Abstract
Background
The survival of antibody isotypes specific to pertussis toxin (PT) and filamentous hemagglutinin (FHA) from mother’s own milk (MBM) and donor breast milk (DBM) during preterm infant digestion was investigated.
Methods
Feed, gastric, and stool samples were collected from 20 preterm mother–infant pairs at 8–9 days and 21–22 days postpartum. Samples were analyzed via ELISA for anti-FHA or anti-PT immunoglobulin A (IgA), IgM, and IgG.
Results
Anti-PT IgA, anti-FHA IgG, and anti-PT IgG were lower in MBM than DBM at 8–9 days postpartum, whereas anti-FHA IgM was higher in MBM than DBM. Anti-PT IgA, anti-PT IgG, and anti-FHA IgG in DBM decreased in gastric contents at both postpartum times but those antibodies in MBM were stable or increased during gastric digestion. Anti-FHA-specific IgA and IgM were higher in gastric contents from infants fed MBM than from infants fed DBM at 8–9 days. All pertussis antibodies were detected in infant stools at both postpartum times.
Conclusions
Pertussis-specific antibodies from MBM were stable during infant digestion, whereas anti-pertussis IgA and IgG from DBM decreased in gastric contents. The constant region and variable region of antibodies and maternal immunization appear to be the critical factors for their stability to proteolytic digestion and pasteurization.
Impact
-
Pertussis-specific antibodies from mother’s breast milk were stable during infant digestion, whereas anti-pertussis IgA and IgG from donor breast milk decreased in gastric contents.
-
The constant region and variable region of pertussis-specific antibodies and the maternal immunization (previous infections and vaccinations) appear to be the critical factors for their stability to proteolytic digestion and pasteurization.
-
Pertussis-specific antibodies from either mother’s breast milk or donor breast milk survived during preterm infant digestion and both types of milk will compensate for the lower IgG transplacental transfer in preterm infants compared with term infants.
Similar content being viewed by others
Introduction
Infectious diseases are the major cause of morbidity and mortality for infants.1 Human milk provides antibodies that may help prevent pathogen infection. Human milk antibodies need to avoid degradation within the digestive tract to protect the newborn against infection. Few studies have examined the digestion and survival of antibodies within the infant gut.2,3,4,5,6,7 There is a critical need to examine the extent to which milk antibodies specific to pathogens survive and maintain their ability to bind to them and thus allow prevention of infection within the gut. This knowledge will enable greater understanding of the impact of milk antibodies within the infant to prevent infectious diseases.
The Center for Disease Control and Prevention recommends that pregnant women are vaccinated with tetanus-reduced-dose diphtheria and acellular pertussis (Tdap) during the late second or third trimester (27–36 weeks of gestation) to maximize protection of the infant against pertussis illness.8,9 Vaccination during pregnancy increases the maternal production of pertussis-specific immunoglobulin G (IgG), which is transferred across the placenta to the fetus and remains in the infant bloodstream after birth, providing passive protection against pertussis.10 Moreover, vaccination increases the secretion of pertussis-specific antibodies in breast milk, which may also provide passive protection against the pathogen.11 Many animals have transfer of IgG across the intestine as they do not have transplacental transfer,12 whereas humans have transplacental transfer, but their transfer of IgG through the intestine is still unknown. Intestinal absorption of pathogen-specific IgA was demonstrated in three newborns fed with colostrum between 18 and 24 h after birth.13 No study has demonstrated that pertussis-specific antibodies from breast milk can prevent infections in infants.
Pertussis-specific IgA was previously identified in breast milk of postpartum Tdap-vaccinated mothers.11 Anti-filamentous hemagglutinin (anti-FHA)- and anti-pertussis toxin (anti-PT)-specific IgA and anti-FHA IgG levels in breast milk were higher in mothers who were vaccinated with Tdap during pregnancy (23–37 weeks of gestation) than unvaccinated mothers during the first 2 weeks of lactation but not at 4 and 8 weeks of lactation.14 The abundance of pertussis-specific IgA and IgG was the highest in colostrum and declined over time.14 De Schutter et al. demonstrated that anti-PT secretory IgA (SIgA) levels were higher in milk from mothers who received pertussis vaccination during pregnancy or at delivery than in milk from mothers without recent pertussis vaccination.15
Preterm infants receive lower levels of maternal transplacental pertussis-specific IgG than term infants, which increases their risk for pathogen infection in the first 2 months of postnatal age.16 Moreover, very low birth weight infants received their first and second doses of pertussis vaccine 1.3-fold later than normal birth weight infants (6.9 and 9.4 months for preterm versus 5.2 and 7.2 months for term, respectively), which left them at higher risk for pertussis infections until their completed immunization, typically at 24 months.17 Regardless of whether mothers were recently vaccinated, their breast milk typically contains some pertussis-specific Igs as most mothers have been exposed to Bordetella pertussis or been Tdap vaccinated in the past.15 Whether pertussis-specific milk antibodies can be absorbed across the intestine into the blood and protect against B. pertussis remains unknown. Small amounts of milk can reach the infant respiratory tract due to regurgitation and inhalation of breast milk during and after feeding,18 which could contribute to protect against infection in the neonatal respiratory tract. Therefore, milk antibodies may be protective against B. pertussis, but no evidence has yet been provided.
