Abstract
Cerebral ischemia, or stroke, is widespread leading cause of death and disability. Surgical and pharmacological interventions that recover blood flow are the most effective treatment strategies for stroke patients. However, restoring the blood supply is accompanied by severe reperfusion injury, with edema and astrocyte end-feet disruption. Here, we report that the oral administration of CU06-1004 (previously Sac-1004), immediately after onset of ischemia/reperfusion (I/R), ameliorated cerebral damage. CU06-1004 stabilized blood‑brain barrier by inhibiting the disruption of the tight junction-related protein zona occludens-1 and the cortical actin ring in endothelial cells (ECs) after I/R. Interestingly, CU06-1004 significantly suppressed astrocyte end-feet swelling following I/R, by reducing aquaporin 4 and connexin 43 levels, which mediates swelling. Furthermore, the degradation of β1-integrin and β-dystroglycan, which anchors to the cortical actin ring in ECs, was inhibited by CU06-1004 administration after I/R. Consistently, CU06-1004 administration following I/R also suppressed the loss of laminin and collagen type IV, which bind to the cortical actin ring anchoring proteins. Unlike the protective effects of CU06-1004 in ECs, astrocyte viability and proliferation were not directly affected. Taken together, our observations suggest that CU06-1004 inhibits I/R-induced cerebral edema and astrocyte end-feet swelling by maintaining EC junction stability.
Key messages
• CU06-1004 ameliorates I/R-induced cerebral injury.
• EC junction integrity was stabilized by CU06-1004 treatment after I/R.
• CU06-1004 reduces astrocyte end-feet swelling following I/R.
• EC junction stability affects astrocyte end-feet structure maintenance after I/R.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemia, or stroke, has become a leading cause of death and disability, worldwide [1]. Ischemia/reperfusion (I/R) injury is a feature of stroke that occurs when the blood supply is restored after ischemia. Reperfusion can be achieved either through thrombolysis using thrombolytic reagents or the surgical removal of thrombi [2]. Despite known beneficial effects, reperfusion causes detrimental effects, including edema, blood‑brain barrier (BBB) breakdown, and astrocyte end-feet swelling in stroke patients [2,3,4]. However, the underlying mechanisms associated with reperfusion damage remain poorly understood. Therefore, methods to protect against stroke need to be developed to inhibit reperfusion-induced damage.
The dynamic activities of nervous tissue are regulated by blood flow in the brain, which is restricted by the BBB, whose structural components include endothelial cells (ECs), tight junctions, pericytes, and astrocyte end-feet [5]. ECs are connected by tight junction proteins, especially occludin, claudin-5, and zona occludense-1 (ZO-1), and are important for BBB permeability. Whereas, occludin and claudin-5 are transmembrane tight junction components, ZO-1 connects the tight junction to actin filaments [6]. The cortical actin ring is composed of cross-linked actin filaments that stabilize EC junctions [7]. Because EC junctions are disrupted during I/R, preventing the destruction of the cortical actin ring may be a promising therapeutic strategy for ameliorating I/R injury. Although ECs form the vessel walls, astrocyte end-feet closely surround capillaries through the vascular basement membrane, which is composed of extracellular matrix. Astrocyte adhesion regulates the quality of the BBB and can be disrupted by pathological conditions, such as stroke and neurological disorders [5]. Accordingly, investigations into stroke have further extended to include not only ECs but also astrocytes. Following I/R, astrocyte end-feet swelling is one of the earliest responses. Water channels and gap junctions have been reported to play important roles in the mediation of astrocyte end-feet swelling [3]. Aquaporins (AQPs) are bidirectional water channels that allow water diffusion, and AQP4 specifically localizes to astrocyte end-feet. Due to specialized features of end-feet, including a high density of orthogonal particle arrays containing AQP4, decreased polarity and increased expression of AQP4 cause edema following stroke [8]. Gap junctions consist of connexin (CX) proteins and allow the passage of molecules. ECs express CX37, 40, and 43, whereas, astrocytes express CX26, 30, and 43 [9]. Among them, CX43 is thought to be involved in the communication between ECs and astrocytes [10]. CX43 dysfunction leads to neuroinflammation, edema, and BBB disruption [9, 11]. However, the correlation between EC junction disruptions and astrocyte end-feet swelling following I/R remains unknown.
In previous studies, CU06-1004 (previously Sac-1004) enhanced the endothelial barrier, increasing cortical actin ring formation, through cAMP/Rac/cortical actin pathways, which prevented vascular endothelial growth factor-induced actin filament destabilization and reduced I/R-induced BBB breakdown in a rat I/R model [12, 13]. Because the mechanism of astrocyte end-feet disruption after I/R injury and whether the breakdown of EC junctions is closely involved in astrocyte end-feet disruption both remain elusive, here, we investigated the effects of CU06-1004 on edema and astrocyte end-feet swelling during I/R injury. Our data suggest that the EC junction stability is significantly correlated with the maintenance of proper astrocyte end-feet structure after cerebral I/R injury.
