Key Points
-
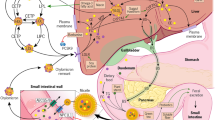
Use of functional foods and dietary supplements is becoming increasingly prevalent among those individuals at risk of cardiovascular disease; however, limited clinical guidance is available for the use of safe and effective supplements
-
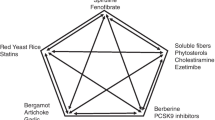
Evidence supports the use of products such as soy protein, green tea, plant sterols, probiotic yogurt, marine-derived omega-3 fatty acids and lovastatin-containing red yeast rice in patients with dyslipidaemia
-
Products such as seaweed, berberine, hawthorn and garlic might confer some limited lipid-lowering benefit in certain patient populations
-
Policosanol, guggulsterone and resveratrol are unlikely to have lipid-lowering effects
-
Functional foods and dietary supplements can be used in addition to pharmacotherapy to provide additional lipid lowering and could potentially reduce medication dose
-
Very few long-term studies have been conducted, which has led to a paucity of information on clinical end points such as mortality and cardiac events
Abstract
Dyslipidaemia is characterized by increased blood levels of total or LDL cholesterol and triglycerides, or decreased HDL cholesterol levels, and is a risk factor for cardiovascular disease. Dyslipidaemia has a high worldwide prevalence, and many patients are turning to alternatives to pharmacotherapy to manage their lipid levels. Lifestyle modification should be emphasized in all patients to reduce cardiovascular risk and can be initiated before pharmacotherapy in primary prevention of cardiovascular disease. Many functional foods and natural health products have been investigated for potential lipid-lowering properties. Those with good evidence for a biochemical effect on plasma lipid levels include soy protein, green tea, plant sterols, probiotic yogurt, marine-derived omega-3 fatty acids and red yeast rice. Other products such as seaweed, berberine, hawthorn and garlic might confer some limited benefit in certain patient groups. Although none of these products can reduce lipid levels to the same extent as statins, most are safe to use in addition to other lifestyle modifications and pharmacotherapy. Natural health products marketed at individuals with dyslipidaemia, such as policosanol, guggulsterone and resveratrol, have minimal definitive evidence of a biochemical benefit. Additional research is required in this field, which should include large, high-quality randomized controlled trials with long follow-up periods to investigate associations with cardiovascular end points.


Similar content being viewed by others
References
World Health Organization. World Health Statistics 2012 http://www.who.int/mediacentre/factsheets/fs310/en/ (2012).
Vazquez-Benitez, G. et al. Preventable major cardiovascular events associated with uncontrolled glucose, blood pressure, and lipids and active smoking in adults with diabetes with and without cardiovascular disease: a contemporary analysis. Diabetes Care 38, 905–912 (2015).
Hubert, H. B., Feinleib, M., McNamara, P. M. & Castelli, W. P. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 67, 968–977 (1983).
Tóth, P. P., Potter, D. & Ming, E. E. Prevalence of lipid abnormalities in the United States: the National Health and Nutrition Examination Survey 2003–2006. J. Clin. Lipidol. 6, 325–330 (2012).
Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 360, 7–22 (2002).
Law, M. R., Wald, N. J. & Rudnicka, A. R. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 326, 1423 (2003).
Hegele, R. A. Plasma lipoproteins: genetic influences and clinical implications. Nat. Rev. Genet. 10, 109–121 (2009).
Miller, M. et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333 (2011).
National Cholesterol Education Program (NECP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106, 3143–3421 (2002).
Eckel, R. H. et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 63, 2960–2984 (2014).
Martirosyan, D. M. & Singh, J. A new definition of functional food by FFC: what makes a new definition unique? Funct. Foods Health Dis. 5, 209–223 (2015).
Department of Health & Human Services, US Food & Drug Administration. What is a dietary supplement? http://www.fda.gov/AboutFDA/Transparency/Basics/ucm195635.htm (2015).
Dickinson, A., Blatman, J., El-Dash, N. & Franco, J. C. Consumer usage and reasons for using dietary supplements: report of a series of surveys. J. Am. Coll. Nutr. 33, 176–182 (2014).
