Abstract
Aims/hypothesis
To determine the effects of marine-derived n-3 polyunsaturated fatty acids (PUFA) on established and emerging lipid and lipoprotein cardiovascular risk markers in patients with type 2 diabetes.
Materials and methods
We performed a systematic review and meta-analysis of randomised controlled trials comparing dietary or non-dietary intake of n-3 PUFA with placebo in patients with type 2 diabetes by searching databases from 1966 to December 2006. Changes in the following variables were recorded triacylglycerol; total cholesterol; HDL, LDL and VLDL and their subfractions; lipid ratios; apolipoproteins; and cholesterol particle sizes.
Results
There were 23 trials on non-dietary supplementation, involving 1,075 subjects with a mean treatment duration of 8.9 weeks, with sufficient data to permit pooling. Compared with placebo, n-3 PUFA had a statistically significant effect on four outcomes, reducing levels of (1) triacylglycerol (18 trials, 969 subjects) by 25% (mean 0.45 mmol/l; 95% CI −0.58 to −0.32; p < 0.00001); (2) VLDL-cholesterol (7 trials, 238 subjects) by 36% (0.07 mmol/l; 95% CI −0.13 to 0.00; p = 0.04); and (3) VLDL-triacylglycerol (6 trials, 178 subjects) by 39.7% (0.44 mmol/l; 95% CI −0.83 to −0.05; p = 0.03); while slightly increasing LDL (16 trials, 565 subjects) by 5.7% (0.11 mmol/l; 95% CI 0.00 to 0.22; p = 0.05). There were no significant effects on total cholesterol, apolipoproteins, lipid subfractions or ratios.
Conclusions/interpretation
In addition to recognised triacylglycerol-lowering effects, n-3 PUFA supplementation decreases VLDL-cholesterol and VLDL-triacylglycerol, but may have an adverse effect on LDL-cholesterol. Larger and longer term clinical trials are required to conclusively establish the effect of n-3 PUFA on cardiovascular risk markers and outcomes in type 2 diabetic patients.
Similar content being viewed by others
Introduction
Marine-derived n-3 (also known as omega-3) polyunsaturated fatty acids (PUFA have been reported to lower the risk of cardiovascular mortality in high-risk and general populations [1], although recent trials [2, 3] and a meta-analysis [4] have cast doubt on the strength of this evidence. There are limited data about the potential benefits for people with type 2 diabetes [5, 6], but two prospective cohort studies among women showed that the risk of coronary heart disease is much lower among women with type 2 diabetes who consume n-3 PUFA [5, 7]. The characteristic dyslipidaemia associated with type 2 diabetes may account for different treatment effects of n-3 PUFA in this population [8], but there are, as yet, no outcome trials in this group.
Although the effects of n-3 PUFA on triacylglycerol (TG), total cholesterol (TC), HDL-cholesterol and LDL-cholesterol have been reviewed systematically in meta-analyses in patients with [9] and without diabetes [10, 11], the only consistent effect reported in patients with type 2 diabetes is a significant reduction in TG concentrations. However, established cardiovascular disease (CVD) risk markers explain only some of the excess risk of CVD in type 2 diabetes [12], which may partly be due to abnormalities in LDL particle size and HDL subfraction concentrations [13], apolipoprotein concentrations [14] and plasma lipase activity [15]. n-3 PUFA supplementation has been shown to have both beneficial and adverse effects [16] on lipid metabolism but no systematic review has assessed its effect on emerging lipid risk markers for cardiovascular disease.
In a previous systematic review, we considered the effects of n-3 PUFA supplementation on thrombogenic and other emerging markers for cardiovascular disease [17]. Here we examine the effects of marine-derived n-3 PUFA supplementation on emerging and established lipid risk markers in patients with type 2 diabetes in randomised placebo-controlled clinical trials, deriving, where possible, pooled estimates of effect size.
Materials and methods
Study protocol
We searched the Cochrane Register of Controlled Trials from 1986, MEDLINE from 1966, Embase from 1966 and the metaRegister of Controlled Trials up to 10 December 2006 for the terms fish oil, nutrition, diet, n-3 fatty acid, polyunsaturated fatty acid, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as most n-3 PUFA supplements contain only EPA and DHA. A standard search filter was used to identify randomised controlled trials among people with type 2 diabetes [18]. Duplicate publications were counted as individual abstracts and publications, but referenced as part of a single trial throughout the paper. Additional trials were identified by searching references cited in the primary trials identified. We restricted our search to trials in humans and included articles in languages other than English.
No restrictions were placed on duration of the trial. Trials were included if they had a control or comparison arm. Trials were excluded if outcome or change data could not be obtained or if they had multiple risk factor intervention on lifestyle factors other than diet and supplementations, unless the effect of the different interventions could be separated.
