Abstract
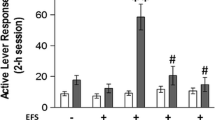
Stress is prevalent in the lives of those with substance use disorders (SUDs) and influences SUD outcomes. Understanding the neurobiological mechanisms through which stress promotes drug use is important for the development of effective SUD interventions. We have developed a model wherein exposure to a stressor, uncontrollable electric footshock, daily at the time of cocaine self-administration escalates intake in male rats. Here we test the hypothesis that stress-induced escalation of cocaine self-administration requires the CB1 cannabinoid receptor. Male Sprague-Dawley rats self-administered cocaine (0.5 mg/kg/inf, i.v.) during 2-h sessions comprised of four 30-min self-administration components separated by 5-min shock sequences or 5-min shock-free periods for 14 days. Footshock produced an escalation of cocaine self-administration that persisted following shock removal. Systemic administration of the cannabinoid receptor type 1 (CB1R) antagonist/inverse agonist, AM251, attenuated cocaine intake only in rats with a history of stress. This effect was localized to the mesolimbic system, as intra-nucleus accumbens (NAc) shell and intra-ventral tegmental area (VTA) micro-infusions of AM251 attenuated cocaine intake only in stress-escalated rats. Cocaine self-administration, regardless of stress history, increased CB1R binding site density in the VTA, but not NAc shell. Following extinction, cocaine-primed reinstatement (10 mg/kg, ip) was increased in rats with prior footshock during self-administration. AM251 attenuated reinstatement only in rats with a stress history. Altogether, these data demonstrate that mesolimbic CB1Rs are required to escalate intake and heighten relapse susceptibility and suggest that repeated stress at the time of cocaine use regulates mesolimbic CB1R activity through a currently unknown mechanism.




Similar content being viewed by others
References
Kosten TR, Kleber HD. Differential diagnosis of psychiatric comorbidity in substance abusers. J Subst Abus Treat. 1988;5:201–6.
Back S, Dansky BS, Coffey SF, Saladin ME, Sonne S, Brady KT. Cocaine dependence with and without posttraumatic stress disorder: a comparison of substance use, trauma history and psychiatric comorbidity. Am J Addict. 2000;9:51–62.
Mahoney JJ, Newton TF, Omar Y, Ross EL, Garza RDL. The relationship between lifetime stress and addiction severity in cocaine-dependent participants. Eur Neuropsychopharmacol. 2013;23:351–7.
Wallace BC. Psychological and environmental determinants of relapse in crack cocaine smokers. J Subst Abus Treat. 1989;6:95–106.
Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacol (Berl). 2011;218:29–37.
Panlilio LV, Stull SW, Bertz JW, Burgess-Hull A, Lanza ST, Curtis BL, et al. Beyond abstinence and relapse II: momentary relationships between stress, craving, and lapse within clusters of patients with similar patterns of drug use. Psychopharmacol (Berl). 2021;238:1513–29.
Waldrop AE, Back SE, Brady KT, Upadhyaya HP, McRae AL, Saladin ME. Daily stressor sensitivity, abuse effects, and cocaine use in cocaine dependence. Addict Behav. 2007;32:3015–25.
Back SE, Hartwell K, DeSantis SM, Saladin M, McRae-Clark A, Price KL, et al. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug Alcohol Depend. 2010;106:21–27.
Panlilio LV, Stull SW, Kowalczyk WJ, Phillips KA, Schroeder JR, Bertz JW, et al. Stress, craving and mood as predictors of early dropout from opioid agonist therapy. Drug Alcohol Depend. 2019;202:200–8.
Banducci AN, Bujarski SJ, Bonn-Miller M, Patel A, Connolly KM. The impact of intolerance of emotional distress and uncertainty on veterans with co-occurring PTSD and substance use disorders. J Anxiety Disord. 2016;41:73–81.
Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacol (Berl). 1999;142:343–51.
Sinha R, Fuse T, Aubin L-, O’Malley SS. Psychological stress, drug-related cues and cocaine craving. Psychopharmacol (Berl). 2000;152:140–8.
Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–31.
Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300.
Mantsch JR, Yuferov V, Mathieu-Kia A, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacol (Berl). 2004;175:26–36.