To bind and neutralize B. pertussis throughout the infant gut, pertussis-specific antibodies from human milk must survive the digestive system. No study has investigated the variation of survival for different antibody isotypes specific to pertussis from breast milk during preterm infant digestion.
Preterm infants are often fed with mother’s own milk (MBM) supplemented with donor breast milk (DBM) or only DBM due to limitations in MBM supply.19,20 DBM processing (including pooling milks from different mothers, Holder pasteurization, and several freeze–thaw cycles) may reduce the levels of milk antibodies. Our previous studies demonstrated that total antibody concentrations (SIgA/IgA, SIgM/IgM, and IgG) and antenatal antibodies specific to influenza A levels (IgA, IgM, and IgG) were higher in MBM than in DBM2 and were higher in gastric contents when infants were fed MBM than when infants were fed DBM at 8–9 days of postnatal age.3 Whether MBM and DBM differ in relative abundance of pertussis-specific antibody is unknown. As the relative abundance of milk antibodies specific to pertussis from MBM or DBM during gastric digestion is unknown, and the differences between MBM and DBM have not been evaluated, the relative abundances of milk anti-pertussis (anti-FHA and anti-PT) antibodies were determined in preterm infants fed MBM and DBM.
Methods
Subjects and enrollment
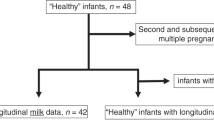
This study was approved by the Institutional Review Boards of the Legacy Health Systems neonatal intensive care unit (NICU) and Oregon State University (1402–2016, first approved on 05/03/17). Written consent to use their milk for research was obtained from those mothers. Samples were collected from 20 premature-delivering mother–infant pairs ranging in gestational age (GA) at birth from 26 to 36 weeks (Supplementary Table S1) in the NICU. Eligibility criteria included having an indwelling nasogastric/orogastric feeding tube, bolus feeding (<60 min infusion tolerated), feeding volumes of at least 4 mL, and mothers who could produce a volume of MBM adequate for one full-volume feed per day. Exclusion criteria included neonates with diagnoses that were incompatible with life, gastrointestinal system anomalies, major gastrointestinal surgery, severe genitourinary anomalies, and significant metabolic or endocrine diseases. Parents of all eligible infants in the NICU were approached for participation after informed consent.
Feeding and sampling
To compare the effects of DBM and MBM, two separate feedings of DBM and MBM without fortification were given to the infants. The schema of the study design is illustrated in Fig. 1. Mothers were able to supply enough milk but agreed to use unfortified DBM (or unfortified MBM) for two of the normal eight daily feeding for this study. At both sample time periods, each infant received two of the normal eight daily feedings as unfortified MBM or DBM on alternate days (randomized order). The order of feeding MBM and DBM was randomized to control for any potential effect of infant day of life on antibody digestion. For the 6 daily feedings, 16 preterm infants were fed fortified MBM (primary) or fortified DBM (if needed). The type of fortifier was a human milk-based fortifier (Prolact 24 calories (Cal) at 8–9 days and Prolact 26 Cal at 21–22 days of postnatal age, Prolacta Bioscience, Duarte, CA) for the extremely/very preterm infants (GA 26–30 weeks, n = 10), whereas that for the moderate/late preterm infants (GA 31–32 weeks, n = 6) was a bovine fortifier (Enfamil 22 Cal or 24 Cal, Mead Johnson Nutrition, Zeeland, MI). Preterm infant with GA 35–36 weeks (n = 4) were fed MBM (primary) or DBM (if needed) without fortification. Milk and gastric samples (2 mL) were collected on 8–9 and 21–22 days postpartum. The pool of DBM was acquired from two batches at Northwest Mother’s Milk Bank. Three-liter batches were pasteurized and frozen in 50-mL doses so that only a small fraction was thawed for each infant feeding. The power analysis based in our previous study indicated that at least 15 infants were required to compare DBM- and MBM-fed infants.20
Preterm infants were fed two separate feedings of mother’s own breast milk (MBM) and donor breast milk (DBM) (randomized) at 8–9 days and 21–22 days of postnatal age. Gastric contents were collected at 30 min after the completion of the feed (separated MBM and DBM) whereas stool samples (mixed MBM and DBM) were collected within 48 h of the gastric sampling at each time point. MBM, DBM in gastric contents and stool samples from preterm infants.
