Abstract
Background
Insulin resistance (IR) is indicated to be linked with adverse outcomes of acute myocardial infarction (AMI), for its pro-inflammatory and pro-thromboplastic function. The triglyceride-glucose (TyG) index is a newly developed substitute marker for IR. The aim of this pooled analysis was to provide a summary of the relationship of TyG index with occurrences of major adverse cardiovascular and cerebrovascular events (MACCEs) among populations suffering from AMI.
Methods
Cohorts reporting multivariate-adjusted hazard ratios of TyG index with MACCEs or its independent events were identified through systematically searching PubMed, MEDLINE, Web of science, Embase and Cochrane databases. Results were combined using a random-effects model.
Results
21 cohorts comprising 20403 individuals were included. Compared to individuals in the lowest TyG category, patients in the highest TyG category exhibited elevated risks of both MACCEs (P < 0.00001) and all-cause death (P < 0.00001). These findings were in line with the results as TyG analyzed as continuous variables (MACCEs: P = 0.006; all-cause death: P < 0.00001). Subgroup analysis demonstrated that diabetic status, type of AMI, nor the reperfusion therapy did not destruct this correlation (for subgroups, all P < 0.05).
Conclusion
All these indicated that higher TyG index could potentially predict MACCEs and all-cause death in patients with AMI as an independent indicator.
Similar content being viewed by others
Introduction
Despite of substantial advances in the recent guideline-recommended treatment, acute myocardial infarction (AMI) remains extensively a major contributor to the mortality and morbidity of cardiovascular diseases globally [1, 2], non-negligibly accounting for recurrent major adverse cardiac and cerebral events (MACCEs) that has been recognizably correlated with worse prognosis in those with AMI [3]. Hence, early identification of potential risk factors for MACCEs in AMI population is imperative for optimal management to further reduce death and disability [4].
Insulin resistance (IR), a hallmark of metabolism disorder, not only functioned as a pivotal mechanism of AMI, but was also related to poorer prognosis [5, 6]. Pathologically, IR could result in vascular inflammation and abnormal coagulation, thereby promoting the accumulation of vascular lipids and thrombosis, ultimately accelerating arteriosclerosis and stenoses [7, 8]. Furthermore, IR hindered the uptake of glucose by ischemic myocardium and thus impeded their ability to generate energy through compensatory glycolysis, subsequently contributing to increased infarct zone and decreased myocardial contractility [5]. To date, population with type 2 diabetes mellitus whom prevalently coming up with IR, has been grouped as the extreme-risk crowd for recurrent MACCEs after AMI [9]. Classically, the hyper insulinemic euglycemic clamp was recognized the gold criterion for evaluating IR [10, 11]; nonetheless, it is too complex to popularize in large-scale clinical practice [12]. The triglyceride–glucose (TyG) index, which is derived from fasting plasma glucose and triglyceride levels, has been proposed as a dependable and accurate substitute marker for IR [13]. Accordingly, accumulating cohorts have been concerning the correlation of TyG index with MACCEs risks in AMI, whereas inconsistent conclusions were obtained: some showed that elevated TyG was apparently in relation to more frequent MACCEs, while others found this association to be non-significant [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34]. Herein, for the first time, we aim to perform a pooled analysis evaluating the predictive value of baseline TyG index on MACCEs in AMI population.
Methods
This review was reported in accordance with the PRISMA guidelines [35].
Study search
We conducted a search in databases including PubMed, MEDLINE, Web of Science, Embase and Cochrane using a combination of terms: (1) “TyG index” OR “triglyceride-glucose index” OR “triglyceride glucose index” and (2) “heart infarction” OR “myocardial infarction” OR “cardiovascular stroke” OR “‘myocardial infarct” OR “myocardial infarct” OR “heart attack” OR “MINOCA” OR “cardiogenic shock”, from inception to October 18, 2023, without language restriction. Mannual hand-searching of reference lists from relevant trials and reviews was supplemented. The full search strategy is expanded in supplementary Table 1. Final inclusion was based on the consensus of two independent reviewers, or a third independent investigator would serve as the referee in case of disagreements.