Materials and methods
Oral administration of CU06-1004
CU06-1004 was synthesized as previously described [12]. Briefly, CU06-1004 was synthesized via tetrahydropyran deprotection and subsequent glycosidation with 4,6-di-O-acetyl-2,3-didieoxyhex-2-enopyran, in the presence of an acid. A working solution of CU06-1004 was prepared in olive oil (O1514; MilliporeSigma, Burlington, MA, USA). Mice were fasted for 4 h before performing ischemia/reperfusion (I/R). Mice awakened from anesthesia 20 min after reperfusion and were then orally administered a single dose of 10 mg/kg CU06-1004 (CU06-1004-I/R group), in a volume of 10 mL/kg. Sham-operated (sham group) and vehicle-treated mice (vehicle-I/R group) were administered the same volume of suspension solution (olive oil, 10 mL/kg).
Brain ischemia/reperfusion (I/R)
Mice were anesthetized by the intraperitoneal injection of tribromoethanol (Avertin, 2.5%). I/R was induced by middle cerebral artery (MCA) occlusion on the left side as previously described [13]. Briefly, the common carotid artery (CCA) was ligated at the distal side of its branching point. The external carotid artery (ECA) was dissected free and ligated at the distal side of the CCA branching point. A 6-0 monofilament suture (602156PK10; Doccol Corp, Sharon, MA, USA) was inserted into the ECA stump and gently advanced 8 mm into the internal carotid artery (ICA). The suture proceeded from the ICA to the MCA. After 1.5 h of ischemia, reperfusion was induced by the withdrawal of the suture. In the sham group, the same surgical procedures were performed without insertion of the suture.
Oxygen-glucose deprivation/reoxygenation (OGD/R)
Human brain microvascular endothelial cells were purchased from Applied Cell Biology Research Institute (Kirkland, WA, USA). OGD/R was performed as previously described [14]. Confluent cells were washed and replaced with glucose-free Dulbecco’s Modified Eagle’s Medium (DMEM). Cells were then incubated with AnaeroPack (AN0025A; Thermo Fisher Scientific) for 6 h. After OGD, cells were reperfused by placing them in endothelial basal medium 2 (CC-3156; Lonza, Walkersville, MD, USA) containing vehicle or CU06-1004 (10 μg/mL) and were incubated in a 95% air/5% CO2 for 16 h. After 16 h, immunofluorescence staining was performed.
Analysis of astrocyte end-feet diameter
Astrocyte end-feet diameter was measured as previously described [15]. After immunostaining of brain slices, the 3-dimensional images were captured using multiple XYZ imaging stacks, orthogonal to the plane. These images were imported into Image J, and the diameter was measured using ruler of X and Y axes.
Isolation of mouse astrocytes
Astrocytes were isolated from four postnatal mice (1–2 days old) as previously described [16,17,18]. Gray cortices were minced, digested with 0.25% trypsin, and centrifuged. Pellets were resuspended in DMEM (HyClone, SH3024301; GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) supplemented with 10% fetal bovine serum (HyClone, SH3008403), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were seeded in T75 culture flasks pre-coated with 20 mg/mL poly-D-lysine. Culture media were changed every 2 days. After achieving confluency around 7–9 days in vitro, microglia were discarded from mixed glial cells via shaking on an orbital shaker at 180 rpm for 30 min. Cells were then washed and separated from oligodendrocyte precursor cells by shaking at 240 rpm for 6 h. The remaining adherent cells were > 98% positive for the astrocytic marker glial fibrillary acidic protein (GFAP).
Animal studies
C57BL6/J male mice (8–10 weeks old) were purchased from RaonBio (Yongin, Korea). Five mice were used for each group. All mice were housed under controlled conditions (24 ± 1 °C, 12 h light/dark cycles, 55% humidity, and specific-pathogen-free) and provided with food and water ad libitum. All animal facilities and experiments were performed in accordance with the Korean Food and Drug Administration guidelines. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Yonsei University (permit number IACUC-A-201904-892-02).
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM). All statistical analyses were performed using Prism 5.0 (GraphPad Software; La Jolla, CA, USA). Data were statistically analyzed by one-way analysis of variance, with post-hoc Bonferroni’s multiple comparison tests, to determine ischemia-related differences among the experimental groups. To identify whether we had sufficient subjects to detect with statistics, a post-hoc power analysis was performed after the study using G-Power 3.1 software (https://download.cnet.com/s/g-power). Values of P < 0.05 were considered to be significant.
Results
CU06-1004 attenuates brain damage after I/R
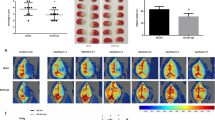
A previous study evaluated the effects of the intravenous injection of CU06-1004 after I/R-induced brain damage [13]. To identify whether similar effects could be observed through other routes, we examined the oral administration of CU06-1004. Infarct volume was examined using 2,3,5-triphenyltetrazolium chloride staining. In the vehicle-I/R group, infarcted regions were observed compared with the sham group. However, in the CU06-1004-I/R group, fewer infarcted regions were observed compared with the vehicle-I/R group (Fig. 1a, b). The effects of CU06-1004 on blood‑brain barrier (BBB) permeability were evaluated with Evans blue (EB) extravasation. EB extravasation was increased in the vehicle-I/R group compared with the sham group. However, CU06-1004 significantly decreased this effect (Fig. 1c−e). Next, we measured neurological deficits. In the vehicle-I/R group, severe neurological deficits were exhibited compared with the sham group. However, CU06-1004 prevented neurological impairments induced by I/R (Fig. 1f). Brain edema was measured by the wet-dry method. In the vehicle-I/R group, brain water content was increased compared with the sham group. In the CU06-1004-I/R group, brain water content was reduced compared with the vehicle-I/R group (Fig. 1g, h). Taken together, these results indicate that the oral administration of CU06-1004 protects against I/R-induced cerebral injury.
