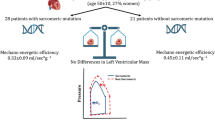
Abstract
Evidence indicates that anatomical and physiological phenotypes of hypertrophic cardiomyopathy (HCM) stem from genetically mediated, inefficient cardiomyocyte energy utilization, and subsequent cellular energy depletion. However, HCM often presents clinically with normal left ventricular (LV) systolic function or hyperkinesia. If energy inefficiency is a feature of HCM, why is it not manifest as resting LV systolic dysfunction? In this Perspectives article, we focus on an idiosyncratic form of reversible systolic dysfunction provoked by LV obstruction that we have previously termed the 'lobster claw abnormality' — a mid-systolic drop in LV Doppler ejection velocities. In obstructive HCM, this drop explains the mid-systolic closure of the aortic valve, the bifid aortic pressure trace, and why patients cannot increase stroke volume with exercise. This phenomenon is characteristic of a broader phenomenon in HCM that we have termed dynamic systolic dysfunction. It underlies the development of apical aneurysms, and rare occurrence of cardiogenic shock after obstruction. We posit that dynamic systolic dysfunction is a manifestation of inefficient cardiomyocyte energy utilization. Systolic dysfunction is clinically inapparent at rest; however, it becomes overt through the mechanism of afterload mismatch when LV outflow obstruction is imposed. Energetic insufficiency is also present in nonobstructive HCM. This paradigm might suggest novel therapies. Other pathways that might be central to HCM, such as myofilament Ca2+ hypersensitivity, and enhanced late Na+ current, are discussed.







Similar content being viewed by others
References
Sherrid, M. et al. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first-line therapy with β-blockade or verapamil. Circ. Heart Fail 6, 694–702 (2013).
O'Mahony, C. et al. The long-term survival and the risks and benefits of implantable cardioverter defibrillators in patients with hypertrophic cardiomyopathy. Heart 98, 116–125 (2012).
Maron, B. J. et al. Implantable cardioverter-defibrillators and prevention of sudden cardiac death in hypertrophic cardiomyopathy. JAMA 298, 405–412 (2007).
Geisterfer-Lowrance, A. A. et al. A molecular basis for familial hypertrophic cardiomyopathy: a β cardiac myosin heavy chain gene missense mutation. Cell 62, 999–1006 (1990).
Thierfelder, L. et al. α-tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: a disease of the sarcomere. Cell 77, 701–712 (1994).
Marston, S. et al. Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ. Res. 105, 219–222 (2009).
van Dijk, S. J. et al. Contractile dysfunction irrespective of the mutant protein in human hypertrophic cardiomyopathy with normal systolic function. Circ. Heart Fail. 5, 36–46 (2012).
Ferrantini, C. et al. Mechanical and energetic consequences of HCM-causing mutations. J. Cardiovasc. Transl. Res. 2, 441–451 (2009).
Witjas-Paalberends, E. R. et al. Faster cross-bridge detachment and increased tension cost in human hypertrophic cardiomyopathy with the R403Q MYH7 mutation. J. Physiol. 592, 3257–3272 (2014).
Redwood, C. S. et al. Properties of mutant contractile proteins that cause hypertrophic cardiomyopathy. Cardiovasc. Res. 44, 20–36 (1999).
Neubauer, S. The failing heart — an engine out of fuel. N. Engl. J. Med. 356, 1140–1151 (2007).
Ashrafian, H. et al. Hypertrophic cardiomyopathy: a paradigm for myocardial energy depletion. Trends Genet. 19, 263–268 (2003).
Crilley, J. G. et al. Hypertrophic cardiomyopathy due to sarcomeric gene mutations is characterized by impaired energy metabolism irrespective of the degree of hypertrophy. J. Am. Coll. Cardiol. 41, 1776–1782 (2003).
Witjas-Paalberends, E. R. et al. Gene-specific increase in the energetic cost of contraction in hypertrophic cardiomyopathy caused by thick filament mutations. Cardiovasc. Res. 103, 248–257 (2014).
