzheimer’s disease. Brain Pathol. 23, 303–310 (2013).
Attwell, D., Mishra, A., Hall, C. N., O’Farrell, F. M. & Dalkara, T. What is a pericyte? J. Cereb. Blood Flow Metab. 36, 451–455 (2016).
Ribatti, D., Nico, B. & Crivellato, E. The role of pericytes in angiogenesis. Int. J. Dev. Biol. 55, 261–268 (2011).
Rustenhoven, J., Jansson, D., Smyth, L. C. & Dragunow, M. Brain pericytes as mediators of neuroinflammation. Trends Pharmacol. Sci. 38, 291–304 (2017).
Luissint, A. C., Artus, C., Glacial, F., Ganeshamoorthy, K. & Couraud, P. O. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS 9, 23 (2012).
Anderson, J. M. & Van Itallie, C. M. Physiology and function of the tight junction. CSH Perspect. Biol. 1, a002584 (2009).
Dong, X. Current strategies for brain drug delivery. Theranostics 8, 1481–1493 (2018).
Ahlawat, J. et al. Nanocarriers as potential drug delivery candidates for overcoming the blood-brain barrier: challenges and possibilities. ACS Omega 5, 12583–12595 (2020).
Gumbiner, B. Structure, biochemistry, and assembly of epithelial tight junctions. Am. J. Physiol. 253, C749–C758 (1987).
Gumbiner, B. M. Breaking through the tight junction barrier. J. Cell Biol. 123, 1631–1633 (1993).
Tsukita, S., Furuse, M. & Itoh, M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2, 285–293 (2001).
Haseloff, R. F., Dithmer, S., Winkler, L., Wolburg, H. & Blasig, I. E. Transmembrane proteins of the tight junctions at the blood-brain barrier: structural and functional aspects. Semin. Cell Dev. Biol. 38, 16–25 (2015).
Furuse, M. et al. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell Biol. 123, 1777–1788 (1993).
Persidsky, Y., Ramirez, S. H., Haorah, J. & Kanmogne, G. D. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 1, 223–236 (2006).
Ikenouchi, J. et al. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J. Cell Biol. 171, 939–945 (2005).
Kojima, T. et al. Expression of tricellulin in epithelial cells and non-epithelial cells. Histol. Histopathol. 28, 1383–1392 (2013).
Ebnet, K. Junctional adhesion molecules (JAMs): cell adhesion receptors with pleiotropic functions in cell physiology and development. Physiol. Rev. 97, 1529–1554 (2017).
Chan, Y. et al. Yi-Zhi-Fang-Dai formula exerts a protective effect on the injury of tight junction scaffold proteins in vitro and in vivo by mediating autophagy through regulation of the RAGE/CaMKKβ/AMPK/mTOR Pathway. Biol. Pharm. Bull. 43, 1847–1858 (2020).
Zhong, Y. et al. Monoclonal antibody 7H6 reacts with a novel tight junction-associated protein distinct from ZO-1, cingulin and ZO-2. J. Cell Biol. 120, 477–483 (1993).
Tajes, M. et al. The blood-brain barrier: structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 31, 152–167 (2014).
Liebner, S. et al. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 135, 311–336 (2018).
Teleanu, D. M., Chircov, C., Grumezescu, A. M., Volceanov, A. & Teleanu, R. I. Blood-brain delivery methods using nanotechnology. Pharmaceutics 10, 269 (2018).
Willis, C. L., Meske, D. S. & Davis, T. P. Protein kinase C activation modulates reversible increase in cortical blood-brain barrier permeability and tight junction protein expression during hypoxia and posthypoxic reoxygenation. J. Cereb. Blood Flow Metab. 30, 1847–1859 (2010).
Keep, R. F. et al. Brain endothelial cell junctions after cerebral hemorrhage: Changes, mechanisms and therapeutic targets. J. Cereb. Blood Flow Metab. 38, 1255–1275 (2018).
Krol, S. et al. Therapeutic benefits from nanoparticles: the potential significance of nanoscience in diseases with compromise to the blood brain barrier. Chem. Rev. 113, 1877–1903 (2013).
Cai, N. et al. Roflumilast, a cyclic nucleotide phosphodiesterase 4 inhibitor, protects against cerebrovascular endothelial injury following cerebral ischemia/reperfusion by activating the Notch1/Hes1 pathway. Eur. J. Pharmacol. 926, 175027 (2022).
Zhao, Y. et al. Factors influencing the blood-brain barrier permeability. Brain Res. 1788, 147937 (2022).
Kwak, M. et al. Adherens junctions organize size-selective proteolytic hotspots critical for Notch signalling. Nat. Cell Biol. 24, 1739–1753 (2022).
Malinova, T. S. & Huveneers, S. Sensing of cytoskeletal forces by asymmetric adherens junctions. Trends Cell Biol. 28, 328–341 (2018).
Stamatovic, S. M., Johnson, A. M., Keep, R. F. & Andjelkovic, A. V. Junctional proteins of the blood-brain barrier: New insights into function and dysfunction. Tissue Barriers 4, e1154641 (2016).
Shen, D. et al. Metformin preserves VE-cadherin in choroid plexus and attenuates hydrocephalus via VEGF/VEGFR2/p-Src in an intraventricular hemorrhage rat model. Int. J. Mol. Sci. 23, 8552 (2022).
Gioelli, N. et al. Neuropilin 1 and its inhibitory ligand mini-tryptophanyl-tRNA synthetase inversely regulate VE-cadherin turnover and vascular permeability. Nat. Commun. 13, 4188 (2022).
Ali, J., Liao, F., Martens, E. & Muller, W. A. Vascular endothelial cadherin (VE-cadherin): cloning and role in endothelial cell-cell adhesion. Microcirculation 4, 267–277 (1997).
Rho, S. S., Ando, K. & Fukuhara, S. Dynamic regulation of vascular permeability by vascular endothelial cadherin-mediated endothelial cell-cell junctions. J. Nippon Med. Sch. 84, 148–159 (2017).
Kuo, W. T., Odenwald, M. A., Turner, J. R. & Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 1514, 21–33 (2022).
Liang, J. et al. PEDF protects endothelial barrier integrity during acute myocardial infarction via 67LR. Int. J. Mol. Sci. 24, 2787 (2023).
Brandtner, A. K., Lehner, G. F., Pircher, A., Feistritzer, C. & Joannidis, M. Differential procoagulatory response of microvascular, arterial and venous endothelial cells upon inflammation in vitro. Thromb. Res. 205, 70–80 (2021).
Zucchini, C. et al. CD99 suppresses osteosarcoma cell migration through inhibition of ROCK2 activity. Oncogene 33, 1912–1921 (2014).
Dash, S., Duraivelan, K. & Samanta, D. Cadherin-mediated host-pathogen interactions. Cell. Microbiol. 23, e13316 (2021).
Turowski, P. & Kenny, B. A. The blood-brain barrier and methamphetamine: open sesame? Front. Neurosci. 9, 156 (2015).
Segarra, M., Aburto, M. R. & Acker-Palmer, A. Blood-brain barrier dynamics to maintain brain homeostasis. Trends Neurosci. 44, 393–405 (2021).
Bhattacharya, A., Kaushik, D. K., Lozinski, B. M. & Yong, V. W. Beyond barrier functions: Roles of pericytes in homeostasis and regulation of neuroinflammation. J. Neurosci. Res. 98, 2390–2405 (2020).