The protocol of feeding is described in our previous study.2 Milk (either MBM or DBM) was fed to the infant via the nasogastric tube with a feeding pump set to deliver the entire bolus over 30–60 min. A sample of the gastric fluid was collected 30 min after the completion of the feed (1–1.5 h after feed initiation). Stool (1 g) was collected within 48 h of the gastric sampling time point and was recovered from the diaper and scraped into a sterile jar. Stool sample collection was not specific to DBM/MBM and thus represents stools deriving from a mixture of DBM and MBM feeding. After collection of each sample type (feed, gastric, and stool), samples were placed immediately on ice and stored at −80 °C in the NICU. Samples were transported on dry ice to the Dallas laboratory at Oregon State University for sample analysis.
Sample preparation and enzyme-linked immunosorbent assays (ELISAs)
Feed (MBM and DBM) and gastric samples were thawed at 4 °C and centrifuged at 3500 × g for 30 min at 4 °C. The infranate was collected, separated into aliquots, and stored at −80 °C. Frozen stool samples (0.1 g) were diluted in 700 μL of phosphate-buffered saline pH 7.4 (Thermo Fisher Scientific, Waltham, MA) with 0.05% Tween-20 (Bio-Rad Laboratories, Irvine, CA) (PBST) and 3% fraction V bovine serum albumin solution (Innovative Research, Novi, MI). Diluted stool samples were mixed by vortex for 2 min, and the vials were centrifuged at 3500 × g for 20 min at 4 °C. The supernatant was collected, separated into aliquots, and stored at −80 °C.
The spectrophotometric ELISAs were recorded with a microplate reader (Spectramax M2, Molecular Devices, Sunnyvale, CA) with two replicates of blanks, standards, and samples. Clear flat-bottom 96-well plates (Thermo Fisher Scientific, Waltham, MA) were coated with 0.75 μg/mL (100 μL) of purified FHA or PT (Enzo Life Sciences, Inc., Farmingdale, NY) from B. pertussis. Plates were incubated overnight at 4 °C. After incubation, plates were washed three times with PBST. Blocking buffer (100 μL PBST with 3% fraction V bovine serum albumin solution) was added in all wells and incubated for 1 h at room temperature. Standards were prepared using human serum from a female adult who received the Tdap vaccine (8 μg of inactivated PT and 8 μg of FHA per 0.5 mL, BOOSTRIX, Supplementary Table S2) 4 months prior. Blood was collected into 6 BD vacutainer serum separation tube (SSTTM) (Becton Dickinson, Franklin Lakes, NJ), incubated at room temperature for 5 min, and then centrifuged at 3900 × g for 5 min. Serum was aliquoted into several vials and stored at −80 °C until used. The relative abundance of pertussis antibodies was derived by interpolation from the standard curve generated from human serum with the assigned quantity of anti-FHA and anti-PT antibodies expressed in ELISA units/mL (EU/mL). The standard curves were prepared using a dilution series of standard human serum in blocking buffer, and the final concentration covered a range from 12.5× (4000 EU/mL) to 102,400× (0.48 EU/mL). Feed (MBM or DBM) and gastric samples were diluted 10× with blocking buffer for anti-FHA- and anti-PT-specific IgA, IgM, and IgG measurements, whereas a 2× dilution was used for the prediluted stool samples. For each step (addition of 100 μL standards/samples and secondary antibodies at 1 μg/mL), washing and incubation for 1 h at room temperature were performed. The detection antibodies were goat anti-human IgA alpha-chain:horseradish peroxidase (HRP) for anti-pertussis IgA, goat anti-human IgM mu-chain:HRP for anti-pertussis IgM, and goat anti-human IgG gamma-chain:HRP for anti-pertussis IgG (Bio-Rad Laboratories). The substrate 3,3’,5,5’-tetramethylbenzidine (Thermo Fisher Scientific) (1×, 100 μL) was added for 5 min at room temperature followed by addition of 2 N sulfuric acid (100 μL) to stop the reaction. Optical density was measured at 450 nm.
Pasteurization effect on antibodies specific to pertussis
To examine the effects of pasteurization directly, a human milk sample was collected from one mother who was vaccinated with the Tdap vaccine (BOOSTRIX) at 25 weeks of pregnancy and gave birth at term. At 12 days postpartum, the mother pumped and collected 150 mL of milk into a sterile plastic bag. Six 1-mL aliquots were centrifuged at 4000 × g for 30 min, 4 °C and infranates were collected (skim). Three of these aliquots were used for the control raw human milk (RHM) and 3 others were pasteurized at 62.5 °C for 30 min, referred to as the pasteurized skimmed human milk (PSHM). Three other 1-mL aliquots were pasteurized whole (referred to as pasteurized whole human milk (PWHM)) and centrifuged at 4000 × g for 30 min at 4 °C to collect skim milk prior to ELISA.