Study selection
Studies fulfilling the following criteria (according to the PECO framework) were included: (P) types of participants: consecutive adults diagnosed with AMI at baseline; (E) exposures and (C) comparators: high versus low TyG index; (O) outcomes: the primiary outcome was major adverse cardiac and cerebrovascular events (MACCEs), and the secondary outcomes were the independent events of MACCEs. TyG index was calculated via ‘ln [TG (mg/dl) * FBG (mg/dl)/2]’. The MACCEs was characterized as a combination of all-cause death, nonfatal stroke, nonfatal myocardial infarction, rehospitalization for HF, and revascularization. Exclusion criteria were set as: Cross-sectional studies for high bias risks; studies not reporting multivariable adjusted association for the correlation of TyG index with AMI prognosis; studies ongoing or in English. In case of overlap in the populations of different studies from the same registry or group, only the largest size was included.
Data extraction and quality assessment
Two independent reviewers extracted and checked data. Data abstracted included: (a) first author’s name, and publication year; (b) research design, and follow-up duration; (c) patients characteristics, including study region, sample size, age, sex, type of AMI, diabetes proportion; (d) patterns of TyG index analysis; (e) outcomes reported; and (f) confounding factors adjusted. The quality (article selection, comparability, and outcomes) were assessed using the Newcastle–Ottawa Scale (NOS) [36].
Statistical analyses
HRs and the 95% confidence intervals (CIs) were used as indicators regarding the correlation of TyGs with MACCEs in individuals with AMI. For cohorts with TyGs analyzed as categorical variables, HRs of MACCEs occurrences in populations with the highest TyGs versus with the lowest were collected. For cohorts with TyGs analyzed as continuous variables, HRs of MACCEs incidences per 1 unit increment of TyGs was collected. Before pooled analyses, HRs were logarithmically converted. The Cochran’s Q test was utilized to calculate the I2 statistics, and heterogeneity revealed statistically significant when I2 > 50. Random-effects model was applied to pool HRs. Subgroup analyses were carried out based on study characteristics, including diabetic status, treatment for coronary occlusion, and type of AMI, on the correlation of TyG with MACCEs risk. Publication bias was evaluated graphically via funnel plots. Sensitivity analysis was conducted using one-by-one elimination method. RevMan (Version 5.4) and STATA (Version 18) were applied to conduct these analyses.
Results
Study search
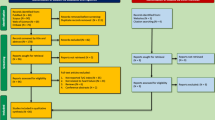
In all, 398 studies were identified after excluding duplications. Among, 67 articles underwent full-text scanning, and of those, a total of 21 cohorts were finally included [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34] (Fig. 1).
Study characteristics and quality assessment
Overall, 22 cohorts (16 retrospective and 5 prospective) comprising a total of 20403 participants diagnosed with AMI at baseline were included in our current review. All articles were published between 2019 and 2023. Among, majority of included studies, 19 in 21, were from China [14,15,16,17,18,19,20,21,22,23,24, 26,27,28,29,30, 32,33,34], while another 2 were performed in Turkey [25, 31]. Sample sizes varied from 131 to 3181, and the mean age varied from 44.5 to 76.5 years, with male proportion varying from 55.9 to 81.8% and DM proportion varying from 0 to 100%. TyG index was analysed as categorical variables in six cohorts [14, 17, 21, 30, 31, 34], as continuous variables in twelve cohorts [15, 16, 18, 20, 22,23,24,25,26,27,28, 32], and as both in three cohorts [19, 29, 33]. The follow-up periods varied from 12.0 to 51.7 years. Age, sex, medical history, laboratory findings, angiographic findings, and in-hospital medications were adjusted to varying degrees when the correlation of TyGs with MACCEs or all-cause death in AMIs were reported (Table 1). The NOS scores were nine for all cohorts, suggesting good quality (Table 2).