Fong, C. W. Permeability of the blood–brain barrier: molecular mechanism of transport of drugs and physiologically important compounds. J. Membr. Biol. 248, 651–669 (2015).
Galea, I. The blood–brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 18, 2489–2501 (2021).
Sonar, S. A. & Lal, G. Blood–brain barrier and its function during inflammation and autoimmunity. J. Leukoc. Biol. 103, 839–853 (2018).
Harilal, S. et al. Revisiting the blood-brain barrier: A hard nut to crack in the transportation of drug molecules. Brain Res. Bull. 160, 121–140 (2020).
Yang, X., Wang, Q. & Cao, E. Structure of the human cation–chloride cotransporter NKCC1 determined by single-particle electron cryo-microscopy. Nat. Commun. 11, 1016 (2020).
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R. & Zlokovic, B. V. Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78 (2019).
DeMaio, A., Mehrotra, S., Sambamurti, K. & Husain, S. The role of the adaptive immune system and T cell dysfunction in neurodegenerative diseases. J. Neuroinflammation 19, 251 (2022).
Yang, J., Luly, K. M. & Green, J. J. Nonviral nanoparticle gene delivery into the CNS for neurological disorders and brain cancer applications. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 15, e1853 (2022).
Pardridge, W. M. CSF, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 13, 963–975 (2016).
Fowler, M. J. et al. Intrathecal drug delivery in the era of nanomedicine. Adv. Drug Deliv. Rev. 165-166, 77–95 (2020).
Porensky, P. N. & Burghes, A. H. Antisense oligonucleotides for the treatment of spinal muscular atrophy. Hum. Gene Ther. 24, 489–498 (2013).
Lonser, R. R., Sarntinoranont, M., Morrison, P. F. & Oldfield, E. H. Convection-enhanced delivery to the central nervous system. J. Neurosurg. 122, 697–706 (2015).
Mehta, A. M., Sonabend, A. M. & Bruce, J. N. Convection-enhanced delivery. Neurotherapeutics 14, 358–371 (2017).
Gurke, J. et al. Hybrid fabrication of multimodal intracranial implants for electrophysiology and local drug delivery. Mater. Horiz. 9, 1727–1734 (2022).
Campbell, A. & Wu, C. Chronically implanted intracranial electrodes: Tissue reaction and electrical changes. Micromachines 9, 430 (2018).
Lozano, A. M. et al. Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15, 148–160 (2019).
Cagnan, H., Denison, T., McIntyre, C. & Brown, P. Emerging technologies for improved deep brain stimulation. Nat. Biotechnol. 37, 1024–1033 (2019).
Liu, Y. et al. Microneedle-mediated vascular endothelial growth factor delivery promotes angiogenesis and functional recovery after stroke. J. Control. Release 338, 610–622 (2021).
Abdelkader, H. et al. Polymeric long-acting drug delivery systems (LADDS) for treatment of chronic diseases: Inserts, patches, wafers, and implants. Adv. Drug Deliv. Rev. 177, 113957 (2021).
Shapira-Furman, T. et al. Biodegradable wafers releasing Temozolomide and Carmustine for the treatment of brain cancer. J. Control. Release 295, 93–101 (2019).
Wang, Z. et al. Silk microneedle patch capable of on-demand multidrug delivery to the brain for glioblastoma treatment. Adv. Mater. 34, 2106606 (2022).
Hladky, S. B. & Barrand, M. A. Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 15, 30 (2018).
Hersh, A. M., Alomari, S. & Tyler, B. M. Crossing the blood-brain barrier: advances in nanoparticle technology for drug delivery in neuro-oncology. Int. J. Mol. Sci. 23, 4153 (2022).
Brasnjevic, I., Steinbusch, H. W. M., Schmitz, C. & Martinez Martinez, P. Delivery of peptide and protein drugs over the blood–brain barrier. Prog. Neurobiol. 87, 212–251 (2009).
Gu, Y. et al. Minoxidil sulfate induced the increase in blood-brain tumor barrier permeability through ROS/RhoA/PI3K/PKB signaling pathway. Neuropharmacology 75, 407–415 (2013).
Han, L. et al. Increased nanoparticle delivery to brain tumors by autocatalytic oriming for improved treatment and imaging. ACS Nano 10, 4209–4218 (2016).
Miao, T. et al. Nanoparticles surmounting blood-brain tumor barrier through both transcellular and paracellular pathways to target brain metastases. Adv. Funct. Mater. 29, 1900259 (2019).
Al Hajj, M., Wicha, M. S., Benito Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
van den Broek, S. L., Shalgunov, V. & Herth, M. M. Transport of nanomedicines across the blood-brain barrier: Challenges and opportunities for imaging and therapy. Biomater. Adv. 141, 213125 (2022).
Al Ahmady, Z. S. et al. Selective brain entry of lipid nanoparticles in haemorrhagic stroke is linked to biphasic blood-brain barrier disruption. Theranostics 12, 4477–4497 (2022).
Liu, Z. et al. Effect of baicalin-loaded PEGylated cationic solid lipid nanoparticles modified by OX26 antibody on regulating the levels of baicalin and amino acids during cerebral ischemia-reperfusion in rats. Int. J. Pharm. 489, 131–138 (2015).
Tsuji, A. & Tamai, I. Carrier-mediated or specialized transport of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 36, 277–290 (1999).
Pardridge, W. M. CNS drug design based on principles of blood-brain barrier transport. J. Neurochem. 70, 1781–1792 (1998).
Henkin, R. I. Intranasal delivery to the brain. Nat. Biotechnol. 29, 480 (2011).
Agrawal, M. et al. Nose-to-brain drug delivery: An update on clinical challenges and progress towards approval of anti-Alzheimer drugs. J. Control. Release 281, 139–177 (2018).
Gänger, S. & Schindowski, K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics 10, 116 (2018).
Chatterjee, B. et al. Targeted drug delivery to the brain via intranasal nanoemulsion: Available proof of concept and existing challenges. Int. J. Pharm. 565, 258–268 (2019).
Godfrey, L. et al. Nanoparticulate peptide delivery exclusively to the brain produces tolerance free analgesia. J. Control. Release 270, 135–144 (2018).
Kanazawa, T., Akiyama, F., Kakizaki, S., Takashima, Y. & Seta, Y. Delivery of siRNA to the brain using a combination of nose-to-brain delivery and cell-penetrating peptide-modified nano-micelles. Biomaterials 34, 9220–9226 (2013).
Khatri, K. D. et al. Nanotechnological advances for nose to brain delivery of therapeutics to improve the Parkinson therapy. Curr. Neuropharmacol. 20, 1 (2022).
Kozlovskaya, L., Abou-Kaoud, M. & Stepensky, D. Quantitative analysis of drug delivery to the brain via nasal route. J. Control. Release 189, 133–140 (2014).
Nabi, B., Rehman, S., Khan, S., Baboota, S. & Ali, J. Ligand conjugation: An emerging platform for enhanced brain drug delivery. Brain Res. Bull. 142, 384–393 (2018).
Anthony, D. P. et al. Targeting receptor-ligand chemistry for drug delivery across blood-brain barrier in brain diseases. Life Sci. 274, 119326 (2021).
Prabhu, S. et al. A polymeric temozolomide nanocomposite against orthotopic glioblastoma xenograft: Tumor-specific homing directed by nestin. Nanoscale 9, 10919–10932 (2017).