Statistical analyses
Wilcoxon matched-pairs signed-rank test in the GraphPad Prism software (version 8) were used to compare anti-pertussis antibodies (EU/mL) in milk and in gastric contents between MBM and DBM (type of feeding) within the same mother–infant pairs at 8–9 days and 21–22 days postpartum. All tests were nonparametric as some of the values did not pass the D’Agostino and Pearson normality test. Student’s t tests were used to evaluate the effect of antibiotics that infants received or not in gastric and stool samples from both feeding types (MBM and DBM) (Supplementary Table S3). Student’s t tests were used to determine the effect of the first type of feed (MBM or DBM) on anti-pertussis antibodies in the gastric contents (Supplementary Table S4) and to compare anti-pertussis antibody in feeds and gastric contents from preterm infant fed MBM or DBM between 8–9 and 21–22 days postpartum (Supplementary Table S5). One-way analysis of variance followed by Dunnett’s multiple comparisons test were performed to compare RHM to PSHM and PWHM. Differences were designated significant at p < 0.05. The sample size was selected based on our previous study sample sizes3 and proved to be adequately powered based on the results.
Results
Infant demographics
Demographic details for the preterm-delivering mother–infant pairs are presented in Supplementary Table S1.
Anti-pertussis antibodies in feeds
The relative abundance of PT-specific IgA (Fig. 2a, d) were 3- and 12-fold higher in DBM than in MBM at 8–9 and 21–22 days postpartum, respectively, but did not differ for FHA-specific IgA at either time of postnatal age (Fig. 2a, c). FHA-specific IgM was eightfold higher in MBM than in DBM at 8–9 days postpartum but did not differ at 21–22 days (Fig. 3a, c). PT-specific IgM did not differ between MBM and DBM at either postpartum time (Fig. 3b, d). FHA- and PT-specific IgG was twofold and fourfold higher in DBM than in MBM at 8–9 days postpartum (Fig. 4a, b). PT-specific IgG was twofold higher in DBM than in MBM at 21–22 days postpartum but did not differ for anti-FHA (Fig. 4c, d). The GMRA of each anti-pertussis isotype in feeds are summarized in Supplementary Table S6.
a–d Relative abundance of anti-FHA IgA at 8–9 days postpartum, anti-PT IgA at 8–9 days postpartum, anti-FHA IgA at 21–22 days postpartum, and anti-PT IgA at 21–22 days postpartum. Values are means ± SD, n = 20 for 8–9 days, and n = 16 for 21–22 days postpartum. Asterisks show statistically significant differences between variables (***p < 0.001; **p < 0.01; *p < 0.05) using the Wilcoxon matched-pairs signed-rank test. EU ELISA units/mL.
a–d Relative abundance of anti-FHA IgM at 8–9 days postpartum age, anti-PT IgM at 8–9 days postpartum, anti-FHA IgM at 21–22 days postpartum, and anti-PT IgM at 21–22 days postpartum. Values are means ± SD, n = 20 for 8–9 days, and n = 16 for 21–22 days postpartum. Asterisks show statistically significant differences between variables (***p < 0.001; **p < 0.01; *p < 0.05) using the Wilcoxon matched-pairs signed-rank test. EU ELISA units/mL.
a–d Relative abundance of anti-FHA IgM at 8–9 days postpartum, anti-PT IgM at 8–9 days postpartum, anti-FHA IgM at 21–22 days postpartum, and anti-PT IgM at 21–22 days postpartum. Values are means ± SD, n = 20 for 8–9 days, and n = 16 for 21–22 days postpartum. Asterisks show statistically significant differences between variables (***p < 0.001; **p < 0.01; *p < 0.05) using the Wilcoxon matched-pairs signed-rank test. EU ELISA units/mL.
Anti-pertussis antibodies in gastric contents
At 8–9 days of postnatal age, relative abundance of FHA-specific IgA (Fig. 2a) and FHA-specific IgM (Fig. 3a) was fourfold and fivefold, respectively, higher in gastric contents from infants fed MBM than those fed DBM but did not differ at 21–22 days postpartum (Figs. 2c and 3c). PT-specific IgA and IgM did not differ after feeding infants with MBM and those with DBM at either time postnatal (Figs. 2b, d and 3b, d). FHA- and PT-specific IgG in gastric contents did not differ after feeding infants with MBM or DBM at either postpartum times (Fig. 4). The Geometric mean relative abundance (GMRA) of each anti-pertussis isotype in gastric contents are summarized in Supplementary Table S7.
Gastric digestion of anti-pertussis antibodies from MBM and DBM
Relative abundance of FHA-specific IgA decreased 33% from DBM to gastric contents at 8–9 days postpartum but did not differ at 21–22 days. Anti-FHA IgA did not differ from MBM to gastric contents at either postnatal time (Fig. 2). PT-specific IgA decreased from DBM to gastric contents in infants fed DBM at 8–9 and 21–22 days postpartum, whereas it increased from MBM to gastric contents at both postpartum times (Fig. 2). FHA- and PT-specific IgM did not change from MBM or DBM to gastric contents at 8–9 or 21–22 days, except that PT-IgM increased from MBM to gastric contents at 8–9 days (Fig. 3). FHA- and PT-specific IgG decreased from DBM to gastric contents at 8–9 and 21–22 days postpartum but did not differ for MBM at both postpartum times (Fig. 4). p Values for the difference between feed and gastric contents of each anti-pertussis isotype are summarized in Supplementary Table S8.