Ellegård, L. & Andersson, H. Oat bran rapidly increases bile acid excretion and bile acid synthesis: an ileostomy study. Eur. J. Clin. Nutr. 61, 938–945 (2007).
Chen, W. J., Anderson, J. W. & Jennings, D. Propionate may mediate the hypocholesterolemic effects of certain soluble plant fibers in cholesterol-fed rats. Proc. Soc. Exp. Biol. Med. 175, 215–218 (1984).
Whitehead, A., Beck, E. J., Tosh, S. & Wolever, T. M. Cholesterol-lowering effects of oat β-glucan: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 100, 1413–1421 (2014).
Goff, L. M., Cowland, D. E., Hooper, L. & Frost, G. S. Low glycaemic index diets and blood lipids: a systematic review and meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 23, 1–10 (2013).
Talati, R., Baker, W. L., Pabilonia, M. S., White, C. M. & Coleman, C. I. The effects of barley-derived soluble fiber on serum lipids. Ann. Fam. Med. 7, 157–163 (2009).
Wei, Z. H. et al. Time- and dose-dependent effect of psyllium on serum lipids in mild-to-moderate hypercholesterolemia: a meta-analysis of controlled clinical trials. Eur. J. Clin. Nutr. 63, 821–827 (2009).
Anderson, J. W. et al. Cholesterol-lowering effects of psyllium intake adjunctive to diet therapy in men and women with hypercholesterolemia: meta-analysis of 8 controlled trials. Am. J. Clin. Nutr. 71, 472–479 (2000).
Hartley, L., May, M. D., Loveman, E., Colquitt, J. L. & Rees, K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. http://dx.doi.org/10.1002/14651858.CD011472.pub2 (2016).
Anderson, T. J. et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can. J. Cardiol. 29, 151–167 (2013).
Demonty, I. et al. Continuous dose-response relationship of the LDL-cholesterol-lowering effect of phytosterol intake. J. Nutr. 139, 271–284 (2009).
Normén, L., Dutta, P., Lia, A. & Andersson, H. Soy sterol esters and β-sitostanol ester as inhibitors of cholesterol absorption in human small bowel. Am. J. Clin. Nutr. 71, 908–913 (2000).
Baker, W. L., Baker, E. L. & Coleman, C. I. The effect of plant sterols or stanols on lipid parameters in patients with type 2 diabetes: a meta-analysis. Diabetes Res. Clin. Pract. 84, e33–e37 (2009).
Malhotra, A. et al. Dietary interventions (plant sterols, stanols, omega-3 fatty acids, soy protein and dietary fibers) for familial hypercholesterolaemia. Cochrane Database Syst. Rev. http://dx.doi.org/10.1002/14651858.CD001918.pub3 (2014).
Demonty, I. et al. The effect of plant sterols on serum triglyceride concentrations is dependent on baseline concentrations: a pooled analysis of 12 randomised controlled trials. Eur. J. Nutr. 52, 153–160 (2013).
Hubacek, J. A., Berge, K. E., Cohen, J. C. & Hobbs, H. H. Mutations in ATP-cassette binding proteins G5 (ABCG5) and G8 (ABCG8) causing sitosterolemia. Hum. Mutat. 18, 359–360 (2001).
AbuMweis, S. S., Marinangeli, C. P., Frohlich, J. & Jones, P. J. Implementing phytosterols into medical practice as a cholesterol-lowering strategy: overview of efficacy, effectiveness, and safety. Can. J. Cardiol. 30, 1225–1232 (2014).
Eslick, G. D., Howe, P. R., Smith, C., Priest, R. & Bensoussan, A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int. J. Cardiol. 136, 4–16 (2009).
Hartweg, J., Farmer, A. J., Perera, R., Holman, R. R. & Neil, H. A. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia 50, 1593–1602 (2007).
Wei, M. Y. & Jacobson, T. A. Effects of eicosapentaenoic acid versus docosahexaenoic acid on serum lipids: a systematic review and meta-analysis. Curr. Atheroscler. Rep. 13, 474–483 (2011).