Kang, S. et al. Muscone/RI7217 co-modified upward messenger DTX liposomes enhanced permeability of blood-brain barrier and targeting glioma. Theranostics 10, 4308–4322 (2020).
Liu, C. et al. A dual-mediated liposomal drug delivery system targeting the brain: Rational construction, integrity evaluation across the blood-brain barrier, and the transporting mechanism to glioma cells. Int. J. Nanomed. 12, 2407–2425 (2017).
Ruan, S. et al. Acid-responsive transferrin dissociation and GLUT mediated exocytosis for increased blood–brain barrier transcytosis and programmed glioma targeting delivery. Adv. Funct. Mater. 28, 1802227 (2018).
Lei, T. et al. A nanocleaner specifically penetrates the blood‒brain barrier at lesions to clean toxic proteins and regulate inflammation in Alzheimer’s disease. Acta Pharm. Sin. B 11, 4032–4044 (2021).
He, X. et al. Acid-responsive dual-targeted nanoparticles encapsulated aspirin rescue the Immune activation and phenotype in autism spectrum disorder. Adv. Sci. 9, e2104286 (2022).
Scherer, T., Sakamoto, K. & Buettner, C. Brain insulin signalling in metabolic homeostasis and disease. Nat. Rev. Endocrinol. 17, 468–483 (2021).
Nagao, H. et al. Unique ligand and kinase-independent roles of the insulin receptor in regulation of cell cycle, senescence and apoptosis. Nat. Commun. 14, 57 (2023).
Boucher, J., Tseng, Y.-H. & Kahn, C. R. Insulin and insulin-like growth factor-1 receptors Act as ligand-specific amplitude modulators of a common pathway regulating gene transcription. J. Biol. Chem. 285, 17235–17245 (2010).
Ulbrich, K., Knobloch, T. & Kreuter, J. Targeting the insulin receptor: nanoparticles for drug delivery across the blood-brain barrier (BBB). J. Drug Target. 19, 125–132 (2011).
Thorne, R. G., Pronk, G. J., Padmanabhan, V. & Frey, W. H. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience 127, 481–496 (2004).
Goldstein, J. L., Brown, M. S., Anderson, R. G. W., Russell, D. W. & Schneider, W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu. Rev. Cell Biol. 1, 1–39 (1985).
Ying, X. et al. Angiopep-conjugated electro-responsive hydrogel nanoparticles: therapeutic potential for epilepsy. Angew. Chem. Int. Ed. 53, 12436–12440 (2014).
Wang, Y. et al. Electroresponsive nanoparticles improve antiseizure effect of phenytoin in generalized tonic-clonic seizures. Neurotherapeutics 13, 603–613 (2016).
Thirumurugan, S. et al. Angiopep-2-decorated titanium-alloy core-shell magnetic nanoparticles for nanotheranostics and medical imaging. Nanoscale 14, 14789–14800 (2022).
Israel, L. L. et al. Signature effects of vector-guided systemic nano bioconjugate delivery across blood-brain barrier of normal, Alzheimer’s, and tumor mouse models. ACS Nano 16, 11815–11832 (2022).
Ebrahimnejad, P., Taleghani, A. S., Asare-Addo, K. & Nokhodchi, A. An updated review of folate-functionalized nanocarriers: a promising ligand in cancer. Drug Discov. Today 27, 471–489 (2022).
Zhuang, X. et al. Grapefruit-derived nanovectors delivering therapeutic miR17 through an intranasal route inhibit brain tumor progression. Mol. Ther. 24, 96–105 (2016).
Wang, Y. et al. Increased brain uptake of pterostilbene loaded folate modified micellar delivery system. Drug Deliv. 29, 3071–3086 (2022).
Kuplennik, N., Lang, K., Steinfeld, R. & Sosnik, A. Folate receptor α-modified nanoparticles for targeting of the central nervous system. ACS Appl. Mater. Interfaces 11, 39633–39647 (2019).
Liu, H., Su, Y. Y., Jiang, X. C. & Gao, J. Q. Cell membrane-coated nanoparticles: a novel multifunctional biomimetic drug delivery system. Drug Deliv. Transl. Res. 13, 716–737 (2023).
Fang, R. H., Kroll, A. V., Gao, W. & Zhang, L. Cell membrane coating nanotechnology. Adv. Mater. 30, 1706759 (2018).
Zeng, Y. et al. Cell membrane coated-nanoparticles for cancer immunotherapy. Acta Pharm. Sin. B 12, 3233–3254 (2022).
Xia, Y. et al. Engineering macrophages for cancer immunotherapy and drug delivery. Adv. Mater. 32, e2002054 (2020).
Fang, R. H., Gao, W. & Zhang, L. Targeting drugs to tumours using cell membrane-coated nanoparticles. Nat. Rev. Clin. Oncol. 20, 33–48 (2023).
Li, J. et al. Cell-membrane-coated nanoparticles for targeted drug delivery to the brain for the Treatment of neurological diseases. Pharmaceutics 15, 621 (2023).
Tan, S., Wu, T., Zhang, D. & Zhang, Z. Cell or cell membrane-based drug delivery systems. Theranostics 5, 863–881 (2015).
Lee, J. Y. et al. Red blood cell membrane bioengineered Zr-89 labelled hollow mesoporous silica nanosphere for overcoming phagocytosis. Sci. Rep. 9, 7419 (2019).
Chai, Z. et al. A facile approach to functionalizing cell membrane-coated nanoparticles with neurotoxin-derived peptide for brain-targeted drug delivery. J. Control. Release 264, 102–111 (2017).
Zou, Y. et al. Effective and targeted human orthotopic glioblastoma xenograft therapy via a multifunctional biomimetic nanomedicine. Adv. Mater. 30, 1803717 (2018).
Tapeinos, C. et al. Cell membrane-coated magnetic nanocubes with a homotypic targeting ability increase intracellular temperature due to ROS scavenging and act as a versatile theranostic system for glioblastoma multiforme. Adv. Healthc. Mater. 8, 1900612 (2019).
Zhu, J. Y. et al. Preferential cancer cell self-recognition and tumor self-targeting by coating nanoparticles with homotypic cancer cell membranes. Nano Lett. 16, 5895–5901 (2016).
Liu, S. et al. Neutrophil-biomimetic “nanobuffer” for remodeling the microenvironment in the infarct core and protecting neurons in the penumbra via neutralization of detrimental factors to treat ischemic stroke. ACS Appl. Mater. Interfaces 14, 27743–27761 (2022).
Oroojalian, F., Beygi, M., Baradaran, B., Mokhtarzadeh, A. & Shahbazi, M. A. Immune cell membrane-coated biomimetic nanoparticles for targeted cancer therapy. Small 17, 2006484 (2021).
Liang, T. et al. Recent advances in macrophage-mediated drug delivery systems. Int. J. Nanomed. 16, 2703–2714 (2021).
Yin, T. et al. Engineered macrophage-membrane-coated nanoparticles with enhanced PD-1 expression induce immunomodulation for a synergistic and targeted antiglioblastoma activity. Nano Lett. 22, 6606–6614 (2022).
Han, Y. et al. Macrophage membrane-coated nanocarriers co-modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact. Mater. 6, 529–542 (2021).