Statistical analysis
Criteria for assessment of trial quality included randomisation, method of randomisation described, blinding, blinding/objective measurements described and no loss/loss to follow-up reported. A score from 0 to 5 was given for each criterion of quality met (Table 1), with individual quality components indicated in Electronic supplementary material (ESM) Table 1 [19].
From each trial, where given, the mean change and the SD between intervention and control groups were recorded for changes in TG; TC; HDL, LDL, VLDL and their subfractions; lipid ratios; apolipoproteins; and cholesterol particle sizes. In cross-over design trials, only data from the first intervention period were used. Data from trials that did not provide phase-specific results using the appropriate statistical methods were not included. Where serial measurement of an outcome was given during the intervention phase, changes from baseline to the final measurement were assessed. Where the study used two sets of doses, included comparisons of EPA and DHA or more than one control group, a sensitivity analysis was carried out to determine which comparison gave the smallest effect size [20], which was then reported as the main result. A fixed-effect model with weighted mean difference was used where no heterogeneity was observed [21]; however, results using the standardised effect are shown for comparison in Fig. 1. We evaluated potential publication bias using a funnel plot method.
If the SD of the change between intervention and control group for an outcome was not provided, we derived them where possible from the 95% CI or standard error, assuming a degree of correlation of 0.5 between the beginning and end of each intervention [21]. All analyses were done using Review Manager (Version 4.2.7; Update Software, Oxford, UK).
Results
Description of studies
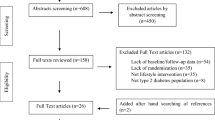
From the electronic searches 886 abstracts were identified, of which 689 papers were excluded on the basis of title and abstract. Of the remaining 197 publications, 164 were excluded because 54 were not randomised, 12 were not placebo-controlled, 46 had multi-factorial interventions from which the effect of n-3 PUFA could not be separated or did not use n-3 PUFA derivatives, 47 included non-type 2 diabetic patients, three did not include human participants and two lacked data or did not report on outcomes that were relevant to this review. In the 34 published papers [22–55] that met the inclusion criteria there were 23 trials of n-3 PUFA supplementation reporting results on 98 established and emerging lipid cardiovascular risk markers and involving 1,075 subjects with type 2 diabetes. The filtering process is indicated in Fig. 2. Table 1 shows the quality score, trial size and dose of n-3 PUFA supplementation used in each trial. The mean dose was 3.5 g/day (median 3 g/day; range 0.9–10 g/day) with a mean intervention duration of 8.9 weeks. The trials included in the pooled analyses were small, with a median of 23 patients. Seventeen of the published papers were duplicate publications of six trials [22, 27, 33, 34, 36–38, 40, 42–46, 48, 51, 54, 55]; multiple papers for each trial are indicated by the following grouping of references: [22, 38], [27, 34, 36, 37], [40, 54, 55], [42, 48, 51], [43, 46], [44, 45]. There was sufficient information on only 12 of the 98 outcomes to allow data to be pooled, the results of which are shown in Fig. 1, while Table 2 lists all the outcomes measured by the respective trials identified in the search.
Trials included both men and women, except for one trial including only men [45]. Two trials excluded pre-menopausal women [51, 54]. One trial compared different preparations of n-3 PUFA to the same control group [54] and another two compared two different control groups with the same n-3 PUFA supplement [29, 50]. Eleven of the trials used a cross-over design, all of these presenting data on the first experimental period [23, 25, 26, 28, 29, 32, 34, 35, 47, 49, 52]. The remaining 12 trials used a parallel design.
Triacylglycerol
Eighteen trials reported changes in TG level among 969 subjects (Fig. 1) [22, 26, 28–32, 34, 35, 39, 41, 45–47, 49–51, 54]. n-3 PUFA lowered TG concentration by 25%, a mean of 0.45 mmol/l (95% CI −0.58, −0.32: p < 0.00001, ESM Fig. 1). Data from five additional trials could not be pooled. Four of these trials reported a reduction in TG after n-3 PUFA supplementation [23–25, 52], while one reported no change [53] (Table 2).
Total cholesterol
Seventeen [22, 26, 28, 30–32, 34, 35, 39, 41, 45–47, 49–51, 54] of 23 trials reported changes in TC among 979 subjects (Fig. 1). n-3 PUFA reduced TC by 0.02 mmol/l (95% CI −0.15 to 0.11; p = 0.80) (ESM Fig. 2). Data from six additional trials could not be pooled. Five of these reported no significant change in TC [23, 29, 45, 52, 53]; one trial reported an increase [24].