Hooper, L. et al. Omega 3 fatty acids for prevention and treatment of cardiovascular disease. Cochrane Database Syst. Rev. http://dx.doi.org/10.1002/14651858.CD003177.pub2 (2004).
Berge, K., Musa-Veloso, K., Harwood, M., Hoem, N. & Burri, L. Krill oil supplementation lowers serum triglycerides without increasing low-density lipoprotein cholesterol in adults with borderline high or high triglyceride levels. Nutr. Res. 34, 126–133 (2014).
Ulven, S. M. et al. Metabolic effects of krill oil are essentially similar to those of fish oil but at lower dose of EPA and DHA, in healthy volunteers. Lipids 46, 37–46 (2011).
Shearer, G. C., Savinova, O. V. & Harris, W. S. Fish oil — how does it reduce plasma triglycerides? Biochim. Biophys. Acta 1821, 843–851 (2012).
Marik, P. E. & Varon, J. Omega-3 dietary supplements and the risk of cardiovascular events: a systematic review. Clin. Cardiol. 32, 365–372 (2009).
Weintraub, H. Update on marine omega-3 fatty acids: management of dyslipidemia and current omega-3 treatment options. Atherosclerosis 230, 381–389 (2013).
McCarthy, M. FDA bans red yeast rice product. Lancet 351, 1637 (1998).
Cicero, A. F. et al. Red yeast rice improves lipid pattern, high-sensitivity C-reactive protein, and vascular remodeling parameters in moderately hypercholesterolemic Italian subjects. Nutr. Res. 33, 622–628 (2013).
Becker, D. J., French, B., Morris, P. B., Silvent, E. & Gordon, R. Y. Phytosterols, red yeast rice, and lifestyle changes instead of statins: a randomized, double-blinded, placebo-controlled trial. Am. Heart J. 166, 187–196 (2013).
Guardamagna, O., Abello, F., Baracco, V., Stasiowska, B. & Martino, F. The treatment of hypercholesterolemic children: efficacy and safety of a combination of red yeast rice extract and policosanols. Nutr. Metab. Cardiovasc. Dis. 21, 424–429 (2011).
Lin, C. C., Li, T. C. & Lai, M. M. Efficacy and safety of Monascus purpureus Went rice in subjects with hyperlipidemia. Eur. J. Endocrinol. 153, 679–686 (2005).
Becker, D. J. et al. Simvastatin versus therapeutic lifestyle changes and supplements: randomized primary prevention trial. Mayo Clin. Proc. 83, 758–764 (2008).
Becker, D. J. et al. Red yeast rice for dyslipidemia in statin-intolerant patients: a randomized trial. Ann. Intern. Med. 150, 830–839 (2009).
Halbert, S. C. et al. Tolerability of red yeast rice (2,400 mg twice daily) versus pravastatin (20 mg twice daily) in patients with previous statin intolerance. Am. J. Cardiol. 105, 198–204 (2010).
Hargrove, J. L., Greenspan, P. & Hartle, D. K. Nutritional significance and metabolism of very long chain fatty alcohols and acids from dietary waxes. Exp. Biol. Med. (Maywood) 229, 215–226 (2004).
Castaño, G. et al. Comparison of the efficacy and tolerability of policosanol with atorvastatin in elderly patients with type II hypercholesterolaemia. Drugs Aging 20, 153–163 (2003).
Torres, O. et al. Treatment of hypercholesterolemia in NIDDM with policosanol. Diabetes Care 18, 393–397 (1995).
Francini-Pesenti, F., Beltramolli, D., Dall'acqua, S. & Brocadello, F. Effect of sugar cane policosanol on lipid profile in primary hypercholesterolemia. Phytother. Res. 22, 318–322 (2008).
Chen, J. T., Wesley, R., Shamburek, R. D., Pucino, F. & Csako, G. Meta-analysis of natural therapies for hyperlipidemia: plant sterols and stanols versus policosanol. Pharmacotherapy 25, 171–183 (2005).