Chu, D., Dong, X., Shi, X., Zhang, C. & Wang, Z. Neutrophil-based drug delivery systems. Adv. Mater. 30, e1706245 (2018).
Pitchaimani, A., Nguyen, T. D. T. & Aryal, S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 160, 124–137 (2018).
Deng, G. et al. Natural-killer-cell-inspired nanorobots with aggregation-induced emission characteristics for near-Infrared-II fluorescence-guided glioma theranostics. ACS Nano 14, 11452–11462 (2020).
Kiessling, M., Herchenhan, E. & Eggert, H. R. Cerebrovascular and metabolic effects on the rat brain of focal Nd:YAG laser irradiation. J. Neurosurg. 73, 909–917 (1990).
Tao, W. & Farokhzad, O. C. Theranostic nanomedicine in the NIR-II window: classification, fabrication, and biomedical applications. Chem. Rev. 122, 5405–5407 (2022).
Xu, C. & Pu, K. Second near-infrared photothermal materials for combinational nanotheranostics. Chem. Soc. Rev. 50, 1111–1137 (2021).
Wu, X. et al. Tether-free photothermal deep-brain stimulation in freely behaving mice via wide-field illumination in the near-infrared-II window. Nat. Biomed. Eng. 6, 754–770 (2022).
Li, B. et al. Photothermal modulation of depression-related ion channel function through conjugated polymer nanoparticles. Adv. Funct. Mater. 31, 2010757 (2021).
Chen, W. et al. Black phosphorus nanosheets as a neuroprotective nanomedicine for neurodegenerative disorder therapy. Adv. Mater. 30, 1703458 (2018).
Wu, D. et al. Nanoengineered on-demand drug delivery system improves efficacy of pharmacotherapy for epilepsy. Sci. Adv. 8, eabm3381 (2022).
Choi, M., Ku, T., Chong, K., Yoon, J. & Choi, C. Minimally invasive molecular delivery into the brain using optical modulation of vascular permeability. Proc. Natl Acad. Sci. USA 108, 9256–9261 (2011).
Jin, L. et al. Fast-acting black-phosphorus-assisted depression therapy with low toxicity. Adv. Mater. 32, 1906050 (2020).
Li, X. et al. Reversibly modulating the blood-brain barrier by laser stimulation of molecular-targeted nanoparticles. Nano Lett. 21, 9805–9815 (2021).
Clement, G. T. & Hynynen, K. A non-invasive method for focusing ultrasound through the human skull. Phys. Med. Biol. 47, 1219 (2002).
Ellens, N. P. K. & Hynynen, K. Power Ultrasonics (eds J A. Gallego-Juárez & K. F. Graff) Ch. 22 (Woodhead Publishing, 2015).
Lynn, J. G. & Putnam, T. J. Histology of cerebral lesions produced by focused ultrasound. Am. J. Pathol. 20, 637–649 (1944).
Aryal, M., Arvanitis, C. D., Alexander, P. M. & McDannold, N. Ultrasound-mediated blood-brain barrier disruption for targeted drug delivery in the central nervous system. Adv. Drug Deliv. Rev. 72, 94–109 (2014).
Gorick, C. M. et al. Applications of focused ultrasound-mediated blood-brain barrier opening. Adv. Drug Deliv. Rev. 191, 114583 (2022).
Liu, Y. et al. Microbubbles in combination with focused ultrasound for the delivery of quercetin-modified sulfur nanoparticles through the blood brain barrier into the brain parenchyma and relief of endoplasmic reticulum stress to treat Alzheimer’s disease. Nanoscale 12, 6498–6511 (2020).
Gorick, C. M. et al. Sonoselective transfection of cerebral vasculature without blood-brain barrier disruption. Proc. Natl Acad. Sci. USA 117, 5644–5654 (2020).
Liu, S. et al. A neuroanatomical basis for electroacupuncture to drive the vagal-adrenal axis. Nature 598, 641–645 (2021).
Zhang, J. et al. Electroacupuncture: a new approach to open the blood-brain barrier in rats recovering from middle cerebral artery occlusion. Acupunct. Med. 36, 377–385 (2018).
Zhang, S. et al. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 190, 69–83 (2022).
Bellettato, C. M. & Scarpa, M. Possible strategies to cross the blood-brain barrier. Ital. J. Pediatr. 44, 131 (2018).
Li, W. et al. BBB pathophysiology–independent delivery of siRNA in traumatic brain injury. Sci. Adv. 7, eabd6889 (2021).
Zhang, X., He, T., Chai, Z., Samulski, R. J. & Li, C. Blood-brain barrier shuttle peptides enhance AAV transduction in the brain after systemic administration. Biomaterials 176, 71–83 (2018).
Obermeier, B., Daneman, R. & Ransohoff, R. M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 19, 1584–1596 (2013).
Wang, D., Tai, P. W. L. & Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 18, 358–378 (2019).
Wang, D. et al. A rationally engineered capsid variant of AAV9 for systemic CNS-directed and peripheral tissue-detargeted gene delivery in neonates. Mol. Ther.-Methods Clin. Dev. 9, 234–246 (2018).
Goertsen, D. et al. AAV capsid variants with brain-wide transgene expression and decreased liver targeting after intravenous delivery in mouse and marmoset. Nat. Neurosci. 25, 106–115 (2022).
Yao, Y. et al. Variants of the adeno-associated virus serotype 9 with enhanced penetration of the blood–brain barrier in rodents and primates. Nat. Biomed. Eng. 6, 1257–1271 (2022).
Coureuil, M., Lécuyer, H., Bourdoulous, S. & Nassif, X. A journey into the brain: insight into how bacterial pathogens cross blood-brain barriers. Nat. Rev. Microbiol. 15, 149–159 (2017).
Sun, R. et al. Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy. Nat. Commun. 13, 5127 (2022).
Chung, T. D. et al. Effects of acute and chronic oxidative stress on the blood-brain barrier in 2D and 3D in vitro models. Fluids Barriers CNS 19, 33 (2022).
Wardill, H. R. et al. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int. J. Cancer 139, 2635–2645 (2016).
Lehner, C. et al. Oxidative stress and blood-brain barrier dysfunction under particular consideration of matrix metalloproteinases. Antioxid. Redox Signal. 15, 1305–1323 (2011).
Kim, Y. et al. Effects of natural polyphenols on oxidative stress-mediated blood-brain barrier dysfunction. Antioxidants 11, 197 (2022).
Ballance, W. C., Qin, E. C., Chung, H. J., Gillette, M. U. & Kong, H. Reactive oxygen species-responsive drug delivery systems for the treatment of neurodegenerative diseases. Biomaterials 217, 119292 (2019).
Nash, A. M. et al. Clinically translatable cytokine delivery platform for eradication of intraperitoneal tumors. Sci. Adv. 8, eabm1032 (2022).
Barenholz, Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J. Control. Release 160, 117–134 (2012).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4, e10143 (2019).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 1, 10–29 (2016).
Allen, T. M. & Cullis, P. R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 65, 36–48 (2013).
Shah, S., Dhawan, V., Holm, R., Nagarsenker, M. S. & Perrie, Y. Liposomes: advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 154-155, 102–122 (2020).
Crommelin, D. J. A., van Hoogevest, P. & Storm, G. The role of liposomes in clinical nanomedicine development. What now? Now what? J. Control. Release 318, 256–263 (2020).
Guimarães, D., Cavaco-Paulo, A. & Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 601, 120571 (2021).