TyG index and the occurrence of MACCEs
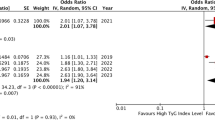
Overall, findings from twelve cohorts demonstrated that in comparison to AMI patients categorized with the lowest TyG [15, 16, 18,19,20, 22, 23, 26,27,28, 32, 33], those in the highest category showed apparently increased risks of MACCEs (HR: 1.65, 95% CI: 1.34-2.04, I2 = 65%, P < 0.00001) (Fig. 2A). Similar results were showed as TyG index analysed as continuous variables [14, 17, 19, 21, 31, 33] (HR: 1.66, 95% CI: 1.15–2.40, I2 = 96%, P = 0.006) (Fig. 2B). Subgroup analyses presented that AMI populations with higher TyG exhibited apparently increased risks of MACCEs independent of diabetic status, type of AMI, and treatment for coronary occlusion (for subgroups, all P < 0.05) (Fig. 3A–C). Sensitivity analysis suggested that the association of TyG index with MACCEs was robust (Supplementary Fig. 1A, B).
TyG index and the occurrence of all-cause death
Overall, results of three cohorts exhibited that in comparison to AMI populations in the lowest TyG category [24, 25, 29], those in the highest category showed significantly increased risk for all-cause death (HR: 2.69, 95% CI: 1.75–4.12, I2 = 27%, P < 0.00001) (Fig. 4A). These findings were in line with results when TyG index analysed as continuous variables [21, 29, 30, 34] (HR: 2.52, 95% CI: 1.90-3.35, I2 = 0%, P < 0.00001) (Fig. 4B). Sensitivity analysis indicated that the correlation of TyG with all-cause death was robust when TyG analysed as continuous variables (Supplementary Fig. 1C, D).
Publication bias
Funnel plots describing the correlation of TyG analysed as categorical and continuous variables with MACCEs were listed in Fig. 5. No significant asymmetry of the funnel plots was observed, suggesting a minimal likelihood of biased publication.
Discussion
In this pooled analysis, we showed that patients with higher TyG index exhibited apparently increased incidences of both MACCEs and all-cause death. For composite MACCEs, subgroup analyses indicated that this relationship was in a stable state as not affected by diabetic status, type of AMI, nor reperfusion treatment of the patients. All findings pointed that higher TyG index could be a potential indicator for elevated risks of MACCEs, as well regarding all-cause death in AMI population.
All along, the hyper insulinemic euglycemic clamp technique is deemed as the “gold criterion” for measurement of insulin sensitivity [37]; nevertheless, the use of this technique was restricted due to its time-consuming, expensive and complicated characteristics [38]. Recently, some other standards effectively diagnosing IR have been gradually developed, for instance, HOMA-IR, QUICKI, and TG/HDL-C [10, 12, 39], thereinto, also including the TyG index that our meta-analysis focused [40]. Current findings confirmed the potential use of the TyG index as a dependable marker for IR. A previous study confirmed both higher sensitivity and specificity using TyG index to detect IR, in comparison to hyper-insulinemic-euglycemic clamp test [41]. Furthermore, evidence explored by Tam et al. also showed a better performance of TyG index in assessing IR than homeostasis model assessment [42]. Methodologically, considering its easily accessible, relevantly safe, and comparatively cheap advantages, we deemed TyG as a not only reliable but also practical index to assess IR in patients with AMI, for often urgent conditions the AMI population placed and thus no ability to satisfy some complex exam procedures.