Mantsch JR, Katz ES. Elevation of glucocorticoids is necessary but not sufficient for the escalation of cocaine self-administration by chronic electric footshock stress in rats. Neuropsychopharmacology. 2007;32:367–76.
Mantsch JR, Baker DA, Serge JP, Hoks MA, Francis DM, Katz ES. Surgical adrenalectomy with diurnal corticosterone replacement slows escalation and prevents the augmentation of cocaine-induced reinstatement in rats self-administering cocaine under long-access conditions. Neuropsychopharmacology. 2008;33:814–26.
Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, et al. Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-Induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology. 2011;36:1444–54.
Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–7.
Hill MN, Ho W-V, Meier SE, Gorzalka BB, Hillard CJ. Chronic corticosterone treatment increases the endocannabinoid 2-arachidonylglycerol in the rat amygdala. Eur J Pharm. 2005;528:99–102.
Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci. 2009;106:4888–93.
Hill MN, Karatsoreos IN, Hillard CJ, McEwen BS. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology. 2010;35:1333–8.
Hill MN, McLaughlin RJ, Pan B, Fitzgerald ML, Roberts CJ, Lee TT-Y, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci. 2011;31:10506–15.
McReynolds JR, Doncheck EM, Li Y, Vranjkovic O, Graf EN, Ogasawara D, et al. Stress promotes drug seeking through glucocorticoid-dependent endocannabinoid mobilization in the prelimbic cortex. Biol Psychiatry. 2018;84:85–94.
Morena M, Patel S, Bains JS, Hill MN. Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology. 2016;41:80–102.
deRoon-Cassini T, Stollenwerk TM, Beatka M, Hillard CJ. Meet your stress management professionals: the endocannabinoids. Trends Mol Med. 2020;26:953–68.
López-Moreno JA, Echeverry-Alzate V, Bühler K. The genetic basis of the endocannabinoid system and drug addiction in humans. J Psychopharmacol. 2012;26:133–43.
Vries TJD, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, et al. A cannabinoid mechanism in relapse to cocaine seeking. Nat Med. 2001;7:1151–4.
Caillé S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific alterations of extracellular endocannabinoid levels in the nucleus accumbens by ethanol, heroin, and cocaine self-administration. J Neurosci. 2007;27:3695–702.
Adamczyk P, McCreary AC, Przegalinski E, Mierzejewski P, Bienkowski P, Filip M. The effects of fatty acid amide hydrolase inhibitors on maintenance of cocaine and food self-administration and on reinstatement of cocaine-seeking and food-taking behavior in rats. J Physiol pharmacology: Off J Pol Physiol Soc PMID - 19826190. 2008;60:119–25.
Orio L, Edwards S, George O, Parsons LH, Koob GF. A role for the endocannabinoid system in the increased motivation for cocaine in extended-access conditions. J Neurosci. 2009;29:4846–57.
Roberts DCS, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17:901–4.
Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56.
Carlezon WA, Devine DP, Wise RA. Habit-forming actions of nomifensine in nucleus accumbens. Psychopharmacol (Berl). 1995;122:194–7.
Pontieri FE, Tanda G, Chiara GD. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci. 1995;92:12304–8.
Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J Neurosci. 2004;24:53–62.
Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–8.
Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–5.
Pan B, Hillard CJ, Liu QS. D2 dopamine receptor activation facilitates endocannabinoid-mediated long-term synaptic depression of GABAergic synaptic transmission in midbrain dopamine neurons via cAMP-protein kinase A signaling. J Neurosci. 2008;28:14018–30.
Oleson EB, Beckert MV, Morra JT, Lansink CS, Cachope R, Abdullah RA, et al. Endocannabinoids shape accumbal encoding of cue-motivated behavior via CB1 receptor activation in the ventral tegmentum. Neuron. 2012;73:360–73.
Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang H, Zhang S, et al. Endocannabinoid actions on cortical terminals orchestrate local modulation of dopamine release in the nucleus accumbens. Neuron. 2017;96:1112–26.e5.
Wang W, Sun D, Pan B, Roberts CJ, Sun X, Hillard CJ, et al. Deficiency in endocannabinoid signaling in the nucleus accumbens induced by chronic unpredictable stress. Neuropsychopharmacology. 2010;35:2249–61.