High-density lipoprotein cholesterol and subfractions
Sixteen trials reported changes in HDL-cholesterol among 882 subjects [22, 26, 28, 30–32, 34, 35, 39, 41, 46, 47, 49–51, 54] (Fig. 1) n-3 PUFA non-significantly increased HDL-cholesterol by 0.02 mmol/l (95% CI −0.01 to 0.06; p = 0.21) (ESM Fig. 3). Five additional trials that were not pooled reported no significant changes in HDL-cholesterol [23–25, 29, 52] (Table 2).
Three trials reported changes in HDL-2 among 72 non-hypertriacylglycerolaemic subjects [32, 47, 54] (Fig. 1). n-3 PUFA supplementation non-significantly increased HDL-2 by 0.03 mmol/l (95% CI −0.03 to 0.09; p = 0.30) (ESM Fig. 4). Four trials reported changes in HDL-3 among 114 non-hypertriacylglycerolaemic subjects [32, 46, 47, 54]. n-3 PUFA supplementation non-significantly decreased HDL-3 by 0.02 mmol/l (95% CI −0.07 to 0.03; p = 0.42) (ESM Fig. 5). The trials reporting HLD-2 and HDL-3 were of 8 weeks duration or less. Three trials reported changes in HDL-TG among 80 non-hypertriacylglycerolaemic subjects [34, 47, 48]. n-3 PUFA non-significantly decreased HDL-TG by 0.01 mmol/l (95% CI −0.05 to 0.03; p = 0.65) (ESM Fig. 6). Data from two additional trials not pooled showed no change in HDL-TG [23, 52].
Low-density lipoprotein and subfractions
Sixteen trials reported changes in LDL-cholesterol among 565 subjects [22, 26, 28–32, 34, 35, 39, 41, 46–49, 54] (Fig. 1). n-3 PUFA supplementation increased plasma LDL-cholesterol by 5.7%, a mean of 0.11 mmol/l (95% CI 0.00 to 0.22; p = 0.05) (Fig. 1, ESM Fig. 7). Data from six additional trials could not be pooled. Three of these reported an increase in LDL-cholesterol [23, 25, 53] and three reported no change [24, 45, 52] (Table 2).
Four trials reported changes in LDL-TG among 106 non-hypertriacylglycerolaemic subjects [34, 47–49] (Fig. 1). n-3 PUFA increased LDL-TG by 0.01 mmol/l (95%CI −0.03 to 0.06; p = 0.61) (ESM Fig. 8). Data from two additional trials could not be pooled, but reported no significant change in LDL-TG [23, 52] (Table 2).
Four trials reported changes in LDL:HDL ratio among 116 subjects [25, 46, 48, 52] involved in trials lasting longer than 8 weeks (Table 2). However, data from these trials could not be pooled. A non-significant change in the LDL: HDL ratio in the n-3 PUFA compared with the control group was reported in three trials, whereas the remaining trial reported a reduction of 0.7% [46].
Very low density lipoprotein cholesterol and subfractions
Seven trials of 238 subjects reported changes in VLDL-cholesterol [25, 28, 34, 39, 47, 49, 52] (Fig. 1). n-3 PUFA significantly decreased VLDL-cholesterol by 36%, a mean of 0.07 mmol/l (95% CI −0.13 to 0.00; p = 0.04) (ESM Fig. 9). Data from one additional trial could not be pooled, but a significant reduction in VLDL-cholesterol was reported [23].
Six trials reported changes in VLDL-TG among 178 subjects [28, 34, 39, 47–49] (Fig. 1). n-3 PUFA reduced VLDL-TG by 39.7%, a mean of −0.44 mmol/l (95% CI −0.83 to −0.05; p = 0.03) (ESM Fig. 10). Data from three additional trials could not be pooled. Of these, two showed a significant decrease in VLDL-TG through n-3 PUFA supplementation [23, 25] and the other showed no change [52] (Table 2). Funnel plots showed a degree of asymmetrical scattering with VLDL-cholesterol and VLDL-TG.
Apolipoproteins
Three trials reported changes in apolipoprotein B (apoB) among 92 non-hypertriacylglycerolaemic subjects [32, 34, 49] (Fig. 1). n-3 PUFA non-significantly increased apoB by 0.03 g/l (95% CI −0.11 to 0.17; p = 0.67) (ESM Fig. 11). Data from four additional trials could not be pooled, but all reported no change in apoB [25, 46, 52, 53] (Table 2).
Three trials reported changes in apolipoprotein AI (apoAI) among 92 non-hypertriacylglycerolaemic subjects [32, 34, 49] (Fig. 1). n-3 PUFA decreased apoAI by 0.02 g/l (95% CI −0.15 to 0.11; p = 0.78) (ESM Fig. 12). Data from four additional trials [25, 46, 52, 53] could not be pooled, but only one reported a significant decrease in apoAI [25].