This is the first systematic review to pool the association of TyG index with the risks of subsequent incidences of MACCEs and all-cause death in AMI population. A previous meta-analysis has illuminated the correlation between TyG index and outcomes in individuals with coronary artery disease [43]. Recent meta-analyses have also identified a positive link between TyG index and the occurrence of atherosclerotic cardiovascular diseases in general population [44, 45]. In comparison to these previous meta-analyses, our study specifically focused on individuals with AMI to provide more precise results for this specific population. Furthermore, the correlation between TyG index and other metabolic cardiovascular disorders, for instance, heart failure [46], coronary artery calcification [47], and arterial stiffness [47, 48], has been previously established. Our study supported the potential value of TyG index to be applied as a predictor of MACCEs risk in AMI patients, by which we were not surprised. Currently, IR has been confirmed that was associated with the worse outcomes following CABG [49]. Then, pathologically, IR might directly result in endothelial dysfunctions [50, 51], hence, aggravating coronary cramp and consequently increasing the occurrence of no-reflow events or microcirculation disturbance after coronary reperfusion, which contributed to pushing ischemia myocardium to the infarcted state and subsequent an intensified infarct size [52,53,54,55]. Moreover, IR has been indicated related to disorders of sympathetic nervous system [56] and imprisons of cardiac autonomic function [57], that would facilitate adverse clinical outcomes during AMI such as malignant arrhythmia, cardiac arrest, sudden death, etc. [58, 59]. In this study, only cohorts were included, thereby effectively avoiding potential recall biases related to cross-sectional design. Furthermore, all the outcomes considered in this analysis were meticulously adjusted for multiple variables. Additionally, subgroup analyses were conducted to ensure the robustness of these findings and ascertain that they were not influenced by patient characteristics such as diabetic status, AMI type, or reperfusion therapy. Nevertheless, further investigations are warranted to determine whether incorporating TyG into existing prediction tools can enhance predictive efficacy for clinical outcomes among individuals with AMI.
There are still some limitations in our meta-analysis. First, there were few studies accessible for this study, and there was substantial heterogeneity among them, which possibly due to variations in the methods of measuring the TyG index, study design, follow-up duration and AMI type among the studies. Further research is necessary to investigate whether different study characteristics such as participant ethnicity, follow-up duration, and concurrent medications could impact these findings. Second, most included studies originated from China, with only 2 out of 21 conducted in European regions. This might limit the generalizability of the predictive value of TyG index for outcomes in AMI populations universally. Third, all research included were cohort studies, thus, causal link between TyG and MACCEs could not be definitively established. Finally, events such as inter-institutional differences in in-hospital management might have prognostic implications in patients with AMI that might weaken the meaningfulness of conclusions in this review to some extent.
Conclusion
In conclusion, based on existing findings from this meta-analysis, higher TyG index might be a reliable indicator for MACCEs in individuals suffering from AMI. Further explorations are imperative and essential to ascertain whether the integration of the TyG index in conjunction with current prediction tools for AMI could enhance their prognostic capabilities.
Data availability
All data generated can be obtained from this published article and its additional information files.
References
Danchin N. Improved long-term survival after acute myocardial infarction: the success of comprehensive care from the acute stage to the long term. Eur Heart J. 2023;44:499–501.
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American heart association. Circulation. 2020;141:e139–e596.
Zhou M, Liu J, Hao Y, Liu J, Huo Y, Smith SC Jr, et al. Prevalence and in-hospital outcomes of diabetes among patients with acute coronary syndrome in China: findings from the improving care for cardiovascular disease in China-acute coronary syndrome project. Cardiovasc Diabetol. 2018;17:147.
Mahtta D, Khalid U, Misra A, Samad Z, Nasir K, Virani SS. Premature atherosclerotic cardiovascular disease: what have we learned recently? Curr Atheroscler Rep. 2020;22:44.
Guo Q, Miao M, Duan L, Liu Y, Qiu Y, Feng X, et al. The relationship between insulin resistance, serum alkaline phosphatase, and left ventricular dysfunction following myocardial infarction. Sci Rep. 2023;13:17974.
Webster KA. Stress hyperglycemia and enhanced sensitivity to myocardial infarction. Curr Hypertens Rep. 2008;10:78–84.
Li T, Wang P, Wang X, Liu Z, Zhang Z, Zhang Y, et al. Inflammation and insulin resistance in diabetic chronic coronary syndrome patients. Nutrients. 2023;15:2808.
Irfan H. Obesity, cardiovascular disease, and the promising role of semaglutide: insights from the SELECT trial. Curr Probl Cardiol. 2024;49:102060.
Ray KK, Colhoun HM, Szarek M, Baccara-Dinet M, Bhatt DL, Bittner VA, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the ODYSSEY OUTCOMES randomised controlled trial. Lancet Diabetes Endocrinol. 2019;7:618–28.