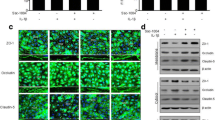
CU06-1004 attenuates brain injury and the disruption of EC junctions after I/R.
Comparison of infarction volumes assessed by TTC staining in the sham, vehicle-I/R, and CU06-1004-I/R groups (a) and quantified (b). Evans blue extravasation from sham, vehicle-I/R, and CU06-1004-I/R groups (c) and the quantification of Evans blue in ipsilateral (d) and contralateral hemispheres (e). Neurological scores for sham, vehicle-I/R, and CU06-1004-I/R groups (f). Brain water content for sham, vehicle-I/R, and CU06–1004-I/R groups. Quantification of water content in the ipsilateral (g) and contralateral hemispheres (h). n = 5 per group. HBMECs were oxygen-glucose deprived for 6 h (i). After reoxygenation and treatment with vehicle or CU06-1004 (10 μg/mL) for 16 h, cells were stained with DAPI, ZO-1, and F-actin. Scale bars, 20 μm. *P < 0.05, **P < 0.01, and ***P < 0.001. Error bars indicate mean ± SEM. ns, not significant; Con, control; OGD/R, oxygen-glucose deprivation/reperfusion; DAPI, 4′,6-diamidino-2-phenylindole; ZO-1, zona occludens-1; F-actin, fibrous actin; 1004, CU06-1004
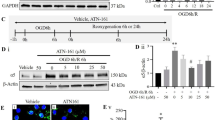
CU06-1004 inhibits EC junction disruption and enhances EC viability after oxygen-glucose deprivation/reoxygenation (OGD/R)
A prior study showed that the treatment of ECs with CU06-1004 inhibited cortical actin ring disruption following vascular endothelial growth factor stimulation [12]. To clarify the role of CU06-1004 in cortical actin ring maintenance, we performed OGD/R as an in vitro I/R model. Vehicle-treated human brain microvascular endothelial cells (HBMECs) showed the breakage of ZO-1 that connects tight junction-related proteins to actin filaments following OGD/R. However, CU06-1004 restored ZO-1, similar to the control group. Furthermore, the induction of stress fiber formation and decreased cortical actin ring formation, which were observed in the vehicle-treated group, were reversed by CU06-1004 treatment (Fig. 1i). HBMEC viability was also suppressed after OGD/R; however, CU06-1004 prevented this effect (Fig. S1a, b). These results demonstrate that CU06-1004 exerts protective effects in ECs by stabilizing tight junctions and the cortical actin ring after OGD/R.
CU06-1004 suppresses astrocyte end-feet swelling and astrocyte activation after I/R
Previous evidence has shown that I/R-induced vascular junction disruption was inhibited by CU06-1004 [13]. Because pericyte attachment is also involved in vessel integrity stabilization, we examined pericyte coverage after I/R. Brains from the vehicle-I/R group showed reduced α-smooth muscle actin (α-SMA), pericyte marker, compared with the sham group. A superimposed lining of α-SMA and cluster of differentiation 31 (CD31), an EC marker, was observed in the CU06-1004-I/R group (Fig. S2a−e), indicating that CU06-1004 significantly blocks the disruption of the BBB structure after I/R injury.
Cerebral edema is one of the earliest responses to I/R due to astrocyte end-feet swelling [3]. End-feet swelling compresses vessels in the ischemic region, resulting in vascular hypoperfusion [19]. We determined end-feet swelling by measuring end-feet diameter in GFAP-labeled cells. Because astrocyte activation is induced by brain injury [3], GFAP levels were also measured. In the vehicle-I/R group, astrocyte abnormalities were evident with increased astrocyte numbers and diameters surrounding ECs. Astrocyte end-feet were swollen, losing fine contact with ECs. These astrocytic changes were significantly reversed by CU06-1004 (Fig. 2a−c).
CU06-1004 inhibits astrocyte end-feet swelling and activation after I/R
a Comparisons of astrocyte changes among the sham, vehicle-I/R, and CU06–1004-I/R groups. Square, enlarged image of the region. Tissues were immunostained for GFAP, as an astrocyte marker, and CD31, as an EC marker. b Quantification of astrocyte end-feet diameter and c GFAP expression levels. n = 5 per group. **P < 0.01 and ***P < 0.001. Scale bars, 20 μm. Error bars indicate mean ± SEM. ns, not significant; DAPI, 4′,6-diamidino-2-phenylindole; GFAP, glial fibrillary acidic protein; CD31, cluster of differentiation 31; 1004, CU06-1004
To further investigate whether CU06-1004 directly affects astrocyte properties, we examined the viability and proliferation of primary astrocytes after CU06-1004 treatment. Isolated primary mouse astrocytes were immunostained for GFAP and CD11b (microglial marker) to confirm cellular identities before experimentation (Fig. S3a). Unlike the protective effects of CU06-1004 in ECs, CU06-1004 did not affect astrocyte properties with or without OGD/R (Fig. S3b−i). Collectively, these results suggest that CU06-1004 likely alleviates astrocyte end-feet destruction by stabilizing the vascular structure after I/R.