Other markers: lipolytic action, ratios and oxidation
Seven trials reported data on 19 additional lipoprotein subfractions, but the data could not be pooled [25, 32, 34, 46, 49, 52, 53] (Table 2). Apolipoprotein A was measured by one trial in ten subjects [32] but no significant change was seen with n-3 PUFA. Apolipoprotein AII was also measured by one trial with 14 patients [52], showing a significant reduction with n-3 PUFA. Lipoprotein lipase was assessed by three trials [32, 42, 53], all showing no change after n-PUFA or compared with control. Two trials [32, 53] measuring lipoprotein(a) could not be pooled due to insufficient data, but both reported no difference between intervention and control groups.
Nine ratio markers reported by eight trials could not be pooled [25, 32, 34, 43, 48–50, 52] (Table 2). apoAI:apoAII was assessed in ten subjects indicating no change with n-3 PUFA [32]. Another of these trials with 44 subjects [43] showed that the LDL:melonaldehyde ratio increased with n-3 PUFA supplementation.
Five trials assessed 11 markers of oxidation [22, 31, 34, 46, 54]. F2 isoprostanes were reduced with n-3 PUFA in a trial of 51 subjects [40], as were lipid peroxides in another of 40 subjects [31] (Table 2). One study measured 27 different lipid particle sizes in 16 subjects [42], showing that n-3 PUFA increased small LDL cholesterol percentage composition. Another two trials showed no difference in LDL particle size with n-3 PUFA [46, 48].
Discussion
Our systematic analysis provides the most comprehensive review of the effects of n-3 PUFA on established and emerging lipid and lipoprotein cardiovascular risk markers in type 2 diabetes. Compared with placebo, n-3 supplementation significantly reduced TGs by 25% (a mean of −0.45 mmol/l), VLDL-cholesterol by 36% (a mean of −0.07 mmol/l) and VLD-TG by 39.7% (a mean of −0.44 mmol/l). There was a modest 5.7% increase in LDL-cholesterol (0.11 mmol/l). n-3 supplementation was not associated with significant changes in plasma cholesterol, HDL-2 and HDL-3, HDL-TG, LDL-TG, LDL:HDL ratio or apolipoprotein concentration.
The limitations of our systematic review include the restricted number of trials that assessed emerging cardiovascular risk markers as outcomes and the small size of trials. Some trials did not describe the methods of randomisation or blinding, so the degree of rigour with which they were conducted was not clear and their results therefore have to be interpreted with caution. It was not possible to pool all the identified outcomes because of non-standardised measurement units, non-reporting of changes in outcomes and non-phase specific data. The funnel plot analysis may indicate bias in reporting for selection or methodology of the trials for VLDL-cholesterol and VLDL-TG. However, we included trials reported in any language to reduce selection and language bias, and an assessment was made of the quality of the trials. Another significant limitation of the reported trials is the short duration of some of them, particularly those investigating lipid sub-fractions. In addition, all of the trials used n-3 PUFA supplementation rather than comparing the effects of different levels of dietary intakes.
We are not aware of other systematic reviews of randomised controlled trials evaluating the effect of marine-derived n-3 PUFA supplementation on emerging lipid markers in type 2 diabetes, although a recent review included patients with diabetes combined with other patient groups in a high-risk group analysis [10]. Three previous systematic reviews evaluated the effect of n-3 PUFA on cardiovascular events, lipid and glycaemic markers in type 2 diabetes [9, 56, 57]. However, we considered additional lipid, lipoprotein and apolipoprotein cardiovascular risk markers, and used changes in the mean from baseline to the end of the trial in the pooled analysis. We have also identified more recently published randomised trials. A fourth systematic review considered the effects of n-3 PUFA on TG, HDL, VLDL and LDL-cholesterol [11] in ten trials including 606 patients with hypertriacylglycerolaemia, but not type 2 diabetes. Three other systematic reviews of effects of n-3 PUFA in the general population [1, 15, 58] considered mortality as their main endpoint but also reported lipid markers. One further systematic review assessed the effect of n-3 PUFA on lipoproteins and their ratios in the general population [59].
Our results are consistent with previous systematic reviews in patients with type 2 diabetes [9, 56, 57] and in the general population [1, 15], showing that TG levels fall significantly after n-3 PUFA supplementation. After adjusting for HDL, increasing levels of TG have been shown to be an independent risk factor for cardiovascular disease in epidemiological studies [60], so lowering of TG levels may be an important therapeutic effect of n-3 PUFA supplementation in patients with type 2 diabetes. TC levels were non-significantly lowered, similar to results of previous systematic reviews in type 2 diabetes [9, 56, 57] and in the general population [1], but were reduced in hypertriacylglycerolaemic patients [11]. HDL was non-significantly increased, as shown in previous systematic reviews of type 2 diabetes [9, 56, 57], in the general population and in type IIa hypercholesterolaemia patients [15]. The observed increase in LDL cholesterol was lower than previously reported [9, 57]. Although it remains a potential adverse effect of n-3 PUFA , this increase of LDL-cholesterol was not observed in pooled randomised controlled trials of hypertriacylglycerolaemic [11] or normolipidaemic patients [1, 15].