Swanson, B. et al. Policosanol for managing human immunodeficiency virus-related dyslipidemia in a medically underserved population: a randomized, controlled clinical trial. Altern. Ther. Health Med. 17, 30–35 (2011).
Francini-Pesenti, F., Brocadello, F., Beltramolli, D., Nardi, M. & Caregaro, L. Sugar cane policosanol failed to lower plasma cholesterol in primitive, diet-resistant hypercholesterolaemia: a double blind, controlled study. Complement. Ther. Med. 16, 61–65 (2008).
Lin, Y. et al. Wheat germ policosanol failed to lower plasma cholesterol in subjects with normal to mildly elevated cholesterol concentrations. Metabolism 53, 1309–1314 (2004).
Dulin, M. F., Hatcher, L. F., Sasser, H. C. & Barringer, T. A. Policosanol is ineffective in the treatment of hypercholesterolemia: a randomized controlled trial. Am. J. Clin. Nutr. 84, 1543–1548 (2006).
Greyling, A., De Witt, C., Oosthuizen, W. & Jerling, J. C. Effects of a policosanol supplement on serum lipid concentrations in hypercholesterolaemic and heterozygous familial hypercholesterolaemic subjects. Br. J. Nutr. 95, 968–975 (2006).
Marinangeli, C. P., Jones, P. J., Kassis, A. N. & Eskin, M. N. Policosanols as nutraceuticals: fact or fiction. Crit. Rev. Food Sci. Nutr. 50, 259–267 (2010).
Kong, W. et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 10, 1344–1351 (2004).
Dong, B., Li, H., Singh, A. B., Cao, A. & Liu, J. Inhibition of PCSK9 transcription by berberine involves down-regulation of hepatic HNF1α protein expression through the ubiquitin-proteasome degradation pathway. J. Biol. Chem. 290, 4047–4058 (2015).
Poirier, S. et al. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J. Biol. Chem. 283, 2363–2372 (2008).
Li, H. et al. Hepatocyte nuclear factor 1α plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J. Biol. Chem. 284, 28885–28895 (2009).
Dong, H., Zhao, Y., Zhao, L. & Lu, F. The effects of berberine on blood lipids: a systemic review and meta-analysis of randomized controlled trials. Planta Med. 79, 437–446 (2013).
Lan, J. et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J. Ethnopharmacol. 161, 69–81 (2015).
Derosa, G., Maffioli, P. & Cicero, A. F. Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin. Biol. Ther. 12, 1113–1124 (2012).
Shi, K. Q. et al. Traditional Chinese medicines benefit to nonalcoholic fatty liver disease: a systematic review and meta-analysis. Mol. Biol. Rep. 39, 9715–9722 (2012).
Yeh, G. Y., Davis, R. B. & Phillips, R. S. Use of complementary therapies in patients with cardiovascular disease. Am. J. Cardiol. 98, 673–680 (2006).
Ha, A. W., Ying, T. & Kim, W. K. The effects of black garlic (Allium satvium) extracts on lipid metabolism in rats fed a high fat diet. Nutr. Res. Pract. 9, 30–36 (2015).
Singh, D. K. & Porter, T. D. Inhibition of sterol 4α-methyl oxidase is the principal mechanism by which garlic decreases cholesterol synthesis. J. Nutr. 136, 759S–764S (2006).
Lin, M. C. et al. Garlic inhibits microsomal triglyceride transfer protein gene expression in human liver and intestinal cell lines and in rat intestine. J. Nutr. 132, 1165–1168 (2002).
Kwon, M. J. et al. Cholesteryl ester transfer protein activity and atherogenic parameters in rabbits supplemented with cholesterol and garlic powder. Life Sci. 72, 2953–2964 (2003).
Mohammadi, A., Bazrafshani, M. R. & Oshaghi, E. A. Effect of garlic extract on some serum biochemical parameters and expression of npc1l1, abca1, abcg5 and abcg8 genes in the intestine of hypercholesterolemic mice. Indian J. Biochem. Biophys. 50, 500–504 (2013).