CU06-1004 reduces increased aquaporin 4 (AQP4) levels after I/R
AQP4 is highly expressed on astrocyte end-feet and regulates water transport between blood and brain tissue [3]. AQP4 is up-regulated by cerebral ischemic injury, especially in astrocyte end-feet adjacent to blood vessels [20, 21]. In the sham group, AQP4 was mainly expressed in astrocyte end-feet. In the vehicle-I/R group, AQP4 was not only localized to astrocyte end-feet but also distributed along astrocytic processes. CU06-1004 administration resulted in AQP4 distribution similar to that in the sham group (Fig. 3a, b). Similar results were also observed in ECs (Fig. S4a, b). We further confirmed AQP4 expression by western blotting. In the ipsilateral hemispheres of the vehicle-I/R group, AQP4 levels were increased compared with those in both the sham group and the corresponding contralateral hemispheres. CU06-1004 significantly reduced AQP4 expression to the sham group in the ipsilateral hemispheres (Fig. 3c−e).
CU06-1004 reduces increased AQP4 expression after I/R. a Comparison of AQP4 expression in the sham, vehicle-I/R, and CU06-1004-I/R groups. Square: enlarged image of the region. Arrows show the presence of AQP4 on astrocytes. b Quantification of AQP4-positive astrocytes. n = 5 per group; scale bars:, 20 μm. c Effects of CU06-1004 administration on AQP4 expression in brain lysates. d, e Quantification of blots using ImageJ software. n = 3 independent experiments. f Effects of CU06-1004 administration on EAAT1/2 expression in brain lysates. g−j Quantification of blots using ImageJ software. n = 3 independent experiments. ***P < 0.001. Error bars indicate mean ± SEM. ns, not significant; DAPI, 4′,6-diamidino-2-phenylindole; AQP4, aquaporin 4; GFAP, glial fibrillary acidic protein; I, ipsilateral hemisphere; C, contralateral hemisphere; 1004, CU06-1004
Astrocyte end-feet swelling can be caused by the increased uptake of excitatory amino acid neurotransmitters, such as glutamate. Glutamate is cleared by excitatory amino acid transporters 1/2 (EAAT1/2) in astrocyte end-feet surrounding neurons [22]. Reduced EAAT1/2 expressions have been reported in cerebral ischemia [23, 24]. In the vehicle-I/R group, ipsilateral hemispheres showed greatly reduced EAAT1/2 expression as previously reported. However, CU06-1004 failed to increase the EAAT1/2 expression (Fig. 3f−j). Taken together, these observations suggest that the effect of CU06-1004 on astrocyte end-feet swelling is likely due to the regulation of AQP4 but not EAAT1/2 in astrocytes.
CU06-1004 alleviates increased connexin (CX) 43 levels after I/R
Cell adhesion molecules, known as CXs, also mediate astrocyte end-feet swelling. Among the CX family, CX43 is expressed in both ECs and astrocytes [25], and astrocytic CX43 levels are increased after I/R injury [26, 27]. In the vehicle-I/R group, CX43-positive astrocytes and ECs were increased compared with the sham group. These changes were significantly reversed by CU06-1004 (Fig. 4a−d). We further confirmed the inhibitory effects of CU06-1004 on CX43 expression by western blotting (Fig. 4e−g).
CU06-1004 alleviates increased CX43 expression after I/R. Comparison of CX43 expression in the sham, vehicle-I/R, and CU06-1004-I/R groups (a, c). Square, enlarged image of the region. Quantification of CX43-positive astrocytes (b) and blood vessels (d). n = 5 per group; scale bars, 20 μm. Effects of CU06–1004 administration on CX43 expression in brain lysates (e). Quantification of blots using ImageJ software (f, g). n = 3 independent experiments. ***P < 0.001. Error bars indicate mean ± SEM. ns, not significant; DAPI, 4′,6-diamidino-2-phenylindole; CX43, connexin 43; GFAP, glial fibrillary acidic protein; CD31, cluster of differentiation 31; I, ipsilateral hemisphere; C, contralateral hemisphere; 1004, CU06-1004
CU06-1004 inhibits degradation of basal membrane and extracellular matrix (ECM) proteins after I/R
The cortical actin ring binds to basal membrane proteins of EC, such as β1-integrin and β-dystroglycan (β-DG). Basal membrane proteins of EC directly anchor to the ECM. Astrocyte end-feet also anchor to ECs through the ECM [28, 29]. Because laminin (LAM), an ECM component in ECs, is important for the localization and expression of AQP4 at astrocyte end-feet [30], we further analyzed whether CU06-1004 affects the expression of basal membrane and ECM proteins after I/R injury. In the vehicle-I/R group, β1-integrin- and β-DG-positive ECs were decreased compared with the sham group. However, CU06-1004 significantly increased β1-integrin- and β-DG-positive ECs compared with the vehicle-I/R group (Fig. 5a−d). A similar result was observed in astrocytes (Fig. S5a−d). Western blotting further confirmed that CU06-1004 inhibited the I/R-induced degradation of these proteins in the ipsilateral hemispheres (Fig. 5e−i). Similarly, the expressions of LAM and collagen type IV (COLIV), which are major components of the ECM, were decreased in the vehicle-I/R group compared with the sham group. CU06-1004 significantly returned LAM and COLIV expression similar to those in the sham group (Fig. 6a−d). These protein levels in brain tissues were further confirmed by western blotting (Fig. 6e−i). Taken together, our results suggest that CU06-1004 inhibits loss of basal membrane and ECM proteins at the BBB, which could contribute to the rescue of astrocyte end-feet structure after I/R injury.