Antimisiaris, S. G. et al. Overcoming barriers by local drug delivery with liposomes. Adv. Drug Deliv. Rev. 174, 53–86 (2021).
Zhang, Z. et al. Brain-targeted drug delivery by manipulating protein corona functions. Nat. Commun. 10, 3561 (2019).
Zhang, Y. et al. Brain-targeted delivery of obidoxime, using aptamer-modified liposomes, for detoxification of organophosphorus compounds. J. Control. Release 329, 1117–1128 (2021).
Kucharz, K. et al. Post-capillary venules are the key locus for transcytosis-mediated brain delivery of therapeutic nanoparticles. Nat. Commun. 12, 4121 (2021).
Zhu, Y., Feijen, J. & Zhong, Z. Dual-targeted nanomedicines for enhanced tumor treatment. Nano Today 18, 65–85 (2018).
Shi, D., Mi, G., Shen, Y. & Webster, T. J. Glioma-targeted dual functionalized thermosensitive Ferri-liposomes for drug delivery through an in vitro blood–brain barrier. Nanoscale 11, 15057–15071 (2019).
Lakkadwala, S., dos Santos Rodrigues, B., Sun, C. & Singh, J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J. Control. Release 307, 247–260 (2019).
Ozdas, M. S. et al. Non-invasive molecularly-specific millimeter-resolution manipulation of brain circuits by ultrasound-mediated aggregation and uncaging of drug carriers. Nat. Commun. 11, 4929 (2020).
Fernandes, M. et al. Novel concept of exosome-like liposomes for the treatment of Alzheimer’s disease. J. Control. Release 336, 130–143 (2021).
Zhang, W., Mehta, A., Tong, Z., Esser, L. & Voelcker, N. H. Development of polymeric nanoparticles for blood–brain barrier transfer—strategies and challenges. Adv. Sci. 8, 2003937 (2021).
Lagreca, E. et al. Recent advances in the formulation of PLGA microparticles for controlled drug delivery. Prog. Biomater. 9, 153–174 (2020).
Semete, B. et al. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomed. -Nanotechnol. 6, 662–671 (2010).
Del Grosso, A. et al. Brain-targeted enzyme-loaded nanoparticles: a breach through the blood-brain barrier for enzyme replacement therapy in Krabbe disease. Sci. Adv. 5, eaax7462 (2019).
Wu, D. et al. Thermo-sensitive micelles extend therapeutic potential for febrile seizures. Signal Transduct. Target. Ther. 6, 296 (2021).
Wang, Y. et al. Nanoparticle-mediated convection-enhanced delivery of a DNA intercalator to gliomas circumvents temozolomide resistance. Nat. Biomed. Eng. 5, 1048–1058 (2021).
Lu, Y. et al. Microthrombus-targeting micelles for neurovascular remodeling and enhanced microcirculatory perfusion in acute ischemic stroke. Adv. Mater. 31, 1808361 (2019).
Lu, Y. et al. Microenvironment remodeling micelles for alzheimer’s disease therapy by early modulation of activated microglia. Adv. Sci. 6, 1801586 (2019).
Lam, F. C. et al. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nat. Commun. 9, 1991 (2018).
Zhou, Y. et al. Blood-brain barrier–penetrating siRNA nanomedicine for Alzheimer’s disease therapy. Sci. Adv. 6, eabc7031 (2020).
Li, J. & Mooney, D. J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1, 16071 (2016).
Picone, P. et al. Nose-to-brain delivery of insulin enhanced by a nanogel carrier. J. Control. Release 270, 23–36 (2018).
Gadhave, D., Rasal, N., Sonawane, R., Sekar, M. & Kokare, C. Nose-to-brain delivery of teriflunomide-loaded lipid-based carbopol-gellan gum nanogel for glioma: Pharmacological and in vitro cytotoxicity studies. Int. J. Biol. Macromol. 167, 906–920 (2021).
Gadhave, D., Gupta, A., Khot, S., Tagalpallewar, A. & Kokare, C. Nose-to-brain delivery of paliperidone palmitate poloxamer-guar gum nanogel: Formulation, optimization and pharmacological studies in rats. Ann. Pharm. Fr. 81, 315–333 (2023).
Huang, G. et al. Nose-to-brain delivery of drug nanocrystals by using Ca2+ responsive deacetylated gellan gum based in situ-nanogel. Int. J. Pharm. 594, 120182 (2021).
Singh, S. et al. Protease responsive nanogels for transcytosis across the blood−brain barrier and intracellular delivery of radiopharmaceuticals to brain tumor cells. Adv. Healthc. Mater. 10, 2100812 (2021).
She, D. et al. Hypoxia-degradable zwitterionic phosphorylcholine drug nanogel for enhanced drug delivery to glioblastoma. Chem. Eng. J. 408, 127359 (2021).
Qi, W. et al. Light-controlled precise delivery of NIR-responsive semiconducting polymer nanoparticles with promoted vascular permeability. Adv. Healthc. Mater. 10, 2100569 (2021).
Curcio, M. et al. Natural polysaccharide carriers in brain delivery: challenge and perspective. Pharmaceutics 12, 1183 (2020).
Agrawal, M. et al. Stimuli-responsive In situ gelling system for nose-to-brain drug delivery. J. Control. Release 327, 235–265 (2020).
Kopeček, J. & Yang, J. Polymer nanomedicines. Adv. Drug Deliv. Rev. 156, 40–64 (2020).
Geng, H. et al. Binding to amyloid-β protein by photothermal blood-brain barrier-penetrating nanoparticles for inhibition and disaggregation of fibrillation. Adv. Funct. Mater. 31, 2102953 (2021).
Goddard, Z. R., Marín, M. J., Russell, D. A. & Searcey, M. Active targeting of gold nanoparticles as cancer therapeutics. Chem. Soc. Rev. 49, 8774–8789 (2020).
Sharifi, M. et al. Plasmonic gold nanoparticles: Optical manipulation, imaging, drug delivery and therapy. J. Control. Release 311-312, 170–189 (2019).
Behroozi, Z. et al. Distribution of gold nanoparticles into the brain: a systematic review and meta-analysis. Nanotoxicology 15, 1059–1072 (2021).
Kwon, S. P. et al. Thrombin-activatable fluorescent peptide incorporated gold nanoparticles for dual optical/computed tomography thrombus imaging. Biomaterials 150, 125–136 (2018).
Meir, R. & Popovtzer, R. Cell tracking using gold nanoparticles and computed tomography imaging. WIREs Nanomed. Nanobiotechnol. 10, e1480 (2018).
Arami, H. et al. Remotely controlled near-infrared-triggered photothermal treatment of brain tumours in freely behaving mice using gold nanostars. Nat. Nanotechnol. 17, 1015–1022 (2022).
Xue, Y., Li, X., Li, H. & Zhang, W. Quantifying thiol–gold interactions towards the efficient strength control. Nat. Commun. 5, 4348 (2014).
Oshra, B., Malka, S., Menachem, M. & Rachela, P. In Proc. SPIE, Nanoscale Imaging, Sensing, and Actuation for Biomedical Applications XVI. 108911H (2019).
Zhang, J. et al. Neuroprotective effects of maize tetrapeptide-anchored gold nanoparticles in Alzheimer’s disease. Colloid Surf. B 200, 111584 (2021).