The extent to which the HDL and LDL lipid sub-fractions may explain the action of n-3 PUFA in reducing cardiovascular risk and how these differ among people with type 2 diabetes to those in other populations remains uncertain. Both HDL-2 and HDL-3 were non-significantly changed, although HDL-2, unlike HDL-3, showed an increase, contrary to a trial of patients with type II-b hyperlipoproteinaemia, characterised by concomitant elevation of plasma TG and LDL [61]. We found that VLDL-cholesterol levels were significantly reduced, a similar finding to that with pooled results of clinical trials in hypertriacylglycerolaemic patients [11] and one that would be expected as a causal effect of the observed reduction of TGs. A corresponding reduction in VLDL-TG, as we found, is part of the mechanism by which n-3 PUFA reduce serum TGs [62], although in a trial of patients with type 11-b hyperlipoproteinaemia VLDL-TG was increased [61].
Measurements of other lipid-related cardiovascular risk markers, such as LDL:HDL ratios and apolipoproteins, have also been proposed as treatment targets. However, we observed no significant increases in the trials that assessed LDL:HDL ratio after n-3 PUFA in comparison with placebo. Changes in LDL:HDL ratio were not observed in a systematic review of the general population [59], although in a trial on hypertriacylglycerolaemic patients with high TC and TG levels, the ratio was increased [63] as would be expected.
We did not observe a significant effect of n-3 PUFA on apoAI and apoB, which is consistent with the results of a trial in hyperlipidaemic patients [64]. The apoB:apoAI ratio is, according to the findings of the large multicentre study INTERHEART, incrementally related to myocardial infarction [65], but only apoAI:apoAII ratio was measured in one trial in this review [52]. Similarly, there were too few trials to pool and comment further on lipid ratios, lipid particle sizes or oxidation factors, although single markers such as F2 isoprostanes [40] and melonaldehyde [22] were decreased in single trials. Observations made from the Framingham Heart Study [66] suggest that cholesterol ratios rather than TC levels provide a better predictor to reduce CHD risk, and a recent trial stratifying CVD risk factors showed that apolipoproteins are more informative of high CVD risk than lipid ratios alone [67]. However, our results were not conclusive on the possibility of the protective mechanism of n-3 PUFA being exerted via these emerging markers in type 2 diabetes, as very few trials measured apolipoproteins, mostly in small numbers of patients. Beneficial effects of n-3 PUFA on established CVD risk factors would be expected to result in improved outcomes. However, any beneficial effect on emerging lipid markers may not result in a reduction in CVD risk because the prognostic significance of these markers in predicting risk is uncertain [68, 69].
In conclusion, our systematic review demonstrates that n-3 PUFA improves TG, VLDL-cholesterol and VLDL-TG, but not other emerging lipid and lipoprotein risk markers. These results, similar to an earlier systematic review [9, 57], highlight the design limitations of many of the published trials in type 2 diabetes. Rigorously designed and conducted randomised controlled trials are now required, measuring both established and emerging cardiovascular risk markers in type 2 diabetes to enable more comprehensive pooled analyses and improve the precision of the effect size estimates. Larger trials, including clinical endpoint trials, of longer duration are needed to establish the role and mechanisms of n-3 PUFA in CHD risk reduction in type 2 diabetes; indeed, four such trials are currently in progress [70–73].