Anti-pertussis antibodies in preterm infant stools derived from mixed MBM/DBM
All antenatal antibody isotypes present in breast milk were detected in the infant stools. The relative abundance of FHA- and PT-specific IgA, IgM, and IgG in infant stools did not differ between 8–9 and 21–22 days postpartum (Supplementary Table S9).
Effect of pasteurization on human milk anti-pertussis antibodies
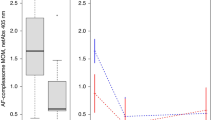
To determine the effect of Holder pasteurization on milk anti-pertussis antibodies, we compared the anti-pertussis antibodies in RHM to PWHM and PSHM. We pasteurized whole and non-fat human milk because previous literature found different results for the antibody concentrations with and without delipidation before pasteurization.21,22 Anti-FHA IgA from RHM decreased 13% in PWHM and 21% in PSHM (Fig. 5). Anti-FHA IgM from RHM decreased 30% in PWHM and 99% in PSHM. Anti-FHA IgG and anti-PT IgG from RHM did not differ in PWHM and PSHM. Anti-PT IgA from RHM increased 72% in PSHM but did not differ in PWHM. Anti-PT IgM from RHM decreased 50% in PWHM and 81% in PSHM.
a–f Reactivity of anti-formaldehyde-treated filamentous hemagglutinin (anti-FHA) IgA, IgM, and IgG and of anti-pertussis toxin (anti-PT) IgA, IgM, and IgG in RHM, PWHM, or PSHM. Milk samples are from one mother who delivered one term infants with 38 weeks of gestational age and 12 days postpartum. Values are means ± SD (n = 3). Asterisks show statistically significant differences between variables (***p < 0.001; **p < 0.01; *p < 0.05).
Discussion
Human milk antibodies specific to pathogens can compensate for the immature immune system in premature infants and reduce the risk of mortality induced by serious infectious diseases. However, in order to function, these antibodies must survive the gastrointestinal conditions. In this study, we examined the survival of pertussis-specific antibodies varying in isotype and protein-binding specificity as a model to better understand the potential of oral antibodies to survive and remain functional across infant digestion. As preterm infants are often fed DBM, this study also aimed to determine whether the relative abundance of antenatal milk anti-pertussis antibodies differed between MBM and DBM and across preterm infant digestion.
Milk anti-pertussis neutralizing antibodies can be specific to FHA, PT, or other pertussis antigens. Anti-FHA- and anti-PT-specific IgA and anti-FHA-specific IgG levels were previously determined in breast milk from women vaccinated with Tdap during pregnancy and non-vaccinated women using ELISA.11,14 The relative abundance of anti-FHA, anti-PT IgA, and anti-FHA IgG measured in MBM cannot be compared between the present study and those measured previously in term-delivering Tdap-vaccinated mothers by Raya et al.,14 Halperin et al.,11 and De Schutter et al.15 because different human serums were used to prepare the standard curve for each anti-pertussis antibody isotype. No commercial standard exists for anti-pertussis IgA, IgM, and IgG; therefore, measuring absolute concentrations was not possible in this study. This investigation is the first to measure the anti-pertussis IgM (anti-FHA and anti-PT) and anti-PT IgG relative abundance in breast milk.
The relative abundance of anti-PT IgA, anti-PT IgG, and anti-FHA IgG were lower in MBM than in DBM at 8–9 and 21–22 days postpartum. Milk anti-PT IgA increased after pasteurization; therefore, we speculate that the higher anti-PT IgA level in DBM might be due to the pasteurization effect, but it is unclear how pasteurization could have increased IgA-binding efficiency (no previous study has demonstrated such phenomenon). Anti-FHA IgG and anti-PT IgG were not affected by pasteurization. The lower abundance of anti-PT IgG and anti-FHA IgG in MBM compared with that in DBM could be due to the differences in vaccination during pregnancy (some mothers may not have received vaccine(s) for pertussis) and differences in maternal immunization (previous infections or vaccinations). Pertussis-specific antibodies did not change with GA in MBM (p > 0.10 for all linear regressions). DBM is generally made with a pooled pasteurized MBM from at least 5–10 different term-delivering mothers. In this study, DBM was from a pooled pasteurized MBM from different mothers, but the number of women was unknown. The vaccination status of the mothers who participated at this study was unknown for both MBM and DBM.