Lee, H. E. et al. Amino-acid- and peptide-directed synthesis of chiral plasmonic gold nanoparticles. Nature 556, 360–365 (2018).
Cai, J. et al. Polarization-sensitive optoionic membranes from chiral plasmonic nanoparticles. Nat. Nanotechnol. 17, 408–416 (2022).
Hou, K. et al. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of Alzheimer’s disease. Nat. Commun. 11, 4790 (2020).
Liu, L. et al. Switchable nanoparticle for programmed gene-chem delivery with enhanced neuronal recovery and CT imaging for neurodegenerative disease treatment. Mater. Horiz. 6, 1923–1929 (2019).
Feng, Q. et al. Self-assembly of gold nanoparticles shows microenvironment-mediated dynamic switching and enhanced brain tumor targeting. Theranostics 7, 1875–1889 (2017).
Gao, X. et al. Guiding brain-tumor surgery via blood–brain-barrier-permeable gold nanoprobes with acid-triggered MRI/SERRS signals. Adv. Mater. 29, 1603917 (2017).
Liu, Y. et al. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid Interface Sci. 552, 388–400 (2019).
Schroeder, V., Savagatrup, S., He, M., Lin, S. & Swager, T. M. Carbon nanotube chemical sensors. Chem. Rev. 119, 599–663 (2019).
Panwar, N. et al. Nanocarbons for biology and medicine: sensing, imaging, and drug delivery. Chem. Rev. 119, 9559–9656 (2019).
Dasari Shareena, T. P., McShan, D., Dasmahapatra, A. K. & Tchounwou, P. B. A review on graphene-based nanomaterials in biomedical applications and risks in environment and health. Nano-Micro Lett. 10, 53 (2018).
Du, J., Xu, N., Fan, J., Sun, W. & Peng, X. Carbon dots for in vivo bioimaging and theranostics. Small 15, 1805087 (2019).
Sanginario, A., Miccoli, B. & Demarchi, D. Carbon nanotubes as an effective opportunity for cancer diagnosis and treatment. Biosensors 7, 9 (2017).
Teradal, N. L. & Jelinek, R. Carbon nanomaterials in biological studies and biomedicine. Adv. Healthc. Mater. 6, 1700574 (2017).
Bagheri, B. et al. Carbon-based nanostructures for cancer therapy and drug delivery applications. J. Mater. Chem. B 10, 9944–9967 (2022).
Ashrafizadeh, M. et al. Carbon dots as versatile nanoarchitectures for the treatment of neurological disorders and their theranostic applications: a review. Adv. Colloid Interfac. 278, 102123 (2020).
Zhou, Y. et al. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood–brain barrier and inhibitors of β-amyloid. Nanoscale 11, 22387–22397 (2019).
Hettiarachchi, S. D. et al. Triple conjugated carbon dots as a nano-drug delivery model for glioblastoma brain tumors. Nanoscale 11, 6192–6205 (2019).
Tabish, T. A. & Narayan, R. J. Crossing the blood–brain barrier with graphene nanostructures. Mater. Today 51, 393–401 (2021).
Liu, J., Dong, J., Zhang, T. & Peng, Q. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J. Control. Release 286, 64–73 (2018).
Wang, K. et al. Intranasal administration of dauricine loaded on graphene oxide: multi-target therapy for Alzheimer’s disease. Drug Deliv. 28, 580–593 (2021).
Tan, M. J. et al. Flexible modulation of CO-release using various nuclearity of metal carbonyl clusters on graphene oxide for stroke remediation. Adv. Healthc. Mater. 7, 1701113 (2018).
Xiong, S. et al. Targeted graphene oxide for drug delivery as a therapeutic nanoplatform against Parkinson’s disease. Biomater. Sci. 9, 1705–1715 (2021).
Su, S. et al. In vitro study of transportation of porphyrin immobilized graphene oxide through blood brain barrier. Mat. Sci. Eng. C.-Mater. 107, 110313 (2020).
Burkhart, A. et al. Expression of iron-related proteins at the neurovascular unit supports reduction and reoxidation of iron for transport through the blood-brain barrier. Mol. Neurobiol. 53, 7237–7253 (2016).
Liu, G. et al. Transferrin modified graphene oxide for glioma-targeted drug delivery: In vitro and in vivo evaluations. ACS Appl. Mater. Interfaces 5, 6909–6914 (2013).
Kim, D. et al. Graphene quantum dots prevent α-synucleinopathy in Parkinson’s disease. Nat. Nanotechnol. 13, 812–818 (2018).
Sung, S. et al. Graphene quantum dots-mediated theranostic penetrative delivery of drug and photolytics in deep tumors by targeted biomimetic nanosponges. Nano Lett. 19, 69–81 (2019).
Henna, T. K. et al. Carbon nanostructures: The drug and the delivery system for brain disorders. Int. J. Pharm. 587, 119701 (2020).
Harsha, P. J. et al. A novel PEGylated carbon nanotube conjugated mangiferin: An explorative nanomedicine for brain cancer cells. J. Drug Deliv. Sci. Technol. 53, 101186 (2019).
Tan, J. M., Foo, J. B., Fakurazi, S. & Hussein, M. Z. Release behaviour and toxicity evaluation of levodopa from carboxylated single-walled carbon nanotubes. Beilstein J. Nanotechnol. 6, 243–253 (2015).
You, Y. et al. Designing dual-functionalized carbon nanotubes with high blood–brain-barrier permeability for precise orthotopic glioma therapy. Dalton Trans. 48, 1569–1573 (2019).
Rasras, S. et al. Single-walled and multiwalled carbon nanotubes induce oxidative stress in isolated rat brain mitochondria. Toxicol. Ind. Health 35, 497–506 (2019).
Facciolà, A. et al. Carbon nanotubes and central nervous system: environmental risks, toxicological aspects and future perspectives. Environ. Toxicol. Pharmacol. 65, 23–30 (2019).
Israel, L. L., Galstyan, A., Holler, E. & Ljubimova, J. Y. Magnetic iron oxide nanoparticles for imaging, targeting and treatment of primary and metastatic tumors of the brain. J. Control. Release 320, 45–62 (2020).
Dadfar, S. M. et al. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 138, 302–325 (2019).
Cardoso, V. F. et al. Advances in magnetic nanoparticles for biomedical applications. Adv. Healthc. Mater. 7, 1700845 (2018).
Rajamani, U. S. G. R. M. P. Biodistribution, clearance and morphological alterations of intravenously administered iron oxide nanoparticles in male wistar rats. Int. J. Nanomed. 14, 9677–9692 (2019).
Qiao, R. et al. Receptor-mediated delivery of magnetic nanoparticles across the blood–brain barrier. ACS Nano 6, 3304–3310 (2012).
Enteshari Najafabadi, R., Kazemipour, N., Esmaeili, A., Beheshti, S. & Nazifi, S. Using superparamagnetic iron oxide nanoparticles to enhance bioavailability of quercetin in the intact rat brain. BMC Pharmacol. Toxicol. 19, 59 (2018).
Aguilera, G. et al. Carboxymethyl cellulose coated magnetic nanoparticles transport across a human lung microvascular endothelial cell model of the blood–brain barrier. Nanoscale Adv. 1, 671–685 (2019).
Nosrati, H. et al. Glutathione (GSH) peptide conjugated magnetic nanoparticles as blood–brain barrier shuttle for MRI-monitored brain delivery of paclitaxel. ACS Biomater. Sci. Eng. 5, 1677–1685 (2019).