Abbreviations
- Apo:
-
apolipoprotein
- CVD:
-
cardiovascular disease
- DHA:
-
docosahexaenoic acid
- EPA:
-
eicosapentaenoic acid
- PUFA:
-
polyunsaturated fatty acids
- TC:
-
total cholesterol
- TG:
-
triacylglycerol
References
Bucher HC, Hengstler P, Schindler C, Meier G (2002) n-3 polyunsaturated fatty acids in coronary heart disease: a meta-analysis of randomized controlled trials. Am J Med 112:298–304
Burr ML, Ashfield-Watt PA, Dunstan FD et al (2003) Lack of benefit of dietary advice to men with angina: results of a controlled trial. Eur J Clin Nutr 57:193–200
Raitt MH, Connor WE, Morris C et al (2005) Fish oil supplementation and risk of ventricular tachycardia and ventricular fibrillation in patients with implantable defibrillators: a randomized controlled trial. JAMA 293:2884–2891
Hooper L, Thompson RL, Harrison RA et al (2006) Risks and benefits of omega 3 fats for mortality, cardiovascular disease, and cancer: systematic review. BMJ 332:752–760
Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE (2003) Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 107:1852–1857
Das UN (2000) Beneficial effect(s) of n-3 fatty acids in cardiovascular diseases: but, why and how? Prostaglandins Leukot Essent Fat Acids 63:351–362
Erkkila AT, Lichtenstein AH, Mozaffarian D, Herrington DM (2004) Fish intake is associated with a reduced progression of coronary artery atherosclerosis in postmenopausal women with coronary artery disease. Am J Clin Nutr 80:626–632
Manzella D, Barbieri M, Rizzo MR et al (2001) Role of free fatty acids on cardiac autonomic nervous system in noninsulin-dependent diabetic patients: effects of metabolic control. J Clin Endocrinol Metab 86:2769–2774
Farmer A, Montori V, Dinneen S, Clar C (2001) Fish oil in people with type 2 diabetes mellitus. Cochrane Database Syst Rev:CD003205
Balk E, Chung M, Lichtenstein A et al (2004) Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Evidence Report/Technology Assessment No. 93. Agency for Healthcare Research and Quality, Tufts-New England Medical Center Evidence-based Practice Center, Rockville, MD
Lewis A, Lookinland S, Beckstrand RL, Tiedeman ME (2004) Treatment of hypertriglyceridemia with omega-3 fatty acids: a systematic review. J Am Acad Nurse Pract 16:384–395
Frayn KN (2002) Insulin resistance, impaired postprandial lipid metabolism and abdominal obesity. A deadly triad. Med Princ Pract 11(Suppl 2):31–40
Mori TA, Burke V, Puddey IB et al (2000) Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose, and insulin in mildly hyperlipidemic men. Am J Clin Nutr 71:1085–1094
Mori TA, Vandongen R, Masarei JR (1990) Fish oil-induced changes in apolipoproteins in IDDM subjects. Diabetes Care 13:725–732
Harris WS (1989) Fish oils and plasma lipid and lipoprotein metabolism in humans: a critical review. J Lipid Res 30:785–807
Leigh-Firbank EC, Minihane AM, Leake DS et al (2002) Eicosapentaenoic acid and docosahexaenoic acid from fish oils: differential associations with lipid responses. Br J Nutr 87:435–445
Hartweg J, Farmer A, Holman R, Neil A (2007) Meta-analysis of the effects of omega-3 polyunsaturated fatty acids on haematological and thrombogenic factors in type 2 diabetes. Diabetologia 50:250–258
Dickersin K, Scherer R, Lefebvre C (1994) Identifying relevant studies for systematic reviews. BMJ 309:1286–1291
Jadad AR, Moore RA, Carroll D et al (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17:1–12
Tramer MR, Reynolds DJ, Moore RA, McQuay HJ (1997) Impact of covert duplicate publication on meta-analysis: a case study. BMJ 315:635–640
Rice JA (1995) Mathematical statistics and data analysis, 2nd ed. Duxbury, Belmont
Alekseeva RI, Sharafetdinov K, Plotnikova OA, Meshcheriakova VA, Mal’tsev GI, Kulakova SN (2000) Effects of diet therapy including eiconol on clinical and metabolic parameters in patients with type 2 diabetes mellitus. Vopr Pitan 69:36–39 [Russian]
Annuzzi G, Rivellese A, Capaldo B et al (1991) A controlled study on the effects of n-3 fatty acids on lipid and glucose metabolism in non-insulin-dependent diabetic patients. Atherosclerosis 87:65–73
Axelrod L, Camuso J, Williams E, Kleinman K, Briones E, Schoenfeld D (1994) Effects of a small quantity of omega-3 fatty acids on cardiovascular risk factors in NIDDM. A randomized, prospective, double-blind, controlled study. Diabetes Care 17:37–44
Boberg M, Pollare T, Siegbahn A, Vessby B (1992) Supplementation with n-3 fatty acids reduces triglycerides but increases PAI-1 in non-insulin-dependent diabetes mellitus. Eur J Clin Invest 22:645–650
Borkman M, Chisholm DJ, Furler SM et al (1989) Effects of fish oil supplementation on glucose and lipid metabolism in NIDDM. Diabetes 38:1314–1319