CU06-1004 prevents the disruption of basal membrane proteins in ECs after I/R. Comparison of β1-integrin (a) and β-DG (c) expression in the sham, vehicle-I/R, and CU06-1004-I/R groups. Square, enlarged image of the region. Quantification of β1-integrin- (b) and β-DG- positive (d) blood vessels. n = 5 per group; scale bars, 20 μm. Effects of CU06–1004 administration on β1-integrin and β-DG expression in brain lysates (e). Quantification of blots using ImageJ software (f−i). n = 3 independent experiments. ***P < 0.001. Error bars indicate mean ± SEM. ns, not significant; DAPI, 4′,6-diamidino-2-phenylindole; DG, dystroglycan; CD31, cluster of differentiation 31; I, ipsilateral hemisphere; C, contralateral hemisphere; 1004, CU06-1004
CU06-1004 inhibits the reduction in extracellular matrix proteins after I/R. Comparison of LAM (a) and COLIV (c) expression in the sham, vehicle-I/R, and CU06-1004-I/R groups. Square, enlarged image of the region. Quantification of LAM- (b) and COLIV-positive (d) blood vessels. n = 5 per group; scale bars, 20 μm. Effects of CU06-1004 administration on LAM and COLIV expression in brain lysates (e). Quantification of blots using ImageJ software (f−i). n = 3 independent experiments. ***P < 0.001. Error bars indicate mean ± SEM. ns, not significant; DAPI, 4′,6-diamidino-2-phenylindole; LAM, laminin; COLIV, collagen type IV; CD31, cluster of differentiation 31; I, ipsilateral hemisphere; C, contralateral hemisphere; 1004, CU06-1004
Discussion
Cerebral ischemia, or stroke, is a common cerebrovascular disease with high morbidity and disability [2]. I/R injury refers to the recovery of blood flow after stroke, which is accompanied by BBB disruptions, oxidative stress injury, and inflammation [31]. BBB disruptions cause edema and hemorrhage, resulting in irreversible brain tissue damage [32]. Thus, the maintenance of BBB integrity may be a key treatment strategy for preventing I/R injury. In previous studies, CU06-1004 was used as a vascular leakage blocker to suppress vascular endothelial growth factor-induced permeability and improved endothelial junctions during I/R injury and tumor angiogenesis [13, 33]. Interestingly, the oral administration of CU06-1004 following I/R significantly suppressed I/R-induced infarct, vascular leakage, behavioral impairment, and astrocyte activation. A single administration of CU06-1004, immediately after onset of reperfusion, inhibited cerebral I/R-induced damage. CU06-1004 stabilizes tight junction-related proteins and the cortical actin ring in ECs [12]. Here, we found that CU06-1004 suppressed OGD/R-induced EC junction disruptions. Therefore, CU06-1004 has a strong capacity to protect ECs from damage and to maintain intact vasculature against I/R injury. In addition to effects on EC junctions, CU06-1004 also preserved intact astrocyte end-feet following I/R. Because CU06-1004 did not directly affect astrocyte viability and proliferation, these effects on astrocyte end-feet maintenance are intriguing. Under normal conditions, astrocyte end-feet closely localize to ECs through interactions between basal membrane and ECM proteins [3]. After I/R injury, the disruption of both EC junctions and astrocytes appeared and was sustained. These abnormalities were reversed by CU06-1004 treatment. Thus, the effects of CU06-1004 on astrocyte end-feet structure are likely mediated by primary effects on EC junctions. Collectively, the direct and indirect effects of CU06-1004 on ECs and astrocytes were able to protect the BBB structure from I/R injury and subsequently reduce edema, astrocyte activation, and inflammation, resulting in the alleviation of neuronal damage. Taken together, these results suggest that the protection of EC junctions is important for the maintenance of astrocyte end-feet and the control of astrocyte activation.