Malekpour-Dehkordi, Z. et al. S-Allylcysteine, a garlic compound, increases ABCA1 expression in human THP-1 macrophages. Phytother. Res. 27, 357–361 (2013).
Hwang, Y. P. et al. S-Allyl cysteine attenuates free fatty acid-induced lipogenesis in human HepG2 cells through activation of the AMP-activated protein kinase-dependent pathway. J. Nutr. Biochem. 24, 1469–1478 (2013).
Morihara, N., Hino, A., Yamaguchi, T. & Suzuki, J. I. Aged garlic extract suppresses the development of atherosclerosis in apolipoprotein E-knockout mice. J. Nutr. 146, 460S–463S (2016).
Lau, B. H. Suppression of LDL oxidation by garlic compounds is a possible mechanism of cardiovascular health benefit. J. Nutr. 136, 765S–768S (2006).
Khoo, Y. S. & Aziz, Z. Garlic supplementation and serum cholesterol: a meta-analysis. J. Clin. Pharm. Ther. 34, 133–145 (2009).
Ried, K., Toben, C. & Fakler, P. Effect of garlic on serum lipids: an updated meta-analysis. Nutr. Rev. 71, 282–299 (2013).
Zeng, T. et al. A meta-analysis of randomized, double-blind, placebo-controlled trials for the effects of garlic on serum lipid profiles. J. Sci. Food Agr. 92, 1892–1902 (2012).
Reinhart, K. M., Talati, R., White, C. M. & Coleman, C. I. The impact of garlic on lipid parameters: a systematic review and meta-analysis. Nutr. Res. Rev. 22, 39–48 (2009).
Stevinson, C., Pittler, M. H. & Ernst, E. Garlic for treating hypercholesterolemia. A meta-analysis of randomized clinical trials. Ann. Intern. Med. 133, 420–429 (2000).
Kwak, J. S. et al. Garlic powder intake and cardiovascular risk factors: a meta-analysis of randomized controlled clinical trials. Nutr. Res. Pract. 8, 644–654 (2014).
Mahdavi-Roshan, M. et al. Effect of garlic powder tablet on carotid intima–media thickness in patients with coronary artery disease: a preliminary randomized controlled trial. Nutr. Health 22, 143–155 (2013).
Ried, K. Garlic lowers blood pressure in hypertensive individuals, regulates serum cholesterol, and stimulates immunity: An updated meta-analysis and review. J. Nutr. 146, 389S–396S (2016).
Galeone, C., Tavani, A., Pelucchi, C., Negri, E. & La Vecchia, C. Allium vegetable intake and risk of acute myocardial infarction in Italy. Eur. J. Nutr. 48, 120–123 (2009).
Ulbricht, C. et al. Guggul for hyperlipidemia: a review by the Natural Standard Research Collaboration. Complement. Ther. Med. 13, 279–290 (2005).
Urizar, N. L. et al. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 296, 1703–1706 (2002).
Cui, J. et al. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 278, 10214–10220 (2003).
Singh, B. B. et al. Ayurvedic and collateral herbal treatments for hyperlipidemia: a systematic review of randomized controlled trials and quasi-experimental designs. Altern. Ther. Health Med. 13, 22–28 (2007).
Nohr, L. A., Rasmussen, L. B. & Straand, J. Resin from the mukul myrrh tree, guggul, can it be used for treating hypercholesterolemia? A randomized, controlled study. Complement. Ther. Med. 17, 16–22 (2009).
Szapary, P. O. et al. Guggulipid for the treatment of hypercholesterolemia: a randomized controlled trial. JAMA 290, 765–772 (2003).
Kiechl, S. et al. Alcohol consumption and atherosclerosis: what is the relation? Prospective results from the Bruneck Study. Stroke 29, 900–907 (1998).
Baur, J. A. & Sinclair, D. A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506 (2006).
Guo, R. et al. Resveratrol suppresses oxidised low-density lipoprotein-induced macrophage apoptosis through inhibition of intracellular reactive oxygen species generation, LOX-1, and the p38 MAPK pathway. Cell Physiol. Biochem. 34, 603–616 (2014).