Ashrafian, H. et al. Disease pathways and novel therapeutic targets in hypertrophic cardiomyopathy. Circ. Res. 109, 86–96 (2011).
Levine, T. B. & Levine, A. B. Metabolic Syndrome and Cardiovascular Disease 2nd ed (Wiley-Blackwell, 2013).
Jung, W. I. et al. 31PNMR spectroscopy detects metabolic abnormalities in asymptomatic patients with hypertrophic cardiomyopathy. Circulation 97, 2536–2542 (1998).
Singh, S. et al. Cardiac energetic impairment in heart disease and the potential role of metabolic modulators: a review for clinicians. Circ. Cardiovasc. Genet. 7, 720–728 (2014).
Neubauer, S. et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 96, 2190–2196 (1997).
Timmer, S. A. et al. Determinants of myocardial energetics and efficiency in symptomatic hypertrophic cardiomyopathy. Eur. J. Nucl. Med. Mol. Imaging 37, 779–788 (2010).
Ishiwata, S. et al. Mechanical efficiency in hypertrophic cardiomyopathy assessed by positron emission tomography with carbon 11 acetate. Am. Heart J. 133, 497–503 (1997).
Timmer, S. A. & Knaapen, P. Coronary microvascular function, myocardial metabolism, and energetics in hypertrophic cardiomyopathy: insights from positron emission tomography. Eur. Heart J. Cardiovasc. Imaging 14, 95–101 (2013).
Blair, E. et al. Mutations in the γ2 subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum. Mol. Genet. 10, 1215–1220 (2001).
Hardie, D. G. et al. AMP-activated protein kinase — development of the energy sensor concept. J. Physiol. 574, 7–15 (2006).
Merante, F. et al. Maternally inherited hypertrophic cardiomyopathy due to a novel T-to-C transition at nucleotide 9997 in the mitochondrial tRNA(glycine) gene. Am. J. Hum. Genet. 55, 437–446 (1994).
Lucas, D. T. et al. Alterations in mitochondrial function in a mouse model of hypertrophic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 284, H575–H583 (2003).
Unno, K. et al. Relation of functional and morphological changes in mitochondria to myocardial contractile and relaxation reserves in asymptomatic to mildly symptomatic patients with hypertrophic cardiomyopathy. Eur. Heart J. 30, 1853–1862 (2009).
Lodi, R. et al. Deficit of in vivo mitochondrial ATP production in patients with Friedreich ataxia. Proc. Natl Acad. Sci. USA 96, 11492–11495 (1999).
Lodi, R. et al. Cardiac energetics are abnormal in Friedreich ataxia patients in the absence of cardiac dysfunction and hypertrophy: an in vivo31P magnetic resonance spectroscopy study. Cardiovasc. Res. 52, 111–119 (2001).
Bunse, M. et al. Cardiac energetics correlates to myocardial hypertrophy in Friedreich's ataxia. Ann. Neurol. 53, 121–123 (2003).
Lan, F. et al. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 12, 101–113 (2013).
Hoskins, A. C. et al. Normal passive viscoelasticity but abnormal myofibrillar force generation in human hypertrophic cardiomyopathy. J. Mol. Cell. Cardiol. 49, 737–745 (2010).
Matsumura, Y. et al. Left ventricular diastolic function assessed using Doppler tissue imaging in patients with hypertrophic cardiomyopathy: relation to symptoms and exercise capacity. Heart 87, 247–251 (2002).
Spindler, M. et al. Diastolic dysfunction and altered energetics in the αMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J. Clin. Invest. 101, 1775–1783 (1998).
Javadpour, M. M. et al. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J. Clin. Invest. 112, 768–775 (2003).
Günal, N. et al. Heart disease in Friedreich's ataxia: a clinical and echocardiographic study. Acta Paediatr. Jpn 38, 308–311 (1996).
Unverferth, D. V. et al. Morphologic and functional characteristics of the heart in Friedreich's ataxia. Am. J. Med. 82, 5–10 (1987).