Wang H, Treadway T, Covey DP, Cheer JF, Lupica CR. Cocaine-induced endocannabinoid mobilization in the ventral tegmental area. Cell Rep. 2015;12:1997–2008.
Tung L, Lu G, Lee Y, Yu L, Lee H, Leishman E, et al. Orexins contribute to restraint stress-induced cocaine relapse by endocannabinoid-mediated disinhibition of dopaminergic neurons. Nat Commun. 2016;7:12199.
Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegaliński E. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res. 2012;1444:45–54.
Soria G, Mendizábal V, Touriño C, Robledo P, Ledent C, Parmentier M, et al. Lack of CB1 cannabinoid receptor impairs cocaine self-administration. Neuropsychopharmacology. 2005;30:1670–80.
Cossu G, Ledent C, Fattore L, Imperato A, Böhme GA, Parmentier M, et al. Cannabinoid CB1 receptor knockout mice fail to self-administer morphine but not other drugs of abuse. Behav Brain Res. 2001;118:61–65.
Martín-García E, Bourgoin L, Cathala A, Kasanetz F, Mondesir M, Gutiérrez-Rodriguez A, et al. Differential control of cocaine self-administration by GABAergic and glutamatergic CB1 cannabinoid receptors. Neuropsychopharmacology. 2016;41:2192–205.
Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411.
Pickel VM, Chan J, Kash TL, Rodríguez JJ, MacKie K. Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience. 2004;127:101–12.
Pickel VM, Chan J, Kearn CS, Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J Comp Neurol. 2006;495:299–313.
Winters BD, Krüger JM, Huang X, Gallaher ZR, Ishikawa M, Czaja K, et al. Cannabinoid receptor 1-expressing neurons in the nucleus accumbens. Proc Natl Acad Sci USA. 2012;109:2717.
Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–7.
Pan B, Zhong P, Sun D, Liu QS. Extracellular signal-regulated kinase signaling in the ventral tegmental area mediates cocaine-induced synaptic plasticity and rewarding effects. J Neurosci. 2011;31:11244–55.
Tong J, Liu X, Vickstrom C, Li Y, Yu L, Lu Y, et al. The Epac-Phospholipase Cε pathway regulates endocannabinoid signaling and cocaine-induced disinhibition of ventral tegmental area dopamine neurons. J Neurosci. 2017;37:3030–44.
Engi SA, Beebe EJ, Ayvazian VM, Cruz FC, Cheer JF, Wenzel JM, et al. Cocaine-induced increases in motivation require 2-arachidonoylglycerol mobilization and CB1 receptor activation in the ventral tegmental area. Neuropharmacology. 2021;193:108625.
Hillard CJ, Edgemond WS, Campbell WB. Characterization of ligand binding to the cannabinoid receptor of rat brain membranes using a novel method: application to anandamide. J neurochemistry. 1995;64:677–83.
Hill MN, Patel S, Carrier EJ, Rademacher DJ, Ormerod BK, Hillard CJ, et al. Downregulation of endocannabinoid signaling in the hippocampus following chronic unpredictable stress. Neuropsychopharmacol (N. Y, N. Y). 2005;30:508–15.
Hill MN, Carrier EJ, Ho W-V, Shi L, Patel S, Gorzalka BB, et al. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–6.
McLaughlin RJ, Hill MN, Dang SS, Wainwright SR, Galea LAM, Hillard CJ, et al. Upregulation of CB1 receptor binding in the ventromedial prefrontal cortex promotes proactive stress-coping strategies following chronic stress exposure. Behav Brain Res. 2013;237:333–7.
Adamczyk P, Faron-Górecka A, Kuśmider M, Dziedzicka-Wasylewska M, Papp M, Filip M. Long-lasting increase in [3H]CP55,940 binding to CB1 receptors following cocaine self-administration and its withdrawal in rats. Brain Res. 2012;1451:34–43.
Castro DC, Berridge KC. Opioid hedonic hotspot in nucleus accumbens shell: mu, delta, and kappa maps for enhancement of sweetness “liking” and “wanting”. J Neurosci. 2014;34:4239–50.
Jong JWD, Fraser KM, Lammel S. Mesoaccumbal dopamine heterogeneity: what do dopamine firing and release have to do with it? Annu Rev Neurosci. 2022;45:109–29.