Cho, I. J., Ahn, J. Y., Kim, S., Choi, M. S. & Ha, T. Y. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem. Biophys. Res. Commun. 367, 190–194 (2008).
Frankel, E. N., Waterhouse, A. L. & Teissedre, P. L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agr. Food Chem. 43, 890–894 (1995).
Sahebkar, A. Effects of resveratrol supplementation on plasma lipids: a systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 71, 822–835 (2013).
Kumar, B. J. & Joghee, N. M. Resveratrol supplementation in patients with type 2 diabetes mellitus: a prospective, open label, randomized controlled trial. Int. Res. J. Pharm. 4, 245–249 (2013).
Movahed, A. et al. Antihyperglycemic effects of short term resveratrol supplementation in type 2 diabetic patients. Evid. Based Complement. Alternat. Med. 2013, 851267 (2013).
Goh, K. P. et al. Effects of resveratrol in patients with type 2 diabetes mellitus on skeletal muscle SIRT1 expression and energy expenditure. Int. J. Sport Nutr. Exerc. Metab. 24, 2–13 (2014).
Ishimwe, N., Daliri, E. B., Lee, B. H., Fang, F. & Du, G. The perspective on cholesterol-lowering mechanisms of probiotics. Mol. Nutr. Food Res. 59, 94–105 (2015).
Asemi, Z. et al. Effect of daily consumption of probiotic yoghurt on lipid profiles in pregnant women: a randomized controlled clinical trial. J. Matern. Fetal Neonatal Med. 25, 1552–1556 (2012).
Sadrzadeh-Yeganeh, H. et al. The effects of probiotic and conventional yoghurt on lipid profile in women. Br. J. Nutr. 103, 1778–1783 (2010).
Fabian, E. & Elmadfa, I. Influence of daily consumption of probiotic and conventional yoghurt on the plasma lipid profile in young healthy women. Ann. Nutr. Metab. 50, 387–393 (2006).
Cho, Y. A. & Kim, J. Effect of probiotics on blood lipid concentrations: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 94, e1714 (2015).
Guo, Z. et al. Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomised controlled trials. Nutr. Metab. Cardiovasc. Dis. 21, 844–850 (2011).
Sun, J. & Buys, N. Effects of probiotics consumption on lowering lipids and CVD risk factors: a systematic review and meta-analysis of randomized controlled trials. Ann. Med. 47, 430–440 (2015).
Holdt, S. L. & Kraan, S. Bioactive compounds in seaweed: functional food applications and legislation. J. Appl. Phycol. 23, 543–597 (2011).
Chen, J., Jiang, Y., Ma, K. Y., Chen, F. & Chen, Z. Y. Microalga decreases plasma cholesterol by down-regulation of intestinal NPC1L1, hepatic LDL receptor, and HMG-CoA reductase. J. Agr. Food Chem. 59, 6790–6797 (2011).
Ku, C. S. et al. Hypolipidemic effect of a blue-green alga (Nostoc commune) is attributed to its nonlipid fraction by decreasing intestinal cholesterol absorption in C57BL/6J mice. J. Med. Food 18, 1214–1222 (2015).
Chen, Z. et al. 24(S)-Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRβ agonist. J. Agr. Food Chem. 62, 6130–6137 (2014).
Kim, M. S., Kim, J. Y., Choi, W. H. & Lee, S. S. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr. Res. Pract. 2, 62–67 (2008).
Panlasigui, L. N., Baello, O. Q., Dimatangal, J. M. & Dumelod, B. D. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac. J. Clin. Nutr. 12, 209–214 (2003).
Kondo, I. et al. Association between food group intake and serum total cholesterol in the Japanese population: NIPPON DATA 80/90. J. Epidemiol. 20 (Suppl. 3), S576–S581 (2010).
Bernstein, A. M., Ding, E. L., Willett, W. C. & Rimm, E. B. A meta-analysis shows that docosahexaenoic acid from algal oil reduces serum triglycerides and increases HDL-cholesterol and LDL-cholesterol in persons without coronary heart disease. J. Nutr. 142, 99–104 (2012).