Anti-FHA and anti-PT IgM did not differ between MBM and DBM and decreased during pasteurization. This result could be due to a combination of pasteurization effect and maternal immunization, suggesting that those antibodies were likely higher in DBM than in MBM before pasteurization.
Anti-FHA IgM abundance was higher in MBM than in DBM at 8–9 days but did not differ at 21–22 days postpartum. Owing to the reduction of anti-FHA IgM during pasteurization, its lower abundance in DBM is likely due to a combined effect of pasteurization (higher in DBM pre-pasteurization than post-pasteurization) and postpartum time (higher in MBM at 8–9 than at 21–22 days) (Supplementary Fig. S2). This result is in agreement with our previous study which demonstrated that the concentrations of total IgM were higher in MBM than in DBM due to the pasteurization effect and early postpartum time of MBM.2
For the first time, this investigation reported that anti-PT IgA, anti-PT IgG, and anti-FHA IgG in DBM was reduced during infant gastric digestion at both postpartum times, whereas those pertussis antibodies were increased or stable in MBM. Anti-FHA-specific IgA and IgM were higher in gastric contents from infants fed MBM than those fed DBM at 8–9 days. These observations suggest that pasteurization of milk anti-pertussis antibodies may reduce their survival during infant digestion. We speculate that the higher digestibility of anti-pertussis antibodies post-pasteurization may be due to the changes in the antibody structure that increase the accessibility of cleavage sites to proteases.
We evaluated whether the first type of feed (MBM or DBM) changed the relative abundance of anti-pertussis antibodies in the gastric contents, which could come from the residual from the first feed. We observed that infants fed the MBM feed first tended (p = 0.060, 1.9-fold) to have higher anti-FHA IgA in DBM gastric contents (at 8–9 days postpartum) than those fed a DBM feed first (Supplementary Table S4). The other antibodies in gastric contents were not affected by the first type of feed at both times of postpartum.
Anti-PT IgA was higher in gastric contents from infants fed MBM at 8–9 days than at 21–22 days (Supplementary Fig. S2) but did not differ in MBM between these postpartum times. This result suggests that early postnatal age allowed for greater survival to gastric digestion of milk anti-PT IgA likely due to the lower amount or activity of pepsin in the preterm infant stomach at 8–9 days of life. Our previous studies demonstrated that total IgA concentration in gastric samples (at 2 h postprandial from preterm infants fed human milk) decreased with the increasing of postnatal age,23 and SIgA concentration in gastric contents from preterm infants fed MBM was also higher at 8–9 days than at 21–22 days postnatal age.2 This reduction of IgA was associated with degradation by proteases (pepsin and/or milk proteases) but not with acid-induced structural deterioration in the stomach.24
This study did not distinguish between preterm infant’s own antibodies and milk antibodies in their gastric contents. No study has demonstrated the specific time when preterm infants are able to produce their own antibodies (especially for SIgA, the most abundant antibody produced in mucosal fluids) at <3 weeks of postnatal age. Anti-PT IgM from DBM reduced with the increasing of GA in gastric contents from preterm infants (p = 0.002, Supplementary Fig. S1), whereas the other pertussis-specific antibodies (from MBM and DBM) did not change with the GA in gastric contents (data not shown). Preterm infants may not be able to produce their own pertussis-specific antibodies as they have not been exposed to B. pertussis or been Tdap vaccinated in the past.
All the milk pertussis antibody types studied most likely survived preterm infant overall digestion to some extent as all were detected in infant stools 24 h post-feeding. Antibodies detected in stool samples may derive from the MBM and/or DBM feeding or be secreted by the infant. However, no correlation was detected for pertussis-specific antibodies in function of GA in the infant stools (data not shown). No difference in pertussis-specific antibodies was observed between 8–9 and 21–22 days in stools. A previous study demonstrated that SIgA specific to Escherichia coli O antigens were detected in the feces of 90% of preterm infants fed human milk fortified with fractions of pasteurized, lyophilized human milk, whereas none was detected in the feces of infants fed cow’s milk-based formula.7 These results indicate that the SIgA detected in the stools of infants fed human milk derived from their mother’s milk rather than via infant production. SIgA specific to E. coli O antigens in infant stools did not differ between collection periods (2.5 and 6 weeks postpartum),7 which is in accordance with our results where no group of anti-pertussis antibodies differed in abundance in the stool between the first and third week postpartum age. However, the amount of water in the stool samples likely varied widely and we did not control for this factor, which could lead to increased variation in the measured values.
No study has demonstrated that milk anti-pertussis antibodies can protect infants against B. pertussis in the respiratory tract. Small amounts of breast milk could reach the infant respiratory tract as a result of regurgitation and inhalation of breast milk during and after feeding18 and provide protection in the respiratory mucosa. Milk antibodies could also be transferred from the gut to the bloodstream. A previous study reported that colostrum poliovirus-specific IgA was recovered in infant stools during the first 18–24 h after birth and the majority of ingested IgA showed up in the infant stool at 2–3 days,13 suggesting that anti-polio IgA was not absorbed by the neonatal intestine. Nutritional intervention studies are needed to confirm whether the milk antibodies specific to pertussis can prevent respiratory infections in infants.