The outermost layer of the BBB is a tightly woven mesh of astrocyte end-feet. Astrocytes are morphologically polarized cells, and nearly every astrocyte extends at least one end-foot that contacts a vessel. Thus, BBB dysfunction is associated with the loss of astrocyte polarity [34]. The end-foot membrane domain is enriched in channels used for water and ion movements [35]. The expression of these channels are increased following stroke, and AQP4, EAAT 1/2, and CX43 have been reported to play important roles in the mediation of astrocyte end-feet swelling [8]. AQP4 is highly expressed on astrocyte end-feet and regulates water transportation. Because AQP4 is normally arranged in a polar manner, the increased mispolarization and expression of AQP4 cause neurological disorders [34]. AQP4 knockout mice show reduced cerebral infarction and edema following ischemia and I/R induction [36, 37]. Increased AQP4 expression following I/R injury was inhibited by CU06-1004, suggesting that CU06-1004 may suppress AQP4-mediated changes in astrocyte volume. Excessive extracellular glutamate enters astrocyte end-feet through EAAT1/2 after neurotransmission. Increased EAAT1/2 expression reduces infarction volume and improves behavior following I/R injury [38, 39]. Vehicle-treated brains showed reduced EAAT1/2 expression; however, CU06-1004 did not alter EAAT1/2 expression following I/R. Because EAATs are mainly expressed in astrocyte end-feet surrounding neurons, CU06-1004 is likely to act on astrocyte end-feet surrounding ECs. Astrocyte end-feet and ECs are interconnected by gap junctions, which allow the non-selective movement of small molecules. Cerebral I/R and OGD/R injuries increase CX43 expression and exacerbate neuroinflammation [40]. CX43 expression was increased after I/R injury, which was inhibited by CU06-1004. Collectively, these results suggest that CU06-1004 inhibits increased AQP4 and CX43 expression without affecting neurotransmission, and this activity contributes to the attenuation of astrocyte end-feet swelling after I/R injury.
The BM is located on the abluminal side of ECs [41]. In the brain, sandwiched between ECs and astrocyte end-feet contributes to BM formation by synthesizing and depositing ECM proteins, such as LAM and COLIV, suggesting that BM participates in BBB maintenance and vascular integrity [42]. ECM proteins can exert these functions by binding receptors, such as integrin and DG, on ECs and astrocytes [43]. According to Sato et al. and Rurak et al., DG knockdown or the impairment of DG binding to LAM causes the delocalization and reduced expression of AQP4 in astrocyte process formations [44, 45]. Lamα2 knockout mice showed increased cerebral edema and reduced BBB integrity and basal membrane protein expression, such as β1-integrin and β-DG [46, 47]. Based on these reports, we postulated that AQP4 and CX43 expression may be modulated by ECM and basal membrane proteins. CU06-1004 suppressed the degradation of β1-integrin, β-DG, LAM, and COLIV following I/R. Collectively, when the cortical actin ring in ECs becomes unstable after I/R, basal membrane proteins that bind to the cortical actin ring become disrupted, resulting in the degradation of ECM proteins that anchor basal membrane proteins. These activities cause instability of junctions between ECs and astrocyte end-feet. AQP4 expression is also increased when the basal membrane proteins in the astrocyte end-feet become degraded [44, 45]. Therefore, when the basal membrane and ECM proteins in ECs become stabilized, the increased AQP4 expression induced by I/R becomes suppressed. In conclusion, stabilizing EC junctions by CU06-1004 can attenuate excessive water movement through AQP4 and contribute to protection of astrocyte-EC interactions. (Fig. S6a, b).
Current treatments for stroke are largely dependent on thrombolytic reagents-mediated thrombolysis. However, this strategy cannot protect against reperfusion-induced injury, which is a critical challenge during stroke treatment [48]. CU06-1004 was previously reported to alleviate reperfusion-induced injury by enhancing EC junctions [13]. Here, we evidently showed that the reperfusion-induced disruptions of EC junctions and astrocyte end-feet were significantly correlated. However, early administrations of CU06-1004 have limitation for treatment of I/R, which may diminish clinical relevance. Therefore, further research is needed to elucidate whether CU06-1004 can be exerted positive effects in clinical conditions, such as long term-induced I/R.
Abbreviations
- α-SMA:
-
α-smooth muscle actin
- AQP:
-
Aquaporin
- BBB:
-
Blood-brain barrier
- BM:
-
Basement membrane
- CD31:
-
Cluster of differentiation 31
- COLIV:
-
Collagen type IV
- CX:
-
Connexin
- DG:
-
Dystroglycan
- EAAT:
-
Excitatory amino acid transporter
- EB:
-
Evans blue
- EC:
-
Endothelial cell
- ECM:
-
Extracellular matrix
- GFAP:
-
Glial fibrillary acidic protein
- HBMEC:
-
Human brain microvascular endothelial cell
- I/R:
-
Ischemia/reperfusion