Zhang, Y. et al. Glioblastoma therapy using codelivery of cisplatin and glutathione peroxidase targeting siRNA from iron oxide nanoparticles. ACS Appl. Mater. Interfaces 12, 43408–43421 (2020).
Zhong, Y. et al. Low-intensity focused ultrasound-responsive phase-transitional nanoparticles for thrombolysis without vascular damage: a synergistic nonpharmaceutical strategy. ACS Nano 13, 3387–3403 (2019).
Xu, H.-L. et al. Glioma-targeted delivery of a theranostic liposome integrated with quantum dots, superparamagnetic iron oxide, and cilengitide for dual-imaging guiding cancer surgery. Adv. Healthc. Mater. 7, 1701130 (2018).
Liu, F. et al. Vessel-targeting nanoclovers enable noninvasive delivery of magnetic hyperthermia–chemotherapy combination for brain cancer treatment. Nano Lett. 21, 8111–8118 (2021).
Sukumar, U. K. et al. Intranasal delivery of targeted polyfunctional gold–iron oxide nanoparticles loaded with therapeutic microRNAs for combined theranostic multimodality imaging and presensitization of glioblastoma to temozolomide. Biomaterials 218, 119342 (2019).
Manzano, M. & Vallet-Regí, M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 30, 1902634 (2020).
Kankala, R. K. et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 32, 1907035 (2020).
Mo, J., He, L., Ma, B. & Chen, T. Tailoring particle size of mesoporous silica nanosystem to antagonize glioblastoma and overcome blood–brain barrier. ACS Appl. Mater. Interfaces 8, 6811–6825 (2016).
Díaz-García, D. et al. Design of mesoporous silica nanoparticles for the treatment of amyotrophic lateral sclerosis (ALS) with a therapeutic cocktail based on leptin and pioglitazone. ACS Biomater. Sci. Eng. 8, 4838–4849 (2022).
Kuang, J. et al. iRGD modified chemo-immunotherapeutic banoparticles for enhanced ommunotherapy against glioblastoma. Adv. Funct. Mater. 28, 1800025 (2018).
Janjua, T. I. et al. Facile synthesis of lactoferrin conjugated ultra small large pore silica nanoparticles for the treatment of glioblastoma. Nanoscale 13, 16909–16922 (2021).
Sun, A. et al. Protective effect of lipoic acid modification on brain dysfunctions of mice induced by mesoporous silica nanoparticles. Chem. Eng. J. 415, 128957 (2021).
Zhu, J. et al. Angiopep-2 modified lipid-coated mesoporous silica nanoparticles for glioma targeting therapy overcoming BBB. Biochem. Biophys. Res. Commun. 534, 902–907 (2021).
Singh, A. K. et al. Lipid-coated MCM-41 mesoporous silica nanoparticles loaded with berberine improved inhibition of acetylcholine esterase and amyloid formation. ACS Biomater. Sci. Eng. 7, 3737–3753 (2021).
Wu, M. et al. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 9, 4777 (2018).
Liu, N. et al. Dual-targeted magnetic mesoporous silica nanoparticles reduce brain amyloid-β burden via depolymerization and intestinal metabolism. Theranostics 12, 6646–6664 (2022).
Turan, O. et al. Treatment of glioblastoma using multicomponent silica nanoparticles. Adv. Ther. 2, 1900118 (2019).
Liu, J., Liew, S. S., Wang, J. & Pu, K. Bioinspired and biomimetic delivery platforms for cancer vaccines. Adv. Mater. 34, 2103790 (2022).
Chang, M. et al. Nanobiomimetic medicine. Adv. Funct. Mater. 32, 2204791 (2022).
Chen, L. et al. Recent progress in targeted delivery vectors based on biomimetic nanoparticles. Signal Transduct. Target. Ther. 6, 225 (2021).
Hu, C. M. J. et al. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108, 10980–10985 (2011).
Zhang, Y., Sun, T. & Jiang, C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B 8, 34–50 (2018).
Tran, P. H. L. et al. Exosomes and nanoengineering: a match made for orecision therapeutics. Adv. Mater. 32, 1904040 (2020).
Edwardson, T. G. W. & Hilvert, D. Virus-inspired function in engineered protein cages. J. Am. Chem. Soc. 141, 9432–9443 (2019).
Herrmann, I. K., Wood, M. J. A. & Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 16, 748–759 (2021).
Li, Z. et al. Chemically and biologically engineered bacteria-based delivery systems for emerging diagnosis and advanced therapy. Adv. Mater. 33, 2102580 (2021).
Gregory, J. V. et al. Systemic brain tumor delivery of synthetic protein nanoparticles for glioblastoma therapy. Nat. Commun. 11, 5687 (2020).
Galstyan, A. et al. Blood–brain barrier permeable nano immunoconjugates induce local immune responses for glioma therapy. Nat. Commun. 10, 3850 (2019).
Yang, T. et al. Delivery of small interfering RNA to inhibit vascular endothelial growth factor in zebrafish using natural brain endothelia cell-secreted exosome nanovesicles for the treatment of brain cancer. AAPS J. 19, 475–486 (2017).
Wang, J. et al. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials 273, 120784 (2021).
Song, Y. et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics 9, 2910–2923 (2019).
Xue, J. et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 12, 692–700 (2017).
Hou, J. et al. Accessing neuroinflammation sites: monocyte/neutrophil-mediated drug delivery for cerebral ischemia. Sci. Adv. 5, eaau8301 (2019).
Lu, G. et al. Engineered biomimetic nanoparticles achieve targeted delivery and efficient metabolism-based synergistic therapy against glioblastoma. Nat. Commun. 13, 4214 (2022).
Jia, Y. et al. Phototheranostics: active targeting of orthotopic glioma using biomimetic oroteolipid nanoparticles. ACS Nano 13, 386–398 (2019).
Geng, X. et al. Active-targeting NIR-II phototheranostics in multiple tumor models using platelet-camouflaged nanoprobes. ACS Appl. Mater. Interfaces 12, 55624–55637 (2020).
Ye, Z. et al. Methotrexate-loaded extracellular vesicles functionalized with therapeutic and targeted peptides for the treatment of glioblastoma multiforme. ACS Appl. Mater. Interfaces 10, 12341–12350 (2018).
Ge, X., Xia, Z. & Guo, S. Recent advances on black phosphorus for biomedicine and biosensing. Adv. Funct. Mater. 29, 1900318 (2019).
Xiong, S. et al. Brain-targeted delivery shuttled by black phosphorus nanostructure to treat Parkinson’s disease. Biomaterials 260, 120339 (2020).
Yang, S. et al. The colossal role of H-MnO2-PEG in ischemic stroke. Nanomed. -Nanotechnol. 33, 102362 (2021).
Gao, Y. et al. NIR-assisted MgO-based polydopamine nanoparticles for targeted treatment of Parkinson’s disease through the blood-brain barrier. Adv. Healthc. Mater. 11, e2201655 (2022).
Li, M. et al. Synthesis of double interfering biodegradable nano-MgO micelle composites and their effect on Parkinson’s disease. ACS Biomater. Sci. Eng. 7, 1216–1229 (2021).