Alves, M. L. et al. Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ. Cardiovasc. Genet. 7, 132–143 (2014).
Tong, C. W. et al. Phosphorylation of cardiac myosin-binding protein-C is a critical mediator of diastolic function. Circ. Heart Fail. 8, 582–594 (2015).
Coppini, R. et al. Late sodium current inhibition reverses electromechanical dysfunction in human hypertrophic cardiomyopathy. Circulation 127, 575–584 (2013).
Olivotto, I. et al. Spectrum and clinical significance of systolic function and myocardial fibrosis assessed by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am. J. Cardiol. 106, 261–267 (2010).
Abozguia, K. et al. Left ventricular strain and untwist in hypertrophic cardiomyopathy: relation to exercise capacity. Am. Heart J. 159, 825–832 (2010).
Pelliccia, F. et al. Cumulative exercise-induced left ventricular systolic and diastolic dysfunction in hypertrophic cardiomyopathy. Int. J. Cardiol. 122, 76–78 (2007).
Sakata, K. et al. Exercise-induced systolic dysfunction in patients with non-obstructive hypertrophic cardiomyopathy and mutations in the cardiac troponin genes. Heart 94, 1282–1287 (2008).
Okeie, K. et al. Left ventricular systolic dysfunction during exercise and dobutamine stress in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 36, 856–863 (2000).
Dass, S. et al. Exacerbation of cardiac energetic impairment during exercise in hypertrophic cardiomyopathy: a potential mechanism for diastolic dysfunction. Eur. Heart J. 36, 1547–1554 (2015).
Sherrid, M. V. et al. Mid-systolic drop in left ventricular ejection velocity in obstructive hypertrophic cardiomyopathy — the lobster claw abnormality. J. Am. Soc. Echocardiogr. 10, 707–712 (1997).
Barac, I. et al. Effect of obstruction on longitudinal left ventricular shortening in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 49, 1203–1211 (2007).
Sherrid, M. V. et al. Reflections of inflections in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 54, 212–219 (2009).
Maron, B. J. et al. Dynamic subaortic obstruction in hypertrophic cardiomyopathy: analysis by pulsed Doppler echocardiography. J. Am. Coll. Cardiol. 6, 1–18 (1985).
Conklin, H. M. et al. Biphasic left ventricular outflow and its mechanism in hypertrophic obstructive cardiomyopathy. J. Am. Soc. Echocardiogr. 17, 375–383 (2004).
Breithardt, O. A. et al. Mid systolic septal deceleration in hypertrophic cardiomyopathy: clinical value and insights into the pathophysiology of outflow tract obstruction by tissue Doppler echocardiography. Heart 91, 379–380 (2005).
Ross, J. Afterload mismatch in aortic and mitral valve disease: implications for surgical therapy. J. Am. Coll. Cardiol. 5, 811–826 (1985).
Beyerbacht, H. P. et al. Aortic valve replacement in patients with aortic valve stenosis improves myocardial metabolism and diastolic function. Radiology 219, 637–643 (2001).
Mahmod, M. et al. Myocardial perfusion and oxygenation are impaired during stress in severe aortic stenosis and correlate with impaired energetics and subclinical left ventricular dysfunction. J. Cardiovasc. Magn. Reson. 16, 29 (2014).
Ashrafian, H. et al. Aortic stenosis with impaired ventricular function manifests impaired cardiac metabolism: implications for prognosis and surgical intervention. Heart 95 (Suppl. I), A1–A87 (2009).
Mahmod, M. et al. Myocardial steatosis and left ventricular contractile dysfunction in patients with severe aortic stenosis. Circ. Cardiovasc. Imag. 6, 808–816 (2013).
Lele, S. S. et al. Exercise capacity in hypertrophic cardiomyopathy. Role of stroke volume limitation, heart rate, and diastolic filling characteristics. Circulation. 92, 2886–2894 (1995).
Cannon, R. O. 3rd et al. Effect of surgical reduction of left ventricular outflow obstruction on hemodynamics, coronary flow, and myocardial metabolism in hypertrophic cardiomyopathy. Circulation 79, 766–775 (1989).