Brennan GM, McVeigh GE, Johnston GD, Hayes JR (1992) Dietary fish oil augments EDRF production or release in patients with non-insulin dependent diabetes mellitus [Abstract]. Br J Clin Pharmacol 33:531
Connor WE, Prince MJ, Ullmann D et al (1993) The hypotriglyceridemic effect of fish oil in adult-onset diabetes without adverse glucose control. Ann N Y Acad Sci 683:337–340
Goh YK, Jumpsen JA, Ryan EA, Clandinin MT (1997) Effect of omega 3 fatty acid on plasma lipids, cholesterol and lipoprotein fatty acid content in NIDDM patients. Diabetologia 40:45–52
Hendra TJ, Britton ME, Roper DR et al (1990) Effects of fish oil supplements in NIDDM subjects. Controlled study. Diabetes Care 13:821–829
Jain S, Gaiha M, Bhattacharjee J, Anuradha S (2002) Effects of low-dose omega-3 fatty acid substitution in type-2 diabetes mellitus with special reference to oxidative stress––a prospective preliminary study. J Assoc Phys India 50:1028–1033
Luo J, Rizkalla SW, Vidal H et al (1998) Moderate intake of n-3 fatty acids for 2 months has no detrimental effect on glucose metabolism and could ameliorate the lipid profile in type 2 diabetic men. Results of a controlled study. Diabetes Care 21:717–724
Maffettone A (1996) Long-term effects (six months) of omega-3 polyunsaturated fatty acids on insulin sensitivity and lipid metabolism in patients with type 2 diabetes and hypertriglyceridemia. G Ital Diabetol 16:185–193
McGrath LT, Brennan GM, Donnelly JP, Johnston GD, Hayes JR, McVeigh GE (1996) Effect of dietary fish oil supplementation on peroxidation of serum lipids in patients with non-insulin dependent diabetes mellitus. Atherosclerosis 121:275–283
McManus RM, Jumpson J, Finegood DT, Clandinin MT, Ryan EA (1996) A comparison of the effects of n-3 fatty acids from linseed oil and fish oil in well-controlled type II diabetes. Diabetes Care 19:463–467
McVeigh GE, Brennan GM, Johnston GD et al (1993) Dietary fish oil augments nitric oxide production or release in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia 36:33–38
McVeigh GE, Brennan GM, Cohn JN, Finkelstein SM, Hayes RJ, Johnston GD (1994) Fish oil improves arterial compliance in non-insulin-dependent diabetes mellitus. Arterioscler Thromb 14:1425–1429
Meshcheriakova VA., Plotnikova OA, Sharafetdinov KH, Alekseeva RI, Mal’tsev GI, Kulakova SN (2001) Comparative study of effects of diet therapy including eiconol or linseed oil on several parameters of lipid metabolism in patients with type 2 diabetes mellitus. Vopr Pitan 70:28–31
Morgan WA, Raskin P, Rosenstock J (1995) A comparison of fish oil or corn oil supplements in hyperlipidemic subjects with NIDDM. Diabetes Care 18:83–86
Mori TA, Woodman RJ, Burke V, Puddey IB, Croft KD, Beilin LJ (2003) Effect of eicosapentaenoic acid and docosahexaenoic acid on oxidative stress and inflammatory markers in treated-hypertensive type 2 diabetic subjects. Free Radic Biol Med 35:772–781
Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V (2006) Effects of n-3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr 84:540–550
Patti L, Maffettone A, Iovine C et al (1999) Long-term effects of fish oil on lipoprotein subfractions and low density lipoprotein size in non-insulin-dependent diabetic patients with hypertriglyceridemia. Atherosclerosis 146:361–367
Pedersen H, Petersen M, Major-Pedersen A et al (2003) Influence of fish oil supplementation on in vivo and in vitro oxidation resistance of low-density lipoprotein in type 2 diabetes. Eur J Clin Nutr 57:713–720
Pelikanova T, Kohout M, Valek J et al (1992) The effect of fish oil on the secretion and effect of insulin in patients with type II diabetes. [Czech]. Cas Lek Ces 131:668–672
Pelikanova T, Kohout M, Valek J, Kazdova L, Base J (1993) Metabolic effects of omega-3 fatty acids in type 2 (non-insulin-dependent) diabetic patients. Ann N Y Acad Sci 683:272–278
Petersen M, Pedersen H, Major-Pedersen A, Jensen T, Marckmann P (2002) Effect of fish oil versus corn oil supplementation on LDL and HDL subclasses in type 2 diabetic patients. Diabetes Care 25:1704–1708
Puhakainen I, Ahola I, Yki-Jarvinen H (1995) Dietary supplementation with n-3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 61:121–126
Rivellese AA, Maffettone A, Iovine C et al (1996) Long-term effects of fish oil on insulin resistance and plasma lipoproteins in NIDDM patients with hypertriglyceridemia. Diabetes Care 19:1207–1213
Schectman G, Kaul S, Kissebah AH (1988) Effect of fish oil concentrate on lipoprotein composition in NIDDM. Diabetes 37:1567–1573
Silvis N, Vorster HH, Mollentze WF, Jager JD, Huisman HW (1988) Metabolic and haemostatic consequences of dietary fibre and n-3 fatty acids in black type 2 (NIDDM) diabetic subjects: a placebo controlled study. Int Clin Nutr Rev 10:362–380