Sanchez-Catalan M, Kaufling J, Georges F, Veinante P, Barrot M. The antero-posterior heterogeneity of the ventral tegmental area. Neuroscience. 2014;282:198–216.
Holly EN, Miczek KA. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacol (Berl). 2015;233:163–86.
Castro DC, Berridge KC. Advances in the neurobiological bases for food ‘liking’ versus ‘wanting’. Physiol Behav. 2014;136:22–30.
Mahler SV, Smith KS, Berridge KC. Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances ‘liking’ of a sweet reward. Neuropsychopharmacology. 2007;32:2267–78.
Mitchell MR, Berridge KC, Mahler SV. Endocannabinoid-enhanced “liking” in nucleus accumbens shell hedonic hotspot requires endogenous opioid signals. Cannabis Cannabinoid Res. 2018;3:166–70.
Reed MD, Hildebrand DGC, Santangelo G, Moffa A, Pira AS, Rycyna L, et al. Assessing contributions of nucleus accumbens shell subregions to reward-seeking behavior. Drug Alcohol Depend. 2015;153:369–73.
Lee DY, Guttilla M, Fung KD, McFeron S, Yan J, Ranaldi R. Rostral–caudal differences in the effects of intra-VTA muscimol on cocaine self-administration. Pharmacol Biochem Behav. 2007;86:542–9.
Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior ventral tegmental area of wistar rats: evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharm Exp Ther. 2005;313:134–45.
Soderman AR, Unterwald EM. Cocaine reward and hyperactivity in the rat: sites of mu opioid receptor modulation. Neuroscience. 2008;154:1506–16.
Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci. 2009;106:4894–9.
Jong JWD, Afjei SA, Dorocic IP, Peck JR, Liu C, Kim CK, et al. A neural circuit mechanism for encoding aversive stimuli in the mesolimbic dopamine system. Neuron. 2019;101:133–51.e7.
Robbe D, Alonso G, Duchamp F, Bockaert J, Manzoni OJ. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J Neurosci. 2001;21:109–16.
Han X, Liang Y, Hempel B, Jordan CJ, Shen H, Bi G, et al. Cannabinoid CB1 receptors are expressed in a subset of dopamine neurons and underlie cannabinoid-induced aversion, hypoactivity, and anxiolytic effects in mice. J Neurosci. 2022;43:373–85.
Secci ME, Mascia P, Sagheddu C, Beggiato S, Melis M, Borelli AC, et al. Astrocytic mechanisms involving kynurenic acid control Δ9-tetrahydrocannabinol-induced increases in glutamate release in brain reward-processing areas. Mol Neurobiol. 2019;56:3563–75.
Zhang L, Zhou Y, Yu Z, Zhang X, Shi J, Shen H. Restoring glutamate homeostasis in the nucleus accumbens via endocannabinoid-mimetic drug prevents relapse to cocaine seeking behavior in rats. Neuropsychopharmacology. 2021;46:970–81.
Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharm. 1998;341:39–44.
Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400.
Hernandez G, Oleson EB, Gentry RN, Abbas Z, Bernstein DL, Arvanitogiannis A, et al. Endocannabinoids promote cocaine-induced impulsivity and its rapid dopaminergic correlates. Biol Psychiatry. 2014;75:487–98.
Solinas M, Justinova Z, Goldberg SR, Tanda G. Anandamide administration alone and after inhibition of fatty acid amide hydrolase (FAAH) increases dopamine levels in the nucleus accumbens shell in rats. J Neurochem. 2006;98:408–19.
Li X, Hoffman AF, Peng XQ, Lupica CR, Gardner EL, Xi ZX. Attenuation of basal and cocaine-enhanced locomotion and nucleus accumbens dopamine in cannabinoid CB1-receptor-knockout mice. Psychopharmacol (Berl). 2009;204:1–11.
Szabo B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–61.
Wang R, Hausknecht KA, Gancarz-Kausch A, Oubraim S, Shen RY, Haj-Dahmane S. Cocaine self-administration abolishes endocannabinoid-mediated long-term depression of glutamatergic synapses in the ventral tegmental area. Eur J Neurosci. 2020;52:4517–24.