- LAM:
-
Laminin
- OGD/R:
-
Oxygen-glucose deprivation/reoxygenation
- TTC:
-
2,3,5-triphenyltetrazolium chloride
- ZO-1:
-
Zona occludens-1
References
Strong K, Mathers C, Bonita R (2007) Preventing stroke: saving lives around the world. Lancet Neurol 6:182–187
L L, X W, Z Y (2016) Ischemia-reperfusion injury in the brain: mechanisms and potential therapeutic strategies. Biochem Pharmacol (Los Angel) 5:4
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, Keep RF, Shi Y (2018) Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol 163:144–171
Da Silva-Candal A, Argibay B, Iglesias-Rey R, Vargas Z, Vieites-Prado A, López-Arias E, Rodríguez-Castro E, López-Dequidt I, Rodríguez-Yáñez M, Piñeiro Y et al (2017) Vectorized nanodelivery systems for ischemic stroke: a concept and a need. J Nanobiotechnology 15:30
Islam MM, Mohamed Z (2015) Computational and pharmacological target of neurovascular unit for drug design and delivery. Biomed Res Int 2015:731292–731210
Wallez Y, Huber P (2008) Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim Biophys Acta 1778:794–809
Fels J, Jeggle P, Liashkovich I, Peters W, Oberleithner H (2014) Nanomechanics of vascular endothelium. Cell Tissue Res 355:727–737
Lafrenaye AD, Simard JM (2019) Bursting at the seams: molecular mechanisms mediating astrocyte swelling. Int J Mol Sci 20:e330
Verkhratsky A, Steardo L, Parpura V, Montana V (2016) Translational potential of astrocytes in brain disorders. Prog Neurobiol 144:188–205
Retamal MA, Schalper KA, Shoji KF, Orellana JA, Bennett MV, Sáez JC (2007) Possible involvement of different connexin43 domains in plasma membrane permeabilization induced by ischemia-reperfusion. J Membr Biol 218:49–63
Kim Y, Davidson JO, Green CR, Nicholson LFB, O'Carroll SJ, Zhang J (2018) Connexins and Pannexins in cerebral ischemia. Biochim Biophys Acta Biomembr 1860:224–236
Maharjan S, Kim K, Agrawal V, Choi HJ, Kim NJ, Kim YM, Suh YG, Kwon YG (2013) Sac-1004, a novel vascular leakage blocker, enhances endothelial barrier through the cAMP/Rac/cortactin pathway. Biochem Biophys Res Commun 435:420–427
Zhang H, Park JH, Maharjan S, Park JA, Choi KS, Park H, Jeong Y, Ahn JH, Kim IH, Lee JC, Cho JH, Lee IK, Lee CH, Hwang IK, Kim YM, Suh YG, Won MH, Kwon YG (2017) Sac-1004, a vascular leakage blocker, reduces cerebral ischemia-reperfusion injury by suppressing blood–brain barrier disruption and inflammation. J Neuroinflammation 14:122
Liao LX, Zhao MB, Dong X, Jiang Y, Zeng KW, Tu PF (2016) TDB protects vascular endothelial cells against oxygen-glucose deprivation/reperfusion-induced injury by targeting miR-34a to increase Bcl-2 expression. Sci Rep 6:37959
McConnell ED, Wei HS, Reitz KM, Kang H, Takano T, Vates GE, Nedergaard M (2016) Cerebral microcirculatory failure after subarachnoid hemorrhage is reversed by hyaluronidase. J Cereb Blood Flow Metab 36:1537–1552
Schildge S, Bohrer C, Beck K, Schachtrup C (2013) Isolation and culture of mouse cortical astrocytes. J Vis Exp 71:e50079
Diniz LP, Tortelli V, Matias I, Morgado J, Bérgamo Araujo AP, Melo HM, Seixas da Silva GS, Alves-Leon SV, de Souza JM, Ferreira ST et al (2017) Astrocyte transforming growth factor Beta 1 protects synapses against Aβ oligomers in Alzheimer’s disease model. J Neurosci 37:6797–6809
Sidoryk-Wegrzynowicz M, Gerber YN, Ries M, Sastre M, Tolkovsky AM, Spillantini MG (2017) Astrocytes in mouse models of tauopathies acquire early deficits and lose neurosupportive functions. Acta Neuropathol Commun 5:89
Ito U, Hakamata Y, Kawakami E, Oyanagi K (2010) Temporary focal cerebral ischemia results in swollen astrocytic end-feet that compress microvessels and lead to focal cortical infarction. J Cereb Blood Flow Metab 31:328–338
Cheng ZJ, Dai TM, Shen YY, He JL, Li J, Tu JL (2018) Atorvastatin pretreatment attenuates ischemic brain edema by suppressing aquaporin 4. J Stroke Cerebrovasc Dis 27:3247–3255
Yang D, Li SY, Yeung CM, Chang RC, So KF, Wong D, Lo AC (2012) Lycium barbarum extracts protect the brain from blood-brain barrier disruption and cerebral edema in experimental stroke. PLoS One 7:e33596
Martín A, Domercq M, Matute C (2018) Inflammation in stroke: the role of cholinergic, purinergic, and glutamatergic signaling. Ther Adv Neurol Disord 11:1756286418774267
Gong HY, Zheng F, Zhang C, Chen XY, Liu JJ, Yue XQ (2016) Propofol protects hippocampal neurons from apoptosis in ischemic brain injury by increasing GLT-1 expression and inhibiting the activation of NMDAR via the JNK/Akt signaling pathway. Int J Mol Med 38:943–950
Qian Y, Tang X, Guan T, Li Y, Sun H (2016) Neuroprotection by combined administration with maslinic acid, a natural product from Olea europaea, and MK-801 in the cerebral ischemia model. Molecules 21:e1093
Tanigami H, Okamoto T, Yasue Y, Shimaoka M (2012) Astroglial integrins in the development and regulation of neurovascular units. Pain Res Treat 2012:964652–964610