Sirtori CR, Paoletti R, Mancini M et al (1997) N-3 fatty acids do not lead to an increased diabetic risk in patients with hyperlipidemia and abnormal glucose tolerance. Italian Fish Oil Multicenter Study. Am J Clin Nutr 65:1874–1881
Vessby B, Boberg M (1990) Dietary supplementation with n-3 fatty acids may impair glucose homeostasis in patients with non-insulin-dependent diabetes mellitus. J Intern Med 228:165–171
Westerveld HT, de Graaf JC, van Breugel HH et al (1993) Effects of low-dose EPA-E on glycemic control, lipid profile, lipoprotein(a), platelet aggregation, viscosity, and platelet and vessel wall interaction in NIDDM. Diabetes Care 16:683–688
Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ (2002) Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 76:1007–1015
Woodman RJ, Mori TA, Burke V et al (2003) Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis 166:85–93
Friedberg CE, Janssen MJ, Heine RJ, Grobbee DE (1998) Fish oil and glycemic control in diabetes. A meta-analysis. Diabetes Care 21:494–500
Montori VM, Farmer A, Wollan PC, Dinneen SF (2000) Fish oil supplementation in type 2 diabetes: a quantitative systematic review. Diabetes Care 23:1407–1415
Studer M, Briel M, Leimenstoll B, Glass TR, Bucher HC (2005) Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med 165:725–730
Mensink RP, Katan MB (1992) Effect of dietary fatty acids on serum lipids and lipoproteins. A meta-analysis of 27 trials. Arterioscler Thromb 12:911–919
Hokanson JE, Austin MA (1996) Plasma triglyceride level is a risk factor for cardiovascular disease independent of high-density lipoprotein cholesterol level: a meta-analysis of population-based prospective studies. J Cardiovasc Risk 3:213–219
Sucic M, Katica D, Kovacevic V (1998) Effect of dietary fish supplementation on lipoprotein levels in patients with hyperlipoproteinemia. Coll Antropol 22:77–83
Harris WS, Bulchandani D (2006) Why do omega-3 fatty acids lower serum triglycerides? Curr Opin Lipidol 17:387–393
Stacpoole PW, Alig J, Ammon L, Crockett SE (1989) Dose-response effects of dietary marine oil on carbohydrate and lipid metabolism in normal subjects and patients with hypertriglyceridemia. Metab Clin Exper 38:946–956
Shidfar F, Keshavarz A, Jallali M, Miri R, Eshraghian M (2003) Comparison of the effects of simultaneous administration of vitamin C and omega-3 fatty acids on lipoproteins, apo A-I, apo B, and malondialdehyde in hyperlipidemic patients. Int J Vitam Nutr Res 73:163–170
Yusuf S, Hawken S, Ounpuu S et al (2004) Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 364:937–952
Natarajan S, Glick H, Criqui M, Horowitz D, Lipsitz SR, Kinosian B (2003) Cholesterol measures to identify and treat individuals at risk for coronary heart disease. Am J Prev Med 25:50–57
Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE (2005) Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA 294:326–333
Wang T, Gona P, Larson MG et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med 355:2631–2639
Ware JH (2006) The limitations of risk factors as prognostic tools. N Engl J Med 355:2615–2617
The AFORRD Trial (Atorvastatin in factorial with omega-3 risk reduction in diabetes) (2004). Register for randomised controlled trials: http://www.controlled-trials.com/ISRCTN76737502, last accessed in April 2007
The ORIGIN Trial (Outcome Reduction with Initial Glargine Intervention) (2205). ClinicalTrials gov Identifier: NCT00069784 : http://www.controlled-trials.com/mrct/trial/OMEGA%2D3%7CDIABETES/1059/61673.html, last accessed in April 2007
The ASCEND Trial (2005). Oxford Clinical Trials Service Unit: http://www.ctsu.ox.ac.uk/ascend/, last accessed in April 2007
Galan P, de Bree A, Mennen L et al (2003) Background and rationale of the SU.FOL.OM3 study: double-blind randomized placebo-controlled secondary prevention trial to test the impact of supplementation with folate, vitamin B6 and B12 and/or omega-3 fatty acids on the prevention of recurrent ischemic events in subjects with atherosclerosis in the coronary or cerebral arteries. J Nutr Health Aging 7:428–435
Acknowledgements
Part of this work was accepted as an abstract at the 65th Annual Meeting of the American Diabetes Association in San Diego, July 2005.
Duality of interest
A. J. Farmer, R. R. Holman and H. A. W. Neil have received research support from Solvay Healthcare, Southampton, UK and Pronova Biocare, Oslo, Norway.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hartweg, J., Farmer, A.J., Perera, R. et al. Meta-analysis of the effects of n-3 polyunsaturated fatty acids on lipoproteins and other emerging lipid cardiovascular risk markers in patients with type 2 diabetes. Diabetologia 50, 1593–1602 (2007). https://doi.org/10.1007/s00125-007-0695-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-007-0695-z