Zhang, Y., Zhang, L., Geng, Y. & Geng, Y. Hawthorn fruit attenuates atherosclerosis by improving the hypolipidemic and antioxidant activities in apolipoprotein E-deficient mice. J. Atheroscler. Thromb. 21, 119–128 (2014).
Rajendran, S., Deepalakshmi, P. D., Parasakthy, K., Devaraj, H. & Devaraj, S. N. Effect of tincture of Crataegus on the LDL-receptor activity of hepatic plasma membrane of rats fed an atherogenic diet. Atherosclerosis 123, 235–241 (1996).
Zhang, Z., Ho, W. K., Huang, Y. & Chen, Z. Y. Hypocholestolemic activity of hawthorn fruit is mediated by regulation of cholesterol-7α-hydroxylase and acyl CoA: cholesterol acyltransferase. Food Res. Int. 35, 885–891 (2002).
Zhang, Z. et al. Hawthorn fruit is hypolipidemic in rabbits fed a high cholesterol diet. J. Nutr. 132, 5–10 (2002).
Dalli, E. et al. Crataegus laevigata decreases neutrophil elastase and has hypolipidemic effect: a randomized, double-blind, placebo-controlled trial. Phytomedicine 18, 769–775 (2011).
US Department of Agriculture. Beverage choices of US adults https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/6_beverage_choices_adults_0708.pdf (2011).
US Department of Agriculture. USDA database for the flavonoid content of selected foods. Release 2.1 https://www.ars.usda.gov/ARSUserFiles/80400525/Data/Flav/Flav02-1.pdf (2007).
Onakpoya, I., Spencer, E., Heneghan, C. & Thompson, M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr. Metab. Cardiovasc. Dis. 24, 823–836 (2014).
Kim, A. et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J. Am. Diet. Assoc. 111, 1720–1729 (2011).
Zheng, X. X. et al. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta-analysis of 14 randomized controlled trials. Am. J. Clin. Nutr. 94, 601–610 (2011).
Suzuki-Sugihara, N. et al. Green tea catechins prevent low-density lipoprotein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 36, 16–23 (2016).
Abe, I. et al. Green tea polyphenols: novel and potent inhibitors of squalene epoxidase. Biochem. Biophys. Res. Commun. 268, 767–771 (2000).
Koo, S. I. & Noh, S. K. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J. Nutr. Biochem. 18, 179–183 (2007).
Zheng, X. X. et al. Effects of green tea catechins with or without caffeine on glycemic control in adults: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 97, 750–762 (2013).
Hartley, L. et al. Green and black tea for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. http://dx.doi.org/10.1002/14651858.CD009934.pub2 (2013).
Hooper, L. et al. Flavonoids, flavonoid-rich foods, and cardiovascular risk: a meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 88, 38–50 (2008).
Wang, D., Chen, C., Wang, Y., Liu, J. & Lin, R. Effect of black tea consumption on blood cholesterol: a meta-analysis of 15 randomized controlled trials. PLoS ONE 9, e107711 (2014).
Zhao, Y., Asimi, S., Wu, K., Zheng, J. & Li, D. Black tea consumption and serum cholesterol concentration: systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 34, 612–619 (2015).
Tang, J. et al. Tea consumption and mortality of all cancers, CVD and all causes: a meta-analysis of eighteen prospective cohort studies. Br. J. Nutr. 114, 673–683 (2015).
Jenkins, D. J. et al. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J. Nutr. 140, 2302S–2311S (2010).
Wu, Z. Y., Wu, X. K. & Zhang, Y. W. Relationship of menopausal status and sex hormones to serum lipids and blood pressure. Int. J. Epidemiol. 19, 297–302 (1990).
Mullen, E., Brown, R. M., Osborne, T. F. & Shay, N. F. Soy isoflavones affect sterol regulatory element binding proteins (SREBPs) and SREBP-regulated genes in HepG2 cells. J. Nutr. 134, 2942–2947 (2004).