Chen Y, Wang L, Zhang L, Chen B, Yang L, Li X, Li Y, Yu H (2018) Inhibition of connexin 43 hemichannels alleviates cerebral ischemia/reperfusion injury via the TLR4 signaling pathway. Front Cell Neurosci 12:372
Xing L, Yang T, Cui S, Chen G (2019) Connexin Hemichannels in astrocytes: role in CNS disorders. Front Mol Neurosci 12:23
Tietz S, Engelhardt B (2015) Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol 209:493–506
Roberts J, Kahle MP, Bix GJ (2012) Perlecan and the blood-brain barrier: beneficial proteolysis? Front Pharmacol 3:155
Menezes MJ, McClenahan FK, Leiton CV, Aranmolate A, Shan X, Colognato H (2014) The extracellular matrix protein laminin α2 regulates the maturation and function of the blood-brain barrier. J Neurosci 34:15260–15280
Cheng X, Zhang F, Li J, Wang G (2019) Galuteolin attenuates cerebral ischemia/reperfusion injury in rats via anti-apoptotic, anti-oxidant, and anti-inflammatory mechanisms. Neuropsychiatr Dis Treat 15:2671–2680
Enzmann G, Kargaran S, Engelhardt B (2018) Ischemia-reperfusion injury in stroke: impact of the brain barriers and brain immune privilege on neutrophil function. Ther Adv Neurol Disord 11:1756286418794184
Agrawal V, Maharjan S, Kim K, Kim NJ, Son J, Lee K, Choi HJ, Rho SS, Ahn S, Won MH, Ha SJ, Koh GY, Kim YM, Suh YG, Kwon YG (2014) Direct endothelial junction restoration results in significant tumor vascular normalization and metastasis inhibition in mice. Oncotarget 5:2761–2777
Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53
Stokum JA, Kurland DB, Gerzanich V, Simard JM (2015) Mechanisms of astrocyte-mediated cerebral edema. Neurochem Res 40:317–328
Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS (2000) Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med 6:159–163
Yao X, Derugin N, Manley GT, Verkman AS (2015) Reduced brain edema and infarct volume in aquaporin-4 deficient mice after transient focal cerebral ischemia. Neurosci Lett 584:368–372
Fontana ACK (2015) Current approaches to enhance glutamate transporter function and expression. J Neurochem 134:982–1007
Harvey BK, Airavaara M, Hinzman J, Wires EM, Chiocco MJ, Howard DB, Shen H, Gerhardt G, Hoffer BJ, Wang Y (2011) Targeted over-expression of glutamate transporter 1 (GLT-1) reduces ischemic brain injury in a rat model of stroke. PLoS One 6:e22135
Yin X, Feng L, Ma D, Yin P, Wang X, Hou S, Hao Y, Zhang J, Xin M, Feng J (2018) Roles of astrocytic connexin-43, hemichannels, and gap junctions in oxygen-glucose deprivation/reperfusion injury induced neuroinflammation and the possible regulatory mechanisms of salvianolic acid B and carbenoxolone. J Neuroinflammation 15:97
Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV (2015) Establishment and dysfunction of the blood-brain barrier. Cell 163:1064–1078
Yao Y (2019) Basement membrane and stroke. J Cereb Blood Flow Metab 39:3–19
Baeten KM, Akassoglou K (2011) Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 71:1018–1039
Sato J, Horibe S, Kawauchi S, Sasaki N, Hirata KI, Rikitake Y (2018) Involvement of aquaporin-4 in laminin-enhanced process formation of mouse astrocytes in 2D culture: roles of dystroglycan and α-syntrophin in aquaporin-4 expression. J Neurochem 147:495–513
Rurak J, Noel G, Lui L, Joshi B, Moukhles H (2007) Distribution of potassium ion and water permeable channels at perivascular glia in brain and retina of the large (myd) mouse. J Neurochem 103:1940–1953
Nickolls AR, Bönnemann CG (2018) The roles of dystroglycan in the nervous system: insights from animal models of muscular dystrophy. Dis Model Mech 11:dmm035931
Nirwane A, Yao Y (2019) Laminins and their receptors in the CNS. Biol Rev 94:283–306
Posada-Duque RA, Barreto GE, Cardona-Gomez GP (2014) Protection after stroke: cellular effectors of neurovascular unit integrity. Front Cell Neurosci 8:231
Funding
This research was supported by the Basic Science Research Program from the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (MSIP; NRF-2019R1A2C3007142), and the Bio & Medical Technology Development Program of the NRF funded by the MSIP (NRF-2015M3A9B6066835). This work was also supported by the Brain Korea 21 (BK21) PLUS program. S. Park, Y. Kim, and C. R. Bae are fellowship awardees by BK21 PLUS program.
Author information
Authors and Affiliations
Contributions
D. Y. Kim and Y. G. Kwon designed the research; D.Y. Kim performed research; D. Y. Kim, H. Zhang, and S. Park analyzed the data; D. Y. Kim and Y. G. Kwon wrote the manuscript; H. Zhang, Y. Kim, C. R. Bae, and Y. G. Kwon contributed advice to the development of the manuscript; Y. M. Kim and Y. G. Kwon contributed to reagents and materials.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest, financial, or otherwise.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, D.Y., Zhang, H., Park, S. et al. CU06-1004 (endothelial dysfunction blocker) ameliorates astrocyte end-feet swelling by stabilizing endothelial cell junctions in cerebral ischemia/reperfusion injury. J Mol Med 98, 875–886 (2020). https://doi.org/10.1007/s00109-020-01920-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-020-01920-z