Bloomgarden ZT. Measures of insulin sensitivity. Clin Lab Med. 2006;26:611–33.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23.
Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26.
Khan SH, Sobia F, Niazi NK, Manzoor SM, Fazal N, Ahmad F. Metabolic clustering of risk factors: evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol Metab Syndr. 2018;10:74.
Luo E, Wang D, Yan G, Qiao Y, Liu B, Hou J, et al. High triglyceride-glucose index is associated with poor prognosis in patients with acute ST-elevation myocardial infarction after percutaneous coronary intervention. Cardiovasc Diabetology. 2019;18:150.
Ma XT, Dong LS, Shao QY, Cheng YJ, Lv S, Sun Y, et al. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc Diabetol. 2020;19:31.
Wang L, Cong HL, Zhang JX, Hu YC, Wei A, Zhang YY, et al. Triglyceride-glucose index predicts adverse cardiovascular events in patients with diabetes and acute coronary syndrome. Cardiovasc Diabetology. 2020;19:80 .
Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. High triglyceride-glucose index is associated with adverse cardiovascular outcomes in patients with acute myocardial infarction. Nutr, Metab, Cardiovasc Dis. 2020;30:2351–62.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Impacts of triglyceride-glucose index on prognosis of patients with type 2 diabetes mellitus and non-ST-segment elevation acute coronary syndrome: results from an observational cohort study in China. Cardiovasc Diabetol. 2020;19:108.
Gao SD, Ma WJ, Huang SZ, Lin XZ, Yu MY. Impact of triglyceride-glucose index on long-term cardiovascular outcomes in patients with myocardial infarction with nonobstructive coronary arteries. Nutr Metab Cardiovasc Dis. 2021;31:3184–92.
Yang J, Tang YD, Zheng YT, Li C, Zhou Q, Gao J, et al. The impact of the triglyceride-glucose index on poor prognosis in nondiabetic patients undergoing percutaneous coronary intervention. Front Endocrinol. 2021. https://doi.org/10.3389/fendo.2021.710240.
Zhang Y, Ding X, Hua B, Liu Q, Gao H, Chen H, et al. Predictive effect of triglyceride-glucose index on clinical events in patients with type 2 diabetes mellitus and acute myocardial infarction: results from an observational cohort study in China. Cardiovasc Diabetol. 2021;20:43.
Zhao Q, Zhang TY, Cheng YJ, Ma Y, Xu YK, Yang JQ, et al. Triglyceride-glucose index as a surrogate marker of insulin resistance for predicting cardiovascular outcomes in nondiabetic patients with non-ST-segment elevation acute coronary syndrome undergoing percutaneous coronary Iintervention. J Atheroscler Thrombosis. 2021;28:1175–94.
Chen L, Ding XH, Fan KJ, Gao MX, Yu WY, Liu HL, et al. Association between triglyceride-glucose index and 2-year adverse cardiovascular and cerebrovascular events in patients with type 2 diabetes mellitus who underwent off-pump coronary artery bypass grafting. Diabetes Metab Syndr Obes-Targets Ther. 2022;15:439–50.
Guo JQ, Ji ZJ, Carvalho A, Qian LL, Ji JJ, Jiang Y, et al. The triglycerides-glucose index and the triglycerides to high-density lipoprotein cholesterol ratio are both effective predictors of in-hospital death in non-diabetic patients with AMI. PeerJ. 2022;10:e14346.
Saylik F, Çinar T, Selçuk M, Tanboga IH. The predictive value of triglyceride-glucose index for in-hospital and one-year mortality in elderly non-diabetic patients with ST-segment elevation myocardial infarction. J Geriatr Cardiol. 2022;19:610–7.
Shao QY, Ma XT, Yang ZQ, Li QX, Wang YF, Liang J, et al. Prognostic significance of multiple triglycerides-derived metabolic indices in patients with acute coronary syndrome. J Geriatr Cardiol. 2022;19:456–68.
Wu ZG, Liu L, Wang WW, Cui HL, Zhang YR, Xu JC, et al. Triglyceride-glucose index in the prediction of adverse cardiovascular events in patients with premature coronary artery disease: a retrospective cohort study. Cardiovasc Diabetol. 2022;21:142.