The goal of this study was not to examine the protection against pertussis and thus the absorption of antibody into the infant’s bloodstream but to compare the survival of pertussis-specific antibodies from MBM and DBM during the infant digestion. Therefore, the neutralizing activity of milk antibodies specific to pathogens is mainly in the gastrointestinal tract lumen and may not need to be absorbed. Milk antibodies could also bind to the surface of pathogen agents and initiate a complement cascade.25 This protection could begin in the infant’s mouth and continue through the intestinal tract until excretion in the stool.
The present study provides knowledge on the effect of maternal vaccination and suggests that maternal immunization could protect infants against pathogens via human milk in addition to the protection from transplacental IgG transfer. We did not distinguish the relative proportion between the transplacental IgG and human milk IgG. The comparison of transplacental IgG versus human milk IgG is not a critical focus in this study because we are mainly looking at the passive immunity via human milk. It is well accepted that transplacental IgG is lower in preterm infants than in term infants16 due to the interruption of pregnancy during preterm birth.26 Moreover, the percentage of IgG among the other isotype forms is only 4% (compared with 18% for IgM/SIgM and 79% SIgA/IgA) in human milk.2 SIgA is the most abundant and efficient antibody in human milk that neutralizes pathogens (immune exclusion) in the infant’s gut to protect against infection.26 Therefore, pertussis-specific antibodies (especially SIgA/IgA) from either MBM or DBM survived during preterm infant digestion and both type of milk compensate for the lower IgG transplacental transfer in preterm infants compared with term infants. Future research should examine the proportion of Tdap-induced protection of the infant derived from antibodies in mother’s milk compared with the transplacental-transferred IgG.
Another limitation was the absence of information on the status of vaccination for the mothers who participated to this study (MBM) or from mothers who donated to the Milk Bank (DBM). A previous study reported that 45.6% of women (among 700 respondents) did not receive Tdap vaccine during their pregnancy from 2017 to 2018.25 Even if we had the vaccination state for each mother, milk antibodies from vaccinated mothers should vary due to their difference of exposition to B. pertussis in the past. Vaccination does not induce the same abundance and functionality of pertussis-specific antibodies in human milk in each individual due to their difference in genes and immune system (previous immunizations, infections, and health conditions).26,27 Although the immune response of two women can be activated to the same antigen (pertussis), the interactions and levels of dendritic cells, T cells, and B cells can vary widely from one individual to another. Variability of antibody production between vaccinated mothers could be related to the differences in vaccine type, dose, or route, which can lead to different initial immune responses.
This report demonstrated that overall pertussis-specific antibodies from MBM were stable during infant digestion, whereas anti-pertussis IgA and IgG from DBM decreased in gastric contents. Among those pertussis-specific antibodies, a large degree of variation in survival was observed during digestion between the antibody’s binding specificity and isotype as well as between individual mother’s milk. Therefore, the constant region and variable region of milk antibody and the maternal immunization (previous infectious and vaccinations) appear to be important factors for their survival during infant digestion and pasteurization. Clinical investigations are needed to determine the required effective dose of milk pertussis-specific antibodies the infants need to ingest to reduce pertussis infection risk.
References
Cohen, M. B. Etiology and mechanisms of acute infectious diarrhea in infants in the United States. J. Pediatr. 118, S34–S39 (1991).
Demers-Mathieu, V. et al. Differences in maternal immunoglobulins within mother’s own breast milk and donor breast milk and across digestion in preterm infants. Nutrients 11, 920 (2019).
Demers-Mathieu, V. et al. Antenatal influenza A-specific IgA, IgM, and IgG antibodies in mother’s own breast milk and donor breast milk, and gastric contents and stools from preterm infants. Nutrients 11, 1567 (2019).
Roos, N. et al. 15N-labeled immunoglobulins from bovine colostrum are partially resistant to digestion in human intestine. J. Nutr. 125, 1238–1244 (1995).
Eibl, M. M., Wolf, H. M., Fürnkranz, H. & Rosenkranz, A. Prevention of necrotizing enterocolitis in low-birth-weight infants by IgA–IgG feeding. N. Engl. J. Med. 319, 1–7 (1988).
Bakker‐Zierikzee, A. M. et al. Faecal SIgA secretion in infants fed on pre‐or probiotic infant formula. Pediatr. Allergy Immunol. 17, 134–140 (2006).
Schanler, R. J., Goldblum, R. M., Garza, C. & Goldman, A. S. Enhanced fecal excretion of selected immune factors in very low birth weight infants fed fortified human milk. Pediatr. Res. 20, 71 (1986).
Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months-Advisory Committee on Immunization Practices (ACIP), 2011. Morb. Mortal. Wkly Rep. 60, 1424–1426 (2011).