Cannon, R. O. 3rd et al. Myocardial ischemia in patients with hypertrophic cardiomyopathy: contribution of inadequate vasodilator reserve and elevated left ventricular filling pressures. Circulation 71, 234–243 (1985).
O'Gara, P. T. et al. Myocardial perfusion abnormalities in patients with hypertrophic cardiomyopathy: assessment with thallium-201 emission computed tomography. Circulation 76, 1214–1223 (1987).
Cecchi, F. et al. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N. Engl. J. Med. 349, 1027–1035 (2003).
Timmer, S. A. et al. Carriers of the hypertrophic cardiomyopathy MYBPC3 mutation are characterized by reduced myocardial efficiency in the absence of hypertrophy and microvascular dysfunction. Eur. J. Heart Fail. 13, 1283–1289 (2011).
Po, J. R. et al. Doppler systolic signal void in hypertrophic cardiomyopathy: apical aneurysm, and severe obstruction without elevated intraventricular velocities. J. Am. Soc. Echocardiogr. 28, 1462–1473 (2015).
Shah, A. et al. Severe symptoms in mid and apical hypertrophic cardiomyopathy. Echocardiography 26, 922–933 (2009).
Maron, M. S. et al. Prevalence, clinical significance and natural history of left ventricular aneurysms in hypertrophic cardiomyopathy. Circulation 18, 1541–1549 (2008).
Dawson, D. K. et al. Tako-tsubo cardiomyopathy: a heart stressed out of energy? JACC Cardiovasc. Imaging 8, 985–987 (2015).
Neil, C. J. et al. Relation of delayed recovery of myocardial function after takotsubo cardiomyopathy to subsequent quality of life. Am. J. Cardiol. 115, 1085–1089 (2015).
Sherrid, M. V. et al. Mechanism of benefit of negative inotropes in obstructive hypertrophic cardiomyopathy. Circulation 97, 41–47 (1998).
Sherrid, M. V. et al. Reversal of acute systolic dysfunction and cardiogenic shock in hypertrophic cardiomyopathy by surgical relief of obstruction. Echocardiography. 28, E174–E179 (2011).
Sasayama, S. et al. Adaptations of the left ventricle to chronic pressure overload. Circ. Res. 38, 172–178 (1976).
Ross, J. Jr. Afterload mismatch and preload reserve: a conceptual framework for the analysis of ventricular function. Prog. Cardiovasc. Dis. 18, 255–264 (1976).
Ross, J. Jr & Braunwald, E. The study of left ventricular function in man by increasing resistance to ventricular ejection with angiotensin. Circulation 26, 739–749 (1964).
Mishiro, Y. et al. Use of angiotensin II stress pulsed tissue Doppler imaging to evaluate regional left ventricular contractility in patients with hypertrophic cardiomyopathy. J. Am. Soc. Echocardiol. 13, 1065–1073 (2000).
Ross, J. Jr et al. Preload, afterload, and the role of afterload mismatch in the descending limb of cardiac function. Eur. J. Cardiol. 4 (Suppl.), 77–86 (1976).
Sirenko, S. G. et al. Differential effect of troponin T mutations on the inotropic responsiveness of mouse hearts — role of myofilament Ca2+ sensitivity increase. J. Physiol. 575, 201–213 (2006).
Knollman, B. C. et al. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J. Biol. Chem. 30, 10039–10048 (2001).
Sequeira, V. et al. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ. Res. 112, 1491–1505 (2013).
Saumarez, R. C. et al. Ventricular fibrillation in hypertrophic cardiomyopathy is associated with increased fractionation of paced right ventricular electrograms. Circulation 86, 467–474 (1992).
O'Mahony, C. et al. The relation of ventricular arrhythmia electrophysiological characteristics to cardiac phenotype and circadian patterns in hypertrophic cardiomyopathy. Europace 14, 724–733 (2012).
Cha, Y. M. et al. Electrophysiologic manifestations of ventricular tachyarrhythmias provoking appropriate defibrillator interventions in high-risk patients with hypertrophic cardiomyopathy. J. Cardiovasc. Electrophysiol. 18, 483–487 (2007).
