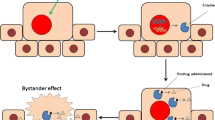
Abstract
Most chemotherapy regimens rely on systemic administration of drugs leading to a wide array of toxicities. Using viral-vector-mediated gene modification of muscle tissues, we have developed a method for gene-directed enzyme prodrug therapy that allows for localized drug administration. An inactive prodrug of geldanamycin was activated locally for inhibition of tumor growth without systemic toxicities. A recombinant adeno-associated virus (rAAV) was used to deliver β-galactosidase (LacZ) to the treatment group and green fluorescent protein to the control group. After 1 week, both groups received adenocarcinoma cells in the same location as the previous rAAV injection. The geldanamycin prodrug was administered 1 h later via intraperitoneal injection. Tumor growth was significantly suppressed in animals whose muscles were gene modified to express β-galactosidase compared with the control. Serum assay to access hepatotoxicity resulted in no significant differences between the animals treated with the inactive or activated form of geldanamycin, indicating minimal damage to non-target organs. Using gene-directed enzyme prodrug therapy, in combination with novel recombinant AAV vectors, we have developed a method for localized activation of chemotherapeutic agents that limits the toxicities seen with traditional systemic administration of these potent drugs.







Similar content being viewed by others
References
Seo SI, Lim SB, Yoon YS, Kim CW, Yu CS, Kim TW et al. Comparison of recurrence patterns between ⩽5 years and >5 years after curative operations in colorectal cancer patients. J Surg Oncol 2013; 108: 9–13.
Heald RJ, Ryall RD . Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986; 1: 1479–1482.
Obrand DI, Gordon PH . Incidence and patterns of recurrence following curative resection for colorectal carcinoma. Dis Colon Rectum 1997; 40: 15–24.
Tjandra JJ, Chan MK . Follow-up after curative resection of colorectal cancer: a meta-analysis. Dis Colon Rectum 2007; 50: 1783–1799.
Tepper OM, Mehrara BJ . Gene therapy in plastic surgery. Plast Reconstr Surg 2002; 109: 716–734.
Michaels J, Dobryansky M, Galiano RD, Ceradini DJ, Bonillas R, Jones D et al. Ex vivo transduction of microvascular free flaps for localized peptide delivery. Ann Plast Surg 2004; 52: 581–584.
Michaels JV, Levine JP, Hazen A, Ceradini DJ, Galiano RD, Soltanian H et al. Biologic brachytherapy: ex vivo transduction of microvascular beds for efficient, targeted gene therapy. Plast Reconstr Surg 2006; 118: 54–65.
Ghali S, Dempsey MP, Jones DM, Grogan RH, Butler PE, Gurtner GC . Plastic surgical delivery systems for targeted gene therapy. Ann Plast Surg 2008; 60: 323–332.
Dempsey MP, Hamou C, Michaels JV, Ghali S, Jazayeri L, Grogan RH et al. Using genetically modified microvascular free flaps to deliver local cancer immunotherapy with minimal systemic toxicity. Plast Reconstr Surg 2008; 121: 1541–1553.
Ghali S, Bhatt KA, Dempsey MP, Jones DM, Singh S, Aarabi S et al. Treating chronic wound infections with genetically modified free flaps. Plast Reconstr Surg 2009; 123: 1157–1168.
Fang L, Sun D . Predictive physiologically based pharmacokinetic model for antibody-directed enzyme prodrug therapy. Drug Metab Dispos 2008; 36: 1153–1165.
Tychopoulos M, Corcos L, Genne P, Beaune P, de Waziers I . A virus-directed enzyme prodrug therapy (VDEPT) strategy for lung cancer using a CYP2B6/NADPH-cytochrome P450 reductase fusion protein. Cancer Gene Ther 2005; 12: 497–508.
Tietze LF, Schmuck K . Prodrugs for targeted tumor therapies: recent developments in ADEPT, GDEPT and PMT. Curr Pharm Des 2011; 17: 3527–3547.