Xiong SQ, Chen Q, Chen X, Hou J, Chen YZ, Long Y, et al. Adjustment of the GRACE score by the triglyceride glucose index improves the prediction of clinical outcomes in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Cardiovasc Diabetol. 2022;21:145.
Fu R, Zhao YY, Cui KY, Yang JG, Xu HY, Yin D, et al. Triglyceride glucose index predicts in-hospital mortality in patients with ST-segment elevation myocardial infarction who underwent primary angiography. J Geriatr Cardiol. 2023;20:185–94.
Hao Q, Yuanyuan Z, Lijuan C. The prognostic value of the triglyceride glucose index in patients with acute myocardial infarction. J Cardiovasc Pharmacol Ther. 2023. https://doi.org/10.1177/10742484231181846.
Karadeniz F, Sancaktepe EA, Karadeniz Y. High triglyceride-glucose index is associated with poor prognosis in patients with acute coronary syndrome in long-term follow-up. Angiology. 2023;74:139–48.
Ye Z, Xu Y, Tang L, Wu M, Wu B, Zhu T, et al. Predicting long-term prognosis after percutaneous coronary intervention in patients with new onset ST-elevation myocardial infarction: development and external validation of a nomogram model. Cardiovasc Diabetol. 2023;22:87.
Zhang Y, Chu C, Zhong Z, Luo YB, Ning FF, Guo N. High triglyceride-glucose index is associated with poor cardiovascular outcomes in Chinese acute coronary syndrome patients without diabetes mellitus who underwent emergency percutaneous coronary intervention with drug-eluting stents. Front Endocrinol (Lausanne). 2023;14:1101952.
Zhao XX, Song L, Li JN, Zhou JY, Li N, Yan SD, et al. Effect of triglyceride-glucose indices and circulating PCSK9-associated cardiovascular risk in STEMI patients with primary percutaneous coronary artery disease: a prospective cohort study. J Inflamm Res. 2023;16:269–82.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71 https://doi.org/10.1136/bmj.n71.
Lo CK, Mertz D, Loeb M. Newcastle-ottawa scale: comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014;14:45.
Rebelos E, Honka MJ. PREDIM index: a useful tool for the application of the euglycemic hyperinsulinemic clamp. J Endocrinol Invest. 2021;44:631–4.
Tura A, Chemello G, Szendroedi J, Göbl C, Færch K, Vrbíková J, et al. Prediction of clamp-derived insulin sensitivity from the oral glucose insulin sensitivity index. Diabetologia. 2018;61:1135–41.
Catanzaro R, Selvaggio F, Sciuto M, Zanoli L, Yazdani A, He F, et al. Triglycerides to high-density lipoprotein cholesterol ratio for diagnosing nonalcoholic fatty liver disease. Minerva Gastroenterol (Torino). 2022;68:261–8.
Ramdas Nayak VK, Satheesh P, Shenoy MT, Kalra S. Triglyceride Glucose (TyG) Index: A surrogate biomarker of insulin resistance. J Pak Med Assoc. 2022;72:986–8.
Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51.
Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35:1605–10.
Liang S, Wang C, Zhang J, Liu Z, Bai Y, Chen Z, et al. Triglyceride-glucose index and coronary artery disease: a systematic review and meta-analysis of risk, severity, and prognosis. Cardiovasc Diabetol. 2023;22:170.
Liu X, Tan Z, Huang Y, Zhao H, Liu M, Yu P, et al. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: a systematic review and meta-analysis. Cardiovasc Diabetol. 2022;21:124.
Ding X, Wang X, Wu J, Zhang M, Cui M. Triglyceride-glucose index and the incidence of atherosclerotic cardiovascular diseases: a meta-analysis of cohort studies. Cardiovasc Diabetol. 2021;20:76.
Khalaji A, Behnoush AH, Khanmohammadi S, Ghanbari Mardasi K, Sharifkashani S, Sahebkar A, et al. Triglyceride-glucose index and heart failure: a systematic review and meta-analysis. Cardiovasc Diabetol. 2023;22:244.