Chan, R. H. et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130, 484–495 (2014).
Wagner, J. A. et al. Calcium-antagonist receptors in the atrial tissue of patients with hypertrophic cardiomyopathy. N. Engl. J. Med. 320, 755–761 (1989).
Brush, J. E. Jr et al. Cardiac norepinephrine kinetics in hypertrophic cardiomyopathy. Circulation 79, 836–844 (1989).
Huke, S. & Knollmann, B. C. Increased myofilament Ca2+-sensitivity and arrhythmia susceptibility. J. Mol. Cell. Cardiol. 48, 824–833 (2010).
Baudenbacher, F. et al. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J. Clin. Invest. 118, 3893–3903 (2008).
Huke, S. et al. Focal energy deprivation underlies arrhythmia susceptibility in mice with calcium-sensitized myofilaments. Circ. Res. 112, 1334–1344 (2013).
Danik, S. B. et al. Electrical remodeling contributes to complex tachyarrhythmias in connexin43-deficient mouse hearts. FASEB J. 22, 1204–1212 (2008).
Watkins, H. et al. Inherited cardiomyopathies. N. Engl. J. Med. 364, 1643–1656 (2011).
Green, E. M. et al. A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351, 617–621 (2016).
Olivotto, I. et al. Novel approach targeting the complex pathophysiology of hypertrophic cardiomyopathy: the impact of Late Sodium Current Inhibition on Exercise Capacity in Subjects with Symptomatic Hypertrophic Cardiomyopathy (LIBERTY-HCM) Trial. Circ. Heart Fail. 9, e002764 (2016).
US National Library of Science. ClinicalTrials.govhttp://www.clinicaltrials.gov/ct2/show/NCT02291237 (2016).
Ormerod, J. O. et al. Impaired energetics in heart failure — a new therapeutic target. Pharmacol. Ther. 119, 264–274 (2008).
Tardiff, J. et al. Targets for therapy in sarcomeric cardiomyopathies. Cardiovasc. Res. 105, 457–470 (2015).
Abozguia, K. et al. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation 122, 1562–1569 (2010).
Gehmlich, K. et al. Changes in the cardiac metabolome caused by perhexiline treatment in a mouse model of hypertrophic cardiomyopathy. Mol. Biosyst. 11, 564–573 (2015).
US National Library of Science. ClinicalTrials.govhttp://www.clinicaltrials.gov/ct2/show/NCT01696370 (2013).
US National Library of Science. ClinicalTrials.govhttp://www.clinicaltrials.gov/ct2/show/NCT01721967 (2016).
Ho, C. Y. et al. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: a pilot randomized trial to modify disease expression. JACC Heart Fail. 3, 180–188 (2015).
Author information
Authors and Affiliations
Contributions
All the authors researched data for the article, discussed its content, wrote the manuscript, and reviewed and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
M.P.F. is the inventor of method of use patents for perhexiline in heart muscle diseases; these patents are owned by Heart Metabolics in which he has no financial interest. M.V.S. has provided protocol consultation to Heart Metabolics. J.O.M.O. declares no competing interests.
Rights and permissions
About this article
Cite this article
Ormerod, J., Frenneaux, M. & Sherrid, M. Myocardial energy depletion and dynamic systolic dysfunction in hypertrophic cardiomyopathy. Nat Rev Cardiol 13, 677–687 (2016). https://doi.org/10.1038/nrcardio.2016.98
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.98
- Springer Nature Limited
This article is cited by
-
The triglyceride-glucose index as a potential protective factor for hypertrophic obstructive cardiomyopathy without diabetes: evidence from a two-center study
Diabetology & Metabolic Syndrome (2023)
-
Metabolic characterization of hypertrophic cardiomyopathy in human heart
Nature Cardiovascular Research (2022)
-
Coronary arterial vasculature in the pathophysiology of hypertrophic cardiomyopathy
Pflügers Archiv - European Journal of Physiology (2019)