Abstract
Background
Sulfurous mineral water and its main active ingredient sodium hydrosulfide (NaHS) are major sources of H2S. The present study aimed to explore their protective effect on one of the serious long-term complications of diabetes; diabetic nephropathy.
Methods
Sulfurous mineral water (as drinking water), NaHS (14 μmol/kg/day; ip), and gliclazide (10 mg/kg; po) were administered daily for 6 weeks to streptozotocin (STZ)-diabetic rats.
Results
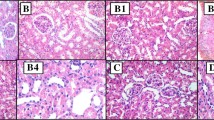
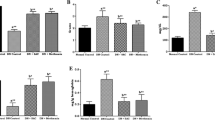
STZ-induced diabetes was associated with body weight reduction, hyperglycemia, overproduction of glycated hemoglobin, as well as decline in serum insulin, C-peptide, and insulin like growth factor-I. Besides, diabetes impaired kidney functions and imposed oxidative and nitrosative stress as manifested by elevated contents of renal thiobarbituric acid reactive substances and nitric oxide, parallel to reduced glutathione content. These deleterious effects were antagonized by sulfurous water and to a better extent by NaHS. Activities of myeloperoxidase and sorbitol dehydrogenase were not altered by STZ or any of the treatments. However, STZ-induced diabetes was accompanied by an increment of aldose reductase which was only mitigated by gliclazide and NaHS. Histopathological examination of kidney sections corroborated the biochemical findings.
Conclusion
This study suggests a novel therapeutic approach for diabetic nephropathy using H2S donors.
Similar content being viewed by others
Abbreviations
- AR:
-
aldose reductase
- HbA1C %:
-
glycated hemoglobin
- IGF-I:
-
insulin like growth factor-I
- MPO:
-
myeloperoxidase
- GSH:
-
reduced glutathione
- NaHS:
-
sodium hydrosulfide
- SD:
-
sorbitol dehydrogenase
- STZ:
-
streptozotocin
- TBARs:
-
thiobarbituric acid reactive substance content
- NOx:
-
total nitrate/nitrite content
References
Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 1999;341(15):1127–33.
Hakim FA, Pflueger A. Role of oxidative stress in diabetic kidney disease. Med Sci Monit 2010;16(2):RA37–48.
Itagaki I, Shimizu K, Kamanaka Y, Ebata K, Kikkawa R, Haneda M, et al. The effect of an aldose reductase inhibitor (epalrestat) on diabetic nephropathy in rats. Diabetes Res Clin Pract 1994;25(3):147–54.
Wang R. Hydrogen sulfide: the third gasotransmitter in biology and medicine. Antioxid Redox Signal 2010;12(9):1061–4.
Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids 2011;41(1):113–21.
Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 2007;6(11):917–35.
Rinaldi L, Gobbi G, Pambianco M, Micheloni C, Mirandola P, Vitale M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab Invest 2006;86(4):391–7.
Lowicka E, Beltowski J. Hydrogen sulfide (H2S) – the third gas of interest for pharmacologists. Pharmacol Rep 2007;59(1):4–24.
Brancaleone V, Roviezzo F, Vellecco V, De Gruttola L, Bucci M, Cirino G. Biosynthesis of H2S is impaired in non-obese diabetic (nod) mice. Br J Pharmacol 2008;155(5):673–80.
Jain SK, Bull R, Rains JL, Bass PF, Levine SN, Reddy S, et al. Low levels of hydrogen sulfide in the blood of diabetes patients and streptozotocin-treated rats causes vascular inflammation? Antioxid Redox Signal 2010;12(11):1333–7.
Gupta AK, Nicol K. The use of sulfur in dermatology. J Drugs Dermatol 2004;3(4):427–31.
Sukenik S, Flusser D, Abu-Shakra M. The role of spa therapy in various rheumatic diseases. Rheum Dis Clin North Am 1999;25(4):883–97.
Costantino M, Giuberti G, Caraglia M, Lombardi A, Misso G, Abbruzzese A, et al. Possible antioxidant role of spa therapy with chlorine–sulphur–bicarbonate mineral water. Amino Acids 2009;36(2):161–5.
Casetta I, Govoni V, Granieri E. Oxidative stress, antioxidants and neurodegenerative diseases. Curr Pharm Des 2005;11(16):2033–52.
Saito M, Kinoshita Y, Satoh I, Shinbori C, Kono T, Hanada T, et al. N-hexacosanol ameliorates streptozotocin-induced diabetic rat nephropathy. Eur J Pharmacol 2006;544(1–3):132–7.
Matthaei S, Horuk R, Olefsky JM. Blood–brain glucose transfer in diabetes mellitus. Decreased number of glucose transporters at blood–brain barrier. Diabetes 1986;35(10):1181–4.
Benedetti S, Benvenuti F, Nappi G, Fortunati NA, Marino L, Aureli T, et al. Antioxidative effects of sulfurous mineral water: protection against lipid and protein oxidation. Eur J Clin Nutr 2009;63(1):106–12.
Geng B, Chang L, Pan C, Qi Y, Zhao J, Pang Y, et al. Endogenous hydrogen sulfide regulation of myocardial injury induced by isoproterenol. Biochem Biophys Res Commun 2004;318(3):756–63.
Attia HN, Al-Rasheed NM, Maklad YA, Ahmed AA, Kenawy SA. Protective effects of combined therapy of gliclazide with curcumin in experimental diabetic neuropathy in rats. Behav Pharmacol 2012;23(2):153–61.
Warenycia MW, Smith KA, Blashko CS, Kombian SB, Reiffenstein RJ. Monoamine oxidase inhibition as a sequel of hydrogen sulfide intoxication: increases in brain catecholamine and 5-hydroxytryptamine levels. Arch Toxicol 1989;63(2):131–6.
Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 1978;86(1):271–8.
Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med 1963;61:882–8.
Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001;5(1):62–71.
Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol 1982;78(3):206–9.
Hoffman PL, Wermuth B, von Wartburg JP. Human brain aldehyde reductases: relationship to succinic semialdehyde reductase and aldose reductase. J Neurochem 1980;35(2):354–66.
Chauncey B, Leite MV, Goldstein L. Renal sorbitol accumulation and associated enzyme activities in diabetes. Enzyme 1988;39(4):231–4.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem 1951;193(1):265–75.
El-Seweidy MM, Sadik NA, Shaker OG. Role of sulfurous mineral water and sodium hydrosulfide as potent inhibitors of fibrosis in the heart of diabetic rats. Arch Biochem Biophys 2011;506(1):48–57.
Xue R, Hao DD, Sun JP, Li WW, Zhao MM, Li XH, et al. Hydrogen sulfide treatment promotes glucose uptake by increasing insulin receptor sensitivity and ameliorates kidney lesions in type 2 diabetes. Antioxid Redox Signal 2013;19(1):5–23.
Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem 1978;253(8):2769–76.
Kook S, Kim GH, Choi K. The antidiabetic effect of onion and garlic in experimental diabetic rats: meta-analysis. J Med Food 2009;12(3):552–60.
Klimek M, Wang S, Ogunkanmi A. Safety and efficacy of red yeast rice (Monascus purpureus) as an alternative therapy for hyperlipidemia. P T 2009;34(6):313–27.
Morsy MD, Hassan WN, Zalat SI. Improvement of renal oxidative stress markers after ozone administration in diabetic nephropathy in rats. Diabetol Metab Syndr 2010;2(1):29.
Xu Y, Osborne BW, Stanton RC. Diabetes causes inhibition of glucose-6-phosphate dehydrogenase via activation of PKA, which contributes to oxidative stress in rat kidney cortex. Am J Physiol Renal Physiol 2005;289(5): F1040–47.
Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes 2008;57(6):1446–54.
Hamilton KL, Butt AG. The molecular basis of renal tubular transport disorders. Comp Biochem Physiol A: Mol Integr Physiol 2000;126(3):305–21.
Forbes JM, Cooper ME, Oldfield MD, Thomas MC. Role of advanced glycation end products in diabetic nephropathy. J Am Soc Nephrol 2003;14(8 Suppl. 3):S254–8.
Karachalias N, Babaei-Jadidi R, Ahmed N, Thornalley PJ. Accumulation of fructosyl-lysine and advanced glycation end products in the kidney, retina and peripheral nerve of streptozotocin-induced diabetic rats. Biochem Soc Trans 2003;31(Pt 6):1423–5.
Ha H, Yu MR, Choi YJ, Kitamura M, Lee HB. Role of high glucose-induced nuclear factor-kappab activation in monocyte chemoattractant protein-1 expression by mesangial cells. J Am Soc Nephrol 2002;13(4):894–902.
Al-Rasheed NM, Willars GB, Brunskill NJ. C-peptide signals via Galpha i to protect against TNF-alpha-mediated apoptosis of opossum kidney proximal tubular cells. J Am Soc Nephrol 2006;17(4):986–95.
Caraglia M, Beninati S, Giuberti G, D’Alessandro AM, Lentini A, Abbruzzese A, et al. Alternative therapy of earth elements increases the chondroprotective effects of chondroitin sulfate in mice. Exp Mol Med 2005;37(5):476–81.
Tripatara P, Patel NS, Brancaleone V, Renshaw D, Rocha J, Sepodes B, et al. Characterisation of cystathionine gamma-lyase/hydrogen sulphide pathway in ischaemia/reperfusion injury of the mouse kidney: an in vivo study. Eur J Pharmacol 2009;606(1–3):205–9.
Jung KJ, Jang HS, Kim JI, Han SJ, Park JW, Park KM. Involvement of hydrogen sulfide and homocysteine transsulfuration pathway in the progression of kidney fibrosis after ureteral obstruction. Biochim Biophys Acta 2013;1832(12): 1989–97.
Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, et al. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med 2003;24(1–3):39–52.
Liu CT, Wong PL, Lii CK, Hse H, Sheen LY. Antidiabetic effect of garlic oil but not diallyl disulfide in rats with streptozotocin-induced diabetes. Food Chem Toxicol 2006;44(8):1377–84.
Nandhini TA, Anuradha CV. Inhibition of lipid peroxidation, protein glycation and elevation of membrane ion pump activity by taurine in RBC exposed to high glucose. Clin Chim Acta 2003;336(1–2):129–35.
Dominguez C, Ruiz E, Gussinye M, Carrascosa A. Oxidative stress at onset and in early stages of type 1 diabetes in children and adolescents. Diabetes Care 1998;21(10):1736–42.
Drel VR, Pacher P, Stevens MJ, Obrosova IG. Aldose reductase inhibition counteracts nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells. Free Radic Biol Med 2006;40(8):1454–65.
Sung JK, Koh JH, Lee MY, Kim BH, Nam SM, Kim JH, et al. Aldose reductase inhibitor ameliorates renal vascular endothelial growth factor expression in streptozotocin-induced diabetic rats. Yonsei Med J 2010;51(3): 385–91.
Kicic E, Palmer TN. Is sorbitol dehydrogenase gene expression affected by streptozotocin-diabetes in the rat? Biochim Biophys Acta 1994;1226(2): 213–8.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Safar, M.M., Abdelsalam, R.M. H2S donors attenuate diabetic nephropathy in rats: Modulation of oxidant status and polyol pathway. Pharmacol. Rep 67, 17–23 (2015). https://doi.org/10.1016/j.pharep.2014.08.001
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1016/j.pharep.2014.08.001