Liu F, Ling Q, Xie S, Xu Y, Liu M, Hu Q, et al. Association between triglyceride glucose index and arterial stiffness and coronary artery calcification: a systematic review and exposure-effect meta-analysis. Cardiovasc Diabetol. 2023;22:111.
Zhong H, Shao Y, Guo G, Zhan Y, Liu B, Shao M, et al. Association between the triglyceride-glucose index and arterial stiffness: a meta-analysis. Med (Baltim). 2023;102:e33194.
Wu Z, Guo D, Chen S, Sun X, Zhang Y, Liu X, et al. Combination of the triglyceride-glucose index and EuroSCORE II improves the prediction of long-term adverse outcomes in patients undergoing coronary artery bypass grafting. Diabetes Metab Res Rev. 2023;39:e3710.
Kuboki K, Jiang ZY, Takahara N, Ha SW, Igarashi M, Yamauchi T, et al. Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation. 2000;101:676–81.
Koffi C, Soleti R, Nitiema M, Mallegol P, Hilairet G, Chaigneau J, et al. Ethanol extract of leaves of cassia siamea lam protects against diabetes-Induced Insulin resistance, hepatic, and endothelial dysfunctions in ob/ob mice. Oxid Med Cell Longev. 2019;2019:6560498.
Wiebe N, Stenvinkel P, Tonelli M. Associations of chronic inflammation, insulin resistance, and severe obesity with mortality, myocardial infarction, cancer, and chronic pulmonary disease. JAMA Netw Open. 2019;2:e1910456.
Xia JG, Li B, Zhang H, Li QX, Lam SM, Yin CL, et al. Precise metabolomics defines systemic metabolic dysregulation distinct to acute myocardial infarction associated with diabetes. Arterioscler Thromb Vasc Biol. 2023;43:581–96.
Dastani M, Mokhtari M, Khameneh Bagheri R, Hassanzadeh P, Jannati Yazdan Abad M, Mohammadpour AH, et al. A Pilot study on correlation between zinc and magnesium serum concentrations and coronary slow flow phenomenon. Acta Biomed. 2021;92:e2021279.
Gocer H, Gunday M, Abusharekh M, Unal M. To show the effect of intermittent fasting during Ramadan on endothelial dysfunction via TIMI frame count. Niger J Clin Pr. 2021;24:943–7.
Kaaja RJ, Pöyhönen-Alho MK. Insulin resistance and sympathetic overactivity in women. J Hypertens. 2006;24:131–41.
Williams S, Raheim SA, Khan MI, Rubab U, Kanagala P, Zhao SS, et al. Cardiac autonomic neuropathy in type 1 and 2 diabetes: epidemiology, pathophysiology and management. Clin Ther. 2022;44:1394–416.
Reed GW, Rossi JE, Cannon CP. Acute myocardial infarction. Lancet. 2017;389:197–210.
Schäfer A. Interventional cardiology: current challenges in acute myocardial infarction. J Clin Med. 2022;11:4504.
Acknowledgements
This work was supported by the Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (ZYLX202111, to X Hou), Beijing Hospitals Authority “Ascent Plan” (FDL20190601, to X Hou), Young Elite Scientists Sponsorship Program by CAST (2022QNRC001, to L Wang), National Natural Science Foundation of China (82200433, to L Wang), and Beijing Hospitals Authority Youth Programme (QML20230602, to L Wang).
Author information
Authors and Affiliations
Contributions
HRL contributed to literature review and writing. LSW contributed to investigation and data curation. XH and ZTD contributed to create figures and tables. CLL and HW contributed to review of all and final revisions. XTH provided resources, performed statistical analysis, and create figures and tables. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, H., Wang, L., Wang, H. et al. The association of triglyceride–glucose index with major adverse cardiovascular and cerebrovascular events after acute myocardial infarction: a meta-analysis of cohort studies. Nutr. Diabetes 14, 39 (2024). https://doi.org/10.1038/s41387-024-00295-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41387-024-00295-1
- Springer Nature Limited