Feng, L. et al. TiO2-nanowired delivery of DL-3-n-butylphthalide (DL-NBP) attenuates blood-brain barrier disruption, brain edema formation, and neuronal damages following concussive head injury. Mol. Neurobiol. 55, 350–358 (2018).
Zou, Y. et al. Blood-brain barrier–penetrating single CRISPR-Cas9 nanocapsules for effective and safe glioblastoma gene therapy. Sci. Adv. 8, eabm8011 (2022).
Lerman, M. J., Lembong, J., Gillen, G. & Fisher, J. P. 3D printing in cell culture systems and medical applications. Appl. Phys. Rev. 5, 041109 (2018).
Jagtiani, E., Yeolekar, M., Naik, S. & Patravale, V. In vitro blood brain barrier models: an overview. J. Control. Release 343, 13–30 (2022).
Wu, J., Wang, C., Sun, J. & Xue, Y. Neurotoxicity of silica nanoparticles: brain localization and dopaminergic neurons damage pathways. ACS Nano 5, 4476–4489 (2011).
Arami, H., Khandhar, A., Liggitt, D. & Krishnan, K. M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 44, 8576–8607 (2015).
Wang, X., Zhong, X., Li, J., Liu, Z. & Cheng, L. Inorganic nanomaterials with rapid clearance for biomedical applications. Chem. Soc. Rev. 50, 8669–8742 (2021).
Boehnke, N. et al. Massively parallel pooled screening reveals genomic determinants of nanoparticle delivery. Science 377, eabm5551 (2022).
Zhu, Y. et al. Multifunctional ginsenoside Rg3-based liposomes for glioma targeting therapy. J. Control. Release 330, 641–657 (2021).
dos Santos Rodrigues, B., Oue, H., Banerjee, A., Kanekiyo, T. & Singh, J. Dual functionalized liposome-mediated gene delivery across triple co-culture blood brain barrier model and specific in vivo neuronal transfection. J. Control. Release 286, 264–278 (2018).
Xu, S. et al. Modulating autophagic flux via ROS-responsive targeted micelles to restore neuronal proteostasis in Alzheimer’s disease. Bioact. Mater. 11, 300–316 (2022).
Yang, P. et al. Neuronal mitochondria-targeted micelles relieving oxidative stress for delayed progression of Alzheimer’s disease. Biomaterials 238, 119844 (2020).
Chen, T. et al. Small-sized mPEG–PLGA nanoparticles of schisantherin A with sustained release for enhanced brain uptake and anti-Parkinsonian activity. ACS Appl. Mater. Interfaces 9, 9516–9527 (2017).
Song, E. et al. Surface chemistry governs cellular tropism of nanoparticles in the brain. Nat. Commun. 8, 15322 (2017).
Sánchez-López, E. et al. Memantine loaded PLGA PEGylated nanoparticles for Alzheimer’s disease: in vitro and in vivo characterization. J. Nanobiotechnol. 16, 32 (2018).
Temizyürek, A. et al. Blood-brain barrier targeted delivery of lacosamide-conjugated gold nanoparticles: Improving outcomes in absence seizures. Epilepsy Res. 184, 106939 (2022).
Yi, Y. et al. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J. Control. Release 295, 268–277 (2019).
Coluccia, D. et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine 14, 1137–1148 (2018).
Guo, Q., Shen, X.-T., Li, Y.-Y. & Xu, S.-Q. Carbon nanotubes-based drug delivery to cancer and brain. Curr. Med. Sci. 37, 635–641 (2017).
Ren, J. et al. The targeted delivery of anticancer drugs to brain glioma by PEGylated oxidized multi-walled carbon nanotubes modified with angiopep-2. Biomaterials 33, 3324–3333 (2012).
Hsieh, T.-Y. et al. Neurotensin-conjugated reduced graphene oxide with multi-stage near-infrared-triggered synergic targeted neuron gene transfection In vitro and in vivo for neurodegenerative disease therapy. Adv. Healthc. Mater. 5, 3016–3026 (2016).
Bielecki, P. A. et al. Immunostimulatory silica nanoparticle boosts innate immunity in brain tumors. Nanoscale Horiz. 6, 156–167 (2021).
Niu, W. et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 21, 1484–1492 (2021).
He, W. et al. Preferential targeting cerebral ischemic lesions with cancer cell-inspired nanovehicle for ischemic stroke treatment. Nano Lett. 21, 3033–3043 (2021).
Wang, C. et al. Camouflaging nanoparticles with brain metastatic tumor cell membranes: a new strategy to traverse blood–brain barrier for imaging and therapy of brain tumors. Adv. Funct. Mater. 30, 1909369 (2020).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
El-Andaloussi, S. et al. Exosome-mediated delivery of siRNA in vitro and in vivo. Nat. Protoc. 7, 2112–2126 (2012).
Yang, C. et al. Glioma-derived exosomes hijack the blood-brain barrier to facilitate nanocapsule delivery via LCN2. J. Control. Release 345, 537–548 (2022).
Xu, F. et al. Engineered extracellular vesicles with SHP2 high expression promote mitophagy for Alzheimer’s disease treatment. Adv. Mater. 34, e2207107 (2022).
Kojima, R. et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 9, 1305 (2018).
Yuan, D. et al. Macrophage exosomes as natural nanocarriers for protein delivery to inflamed brain. Biomaterials 142, 1–12 (2017).
Tian, T. et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150, 137–149 (2018).
Cheng, G. et al. “Swiss Army Knife” black phosphorus-based nanodelivery platform for synergistic antiparkinsonian therapy via remodeling the brain microenvironment. J. Control. Release 353, 752–766 (2023).
Li, Z. et al. Neodymium (3+)-coordinated black phosphorus quantum dots with retrievable NIR/X-Ray pptoelectronic switching effect for anti-glioblastoma. Small 18, e2105160 (2022).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82273861, 82003666, 82022071), Natural Science Foundation of Zhejiang Province (LD22H310003, LQ23H300001), and Young Elite Scientists Sponsorship Program by CAST (YESS20220139). Schematic illustrations in Figs. 1, 2a, 3a, 4a, and 5a are created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Y.W., Z.C., and D.W. conceived this review. D.W. and Q.C. wrote the manuscript. Y.W. revised the manuscript, and other authors discussed and commented on the review. All authors have read and approved the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, D., Chen, Q., Chen, X. et al. The blood–brain barrier: structure, regulation, and drug delivery. Sig Transduct Target Ther 8, 217 (2023). https://doi.org/10.1038/s41392-023-01481-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41392-023-01481-w
- Springer Nature Limited
This article is cited by
-
Dual-targeted delivery of temozolomide by multi-responsive nanoplatform via tumor microenvironment modulation for overcoming drug resistance to treat glioblastoma
Journal of Nanobiotechnology (2024)
-
Tau- and α-synuclein-targeted gold nanoparticles: applications, opportunities, and future outlooks in the diagnosis and therapy of neurodegenerative diseases
Journal of Nanobiotechnology (2024)
-
Anticancer platinum-drug delivered by mesenchymal stromal cells improves its activity on glioblastoma
Cancer Nanotechnology (2024)
-
Cocaine regulates antiretroviral therapy CNS access through pregnane-x receptor-mediated drug transporter and metabolizing enzyme modulation at the blood brain barrier
Fluids and Barriers of the CNS (2024)
-
Molecular insights of exercise therapy in disease prevention and treatment
Signal Transduction and Targeted Therapy (2024)