Shukla, A. et al. Isoflavone-poor soy protein alters the lipid metabolism of rats by SREBP-mediated down-regulation of hepatic genes. J. Nutr. Biochem. 18, 313–321 (2007).
Manzoni, C. et al. Subcellular localization of soybean 7S globulin in HepG2 cells and LDL receptor up-regulation by its alpha' constituent subunit. J. Nutr. 133, 2149–2155 (2003).
Yu, D. et al. Association of soy food intake with risk and biomarkers of coronary heart disease in Chinese men. Int. J. Cardiol. 172, e285–e287 (2014).
Zhang, X. et al. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J. Nutr. 133, 2874–2878 (2003).
Anderson, J. W. & Bush, H. M. Soy protein effects on serum lipoproteins: a quality assessment and meta-analysis of randomized, controlled studies. J. Am. Coll. Nutr. 30, 79–91 (2011).
Qin, Y. et al. Isoflavones for hypercholesterolaemia in adults. Cochrane Database Syst. Rev. http://dx.doi.org/10.1002/14651858.CD009518.pub2 (2013).
Reynolds, K. et al. A meta-analysis of the effect of soy protein supplementation on serum lipids. Am. J. Cardiol. 98, 633–640 (2006).
Taku, K. et al. Soy isoflavones lower serum total and LDL cholesterol in humans: a meta-analysis of 11 randomized controlled trials. Am. J. Clin. Nutr. 85, 1148–1156 (2007).
Tokede, O. A., Onabanjo, T. A., Yansane, A., Gaziano, J. M. & Djoussé, L. Soya products and serum lipids: a meta-analysis of randomised controlled trials. Br. J. Nutr. 114, 831–843 (2015).
Yang, B. et al. Systematic review and meta-analysis of soy products consumption in patients with type 2 diabetes mellitus. Asia Pac. J. Clin. Nutr. 20, 593–602 (2011).
Zhan, S. & Ho, S. C. Meta-analysis of the effects of soy protein containing isoflavones on the lipid profile. Am. J. Clin. Nutr. 81, 397–408 (2005).
Jenkins, D. J. et al. Effects of a dietary portfolio of cholesterol-lowering foods versus lovastatin on serum lipids and C-reactive protein. JAMA 290, 502–510 (2003).
Sax, J. K. Dietary supplements are not all safe and not all food: How the low cost of dietary supplements preys on the consumer. Am. J. Law Med. 41, 374–394 (2015).
Acknowledgements
R.A.H. is supported by the Jacob J. Wolfe Distinguished Medical Research Chair, the Edith Schulich Vinet Research Chair in Human Genetics and the Martha G. Blackburn Chair in Cardiovascular Research. He has received operating grants from the Canadian Institutes of Health Research (Foundation Grant), the Heart and Stroke Foundation of Ontario (T-000353) and Genome Canada through Genome Quebec (award 4530).
Author information
Authors and Affiliations
Contributions
P.M.H. and R.A.H. researched data for the article, contributed to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Animal studies on seaweed feeding (PDF 214 kb)
Rights and permissions
About this article
Cite this article
Hunter, P., Hegele, R. Functional foods and dietary supplements for the management of dyslipidaemia. Nat Rev Endocrinol 13, 278–288 (2017). https://doi.org/10.1038/nrendo.2016.210
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.210
- Springer Nature Limited
This article is cited by
-
Genetic study of the causal effect of lipid profiles on insomnia risk: a Mendelian randomization trial
BMC Medical Genomics (2023)
-
Ecological change of the gut microbiota during pregnancy and progression to dyslipidemia
npj Biofilms and Microbiomes (2023)
-
Supplements for Lipid Lowering: What Does the Evidence Show?
Current Cardiology Reports (2023)
-
Therapeutic potential of herbal medicine for the management of hyperlipidemia: latest updates
Environmental Science and Pollution Research (2022)
-
Screening and Characterization of Some Lactobacillaceae for Detection of Cholesterol-Lowering Activities
Probiotics and Antimicrobial Proteins (2022)