Manzoni OJ, Bockaert J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur J Pharm. 2001;412:3.
Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA. 2002;99:8384–8.
Manz KM, Ghose D, Turner BD, Taylor A, Becker J, Grueter CA, et al. Calcium-permeable AMPA receptors promote endocannabinoid signaling at parvalbumin interneuron synapses in the nucleus accumbens core. Cell Rep. 2020;32:107971.
Fourgeaud L, Mato S, Bouchet D, Hémar A, Worley PF, Manzoni OJ. A single in vivo exposure to cocaine abolishes endocannabinoid-mediated long-term depression in the nucleus accumbens. J Neurosci. 2004;24:6939–45.
Conrad KL, McCutcheon JE, Cotterly LM, Ford KA, Beales M, Marinelli M. Persistent increases in cocaine-seeking behavior after acute exposure to cold swim stress. Biol Psychiatry. 2010;68:303–5.
Ball KT, Stone E, Best O, Collins T, Edson H, Hagan E, et al. Chronic restraint stress during withdrawal increases vulnerability to drug priming-induced cocaine seeking via a dopamine D1-like receptor-mediated mechanism. Drug Alcohol Depend. 2018;187:327–34.
Glynn RM, Rosenkranz JA, Wolf ME, Caccamise A, Shroff F, Smith AB, et al. Repeated restraint stress exposure during early withdrawal accelerates incubation of cue-induced cocaine craving. Addict Biol. 2018;23:80–89.
Garcia-Keller C, Smiley C, Monforton C, Melton S, Kalivas PW, Gass J. N-Acetylcysteine treatment during acute stress prevents stress-induced augmentation of addictive drug use and relapse. Addict Biol. 2020;25:e12798.
Schwendt M, Shallcross J, Hadad NA, Namba MD, Hiller H, Wu L, et al. A novel rat model of comorbid PTSD and addiction reveals intersections between stress susceptibility and enhanced cocaine seeking with a role for mGlu5 receptors. Transl Psychiatry. 2018;8:209.
Sutton MA, Karanian DA, Self DW. Factors that determine a propensity for cocaine-seeking behavior during abstinence in rats. Neuropsychopharmacology. 2000;22:626–41.
Baker DA, Tran-Nguyen T, Fuchs RA, Neisewander JL. Influence of individual differences and chronic fluoxetine treatment on cocaine-seeking behavior in rats. Psychopharmacol (Berl). 2001;155:18–26.
Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci. 2006;26:8531–6.
McReynolds JR, Doncheck EM, Vranjkovic O, Ganzman GS, Baker DA, Hillard CJ, et al. CB1 receptor antagonism blocks stress-potentiated reinstatement of cocaine seeking in rats. Psychopharmacol (Berl). 2016;233:99–109.
Kupferschmidt DA, Klas PG, Erb S. Cannabinoid CB1 receptors mediate the effects of corticotropin-releasing factor on the reinstatement of cocaine seeking and expression of cocaine-induced behavioural sensitization. Br J Pharm. 2012;167:196–206.
Ward SJ, Rosenberg M, Dykstra LA, Walker EA. The CB1 antagonist rimonabant (SR141716) blocks cue-induced reinstatement of cocaine seeking and other context and extinction phenomena predictive of relapse. Drug Alcohol Depend. 2009;105:248–55.
Doncheck EM, Liddiard GT, Konrath CD, Liu X, Yu L, Urbanik LA, et al. Sex, stress, and prefrontal cortex: influence of biological sex on stress-promoted cocaine seeking. Neuropsychopharmacology. 2020;45:1974–85.
Craft RM, Marusich JA, Wiley JL. Sex differences in cannabinoid pharmacology: a reflection of differences in the endocannabinoid system? Life Sci. 2013;92:476–81.
Ney LJ, Matthews A, Bruno R, Felmingham KL. Modulation of the endocannabinoid system by sex hormones: Implications for posttraumatic stress disorder. Neurosci Biobehav Rev. 2018;94:302–20.
Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on hippocampal CB1 receptors in male and female rats. Behav Brain Res. 2009;203:264–9.
Peterson BM, Martinez LA, Meisel RL, Mermelstein PG. Estradiol impacts the endocannabinoid system in female rats to influence behavioral and structural responses to cocaine. Neuropharmacology. 2016;110:118–24.
Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacol (Berl). 2005;180:169–76.
Covington HE 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacol (Berl). 2001;158:388–98.
Quadros IM, Miczek KA. Two modes of intense cocaine bingeing: increased persistence after social defeat stress and increased rate of intake due to extended access conditions in rats. Psychopharmacol (Berl). 2009;206:109–20.
Comings DE, Muhleman D, Gade R, Johnson P, Verde R, Saucier G, et al. Cannabinoid receptor gene (CNR1): association with i.v. drug use. Mol Psychiatry. 1997;2:161–8.
Ballon N, Leroy S, Roy C, Bourdel MC, Charles-Nicolas A, Krebs MO, et al. (AAT)n repeat in the cannabinoid receptor gene (CNR1): association with cocaine addiction in an African-Caribbean population. Pharmacogenomics J. 2006;6:126–30.
Clarke TK, Bloch PJ, Ambrose-Lanci L, Ferraro TN, Berrettini WH, Kampman KM, et al. Further evidence for association of polymorphisms in the CNR1 gene with cocaine addiction: confirmation in an independent sample and meta-analysis. Addict Biol. 2013;18:702–8.
Zuo L, Kranzler HR, Luo X, Yang BZ, Weiss R, Brady K, et al. Interaction between two independent CNR1 variants increases risk for cocaine dependence in European Americans: a replication study in family-based sample and population-based sample. Neuropsychopharmacology. 2009;34:1504–13.
Herman AI, Kranzler HR, Cubells JF, Gelernter J, Covault J. Association study of the CNR1 gene exon 3 alternative promoter region polymorphisms and substance dependence. Am J Med Genet B Neuropsychiatr Genet. 2006;141b:499–503.
Jing L, Qiu Y, Zhang Y, Li JX. Effects of the cannabinoid CB1 receptor allosteric modulator ORG 27569 on reinstatement of cocaine- and methamphetamine-seeking behavior in rats. Drug Alcohol Depend. 2014;143:251–6.
Nguyen T, Gamage TF, Finlay DB, Decker AM, Langston TL, Barrus D, et al. Development of 3-(4-Chlorophenyl)-1-(phenethyl)urea analogues as allosteric modulators of the cannabinoid Type-1 receptor: RTICBM-189 is brain penetrant and attenuates reinstatement of cocaine-seeking behavior. J Med Chem. 2022;65:257–70.
Acknowledgements
We thank Luke Urbanik for assistance with surgeries.
Funding
This work was supported by National Institute on Drug Abuse Grants R01-DA015758 to JRM, R01-DA038663 to JRM and CJH, and K01-DA045295 to JRMc.
Author information
Authors and Affiliations
Contributions
JRMc, CJH, and JRM designed the project and experiments. JRMc and JRM supervised all of the experiments. JRMc, CPW, and DMS conducted the surgeries and JRMc ran the behavioral experiments with assistance from CPW, DMS, RS, and JCM. CPW and RS completed the histology. JRMc ran the behavior and collected tissue for the CB1R binding assay and CJH supervised and LAK completed the binding assays and analysis of the binding data. JRMc analyzed and graphed all data. JRMc and JRM wrote the first draft of the manuscript with editing and reviewing by CJH. Funding was acquired by JRMc, CJH, and JRM.
Corresponding author
Ethics declarations
Competing interests
JRMc, CPW, DMS, JCM, LAK, and RS declare no potential competing interest. CJH is a member of the scientific board of directors of Phytecs, Inc; has equity in Formulate Biosciences, Inc and has received consultation fees from Pharmavite, LLC. JRM is a co-founder of and has equity in Promentis Pharmaceuticals, Inc.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
McReynolds, J.R., Wolf, C.P., Starck, D.M. et al. Role of mesolimbic cannabinoid receptor 1 in stress-driven increases in cocaine self-administration in male rats. Neuropsychopharmacol. 48, 1121–1132 (2023). https://doi.org/10.1038/s41386-023-01589-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41386-023-01589-1
- Springer Nature Switzerland AG
This article is cited by
-
Novel evidence for endocannabinoid-mediated enhancement of cocaine intake in response to stress
Neuropsychopharmacology (2023)