Cheng H, Cao X, Xian M, Fang L, Cai TB, Ji JJ et al. Synthesis and enzyme-specific activation of carbohydrate–geldanamycin conjugates with potent anticancer activity. J Med Chem 2005; 48: 645–652.
Fang L, Battisti RF, Cheng H, Reigan P, Xin Y, Shen J et al. Enzyme specific activation of benzoquinone ansamycin prodrugs using HuCC49DeltaCH2-beta-galactosidase conjugates. J Med Chem 2006; 49: 6290–6297.
During MJ . Adeno-associated virus as a gene delivery system. Adv Drug Delivery Rev 1997; 27: 83–94.
Lawlor PA, Bland RJ, Mouravlev A, Young D, During MJ . Efficient gene delivery and selective transduction of glial cells in the mammalian brain by AAV serotypes isolated from nonhuman primates. Mol Ther 2009; 17: 1692–1702.
Noe F, Frasca A, Balducci C, Carli M, Sperk G, Ferraguti F et al. Neuropeptide Y overexpression using recombinant adeno-associated viral vectors. Neurotherapeutics 2009; 6: 300–306.
Noe F, Vaghi V, Balducci C, Fitzsimons H, Bland R, Zardoni D et al. Anticonvulsant effects and behavioural outcomes of rAAV serotype 1 vector-mediated neuropeptide Y overexpression in rat hippocampus. Gene Therapy 2010; 17: 643–652.
Kaplitt MG, Leone P, Samulski RJ, Xiao X, Pfaff DW, O'Malley KL et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994; 8: 148–154.
Carruthers KH, During MJ, Muravlev A, Wang C, Kocak E . Fat grafting as a vehicle for the delivery of recombinant adenoassociated viral vectors to achieve gene modification of muscle flaps. Ann Plast Surg 2013; 70: 726–731.
Schnell MA, Hardy C, Hawley M, Propert KJ, Wilson JM . Effect of blood collection technique in mice on clinical pathology parameters. Hum Gene Ther 2002; 13: 155–161.
Supko JG, Hickman RL, Grever MR, Malspeis L . Preclinical pharmacologic evaluation of geldanamycin as an antitumor agent. Cancer Chemother Pharmacol 1995; 36: 305–315.
Fernandes BF, Nikolitch K, Coates J, Novais G, Odashiro A, Odashiro PP et al. Local chemotherapeutic agents for the treatment of ocular malignancies. Surv Ophthalmol 2013; 59: 97–114.
Ponnazhagan S . Adenoassociated virus vectors for genetic immunization. Immunol Res 2002; 26: 247–253.
Acknowledgements
We would like to acknowledge Peng George Wang, PhD, of the Ohio State University Department of Chemistry, for his efforts constructing the geldanamycin prodrug (Compound 25). The project described was supported by Pilot Project No. 11876 Grant UL1TR001070 from the National Center For Advancing Translational Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Advancing Translational Sciences or the National Institutes of Health. This project was supported by the National Center for Research Resources, the National Center for Advancing Translational Sciences and the Office of the Director, National Institutes of Health, through UCSF-CTSI Grant Number KL2 (K12) RR024130. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This study supported through the Institutional Research Grant Number IRG-67-003-47 from the American Cancer Society, administered through the Comprehensive Cancer Center at The Ohio State University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors KHC, GM, MJD, AM, CW and EK have no commercial associations or financial disclosures that might pose or create a conflict of interest with information presented in the attached manuscript.
Rights and permissions
About this article
Cite this article
Carruthers, K., Metzger, G., During, M. et al. Gene-directed enzyme prodrug therapy for localized chemotherapeutics in allograft and xenograft tumor models. Cancer Gene Ther 21, 434–440 (2014). https://doi.org/10.1038/cgt.2014.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cgt.2014.47
- Springer Nature America, Inc.
This article is cited by
-
Vector engineering, strategies and targets in cancer gene therapy
Cancer Gene Therapy (2022)