Centers for Disease Control and Prevention. Pertussis (whooping cough)—what you need to know. http://www.cdc.gov/Features/Pertussis/ (2012).
Healy, C. M., Rench, M. A. & Baker, C. J. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin. Infect. Dis. 56, 539–544 (2012).
Halperin, B. et al. Kinetics of the antibody response to tetanus-diphtheria-acellular pertussis vaccine in women of childbearing age and postpartum women. Clin. Infect. Dis. 53, 885–892 (2011).
Sweet, C., Jakeman, K. J. & Smith, H. Role of milk-derived IgG in passive maternal protection of neonatal ferrets against influenza. J. Gen. Virol. 68, 2681–2686 (1987).
Ogra, S. S., Weintraub, D. & Ogra, P. L. Immunologic aspects of human colostrum and milk: III. Fate and absorption of cellular and soluble components in the gastrointestinal tract of the newborn. J. Immunol. 119, 245–248 (1977).
Raya, B. A. et al. The induction of breast milk pertussis specific antibodies following gestational tetanus–diphtheria–acellular pertussis vaccination. Vaccine 32, 5632–5637 (2014).
De Schutter, S. et al. Quantification of vaccine-induced antipertussis toxin secretory IgA antibodies in breast milk: comparison of different vaccination strategies in women. Pediatr. Infect. Dis. J. 34, e149–e152 (2015).
van den Berg, J. P. et al. Transplacental transport of IgG antibodies specific for pertussis, diphtheria, tetanus, Haemophilus influenzae type b, and Neisseria meningitidis serogroup C is lower in preterm compared with term infants. Pediatr. Infect. Dis. J. 29, 801–805 (2010).
Langkamp, D. L., Hoshaw-Woodard, S., Boye, M. E. & Lemeshow, S. Delays in receipt of immunizations in low-birth-weight children: a nationally representative sample. Arch. Pediatr. Adolesc. Med. 155, 167–172 (2001).
Decarlo, J., Tramer, A. & Startzman, H. Iodized oil aspiration in the newborn. Am. J. Dis. Child. 84, 442–445 (1952).
Sagrera, X. et al. Outbreaks of influenza A virus infection in neonatal intensive care units. Pediatr. Infect. Dis. J. 21, 196–200 (2002).
Carroll, K. & Herrmann, K. R. The cost of using donor human milk in the NICU to achieve exclusively human milk feeding through 32 weeks postmenstrual age. Breastfeed. Med. 8, 286–290 (2013).
Evans, T. J., Ryley, H. C., Neale, L. M., Dodge, J. A. & Lewarne, V. M. Effect of storage and heat on antimicrobial proteins in human milk. Arch. Dis. Child. 53, 239–241 (1978).
Ford, J. E., Law, B. A., Marshall, V. M. & Reiter, B. Influence of the heat treatment of human milk on some of its protective constituents. J. Pediatr. 90, 29–35 (1977).
Demers-Mathieu, V., Underwood, M., Beverly, R., Nielsen, S. & Dallas, D. Comparison of human milk immunoglobulin survival during gastric digestion between preterm and term infants. Nutrients 10, 631 (2018).
Demers-Mathieu, V., Nielsen, S. D., Underwood, M. A., Borghese, R. & Dallas, D. C. Changes in proteases, antiproteases and bioactive proteins from mother’s breast milk to the premature infant stomach. J. Pediatr. Gastroenterol. Nutr. 66, 318–324 (2018).
Boes, M. Role of natural and immune IgM antibodies in immune responses. Mol. Immunol. 37, 1141–1149 (2000).
Zimmermann, P. & Curtis, N. Factors that influence the immune response to vaccination. Clin. Microbiol. Rev. 32, e00084 (2019).
Kollmann, T. R., Kampmann, B., Mazmanian, S. K., Marchant, A. & Levy, O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 46, 350–363 (2017).
Acknowledgements
We thank the Northwest Mother’s Milk Bank for their support. This study was funded by the K99/R00 Pathway to Independence Career Award, Eunice Kennedy Shriver Institute of Child Health & Development of the National Institutes of Health (R00HD079561), and The Gerber Foundation.
Author information
Authors and Affiliations
Contributions
V.D.-M., D.C.D., and R.K.H. designed the clinical study. V.D.-M. conducted the experiments and the statistical analyses and wrote the paper. R.K.H., A.M.M., E.A.M., and R.L.M. provided milk and gastric samples and revised the paper. All authors revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Demers-Mathieu, V., Huston, R.K., Markell, A.M. et al. Impact of pertussis-specific IgA, IgM, and IgG antibodies in mother’s own breast milk and donor breast milk during preterm infant digestion. Pediatr Res 89, 1136–1143 (2021). https://doi.org/10.1038/s41390-020-1031-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-020-1031-2
- Springer Nature America, Inc.