Abstract
The purpose of the study was to investigate the impact of microbes [symbiotic bacteria viz. Rhizobium (Frank) and the arbuscular mycorrhizal fungi (AMF) viz. Funneliformis caledonius (Nicolson & Gerd.) and Glomus bagyarajii Mehrotra] on the growth and physiology of lentil (Lens culinaris Medik.) cultivated in soil alone and soil amended with fly ash. The experiment had twenty-four treatments, twelve in sterilized soil and twelve in unsterilized soil (with six treatments in soil alone and six in soil amended with fly ash in both the sets). Amendment of soil with 25% fly ash significantly increased plant growth. Microbial inoculation further increased the plant growth and physiological parameters studied (plant length, fresh and dry weight, chlorophyll and protein content, and nitrate reductase activity). Microbial parameters like nodule number and fresh weight, mycorrhizal root colonization and spore numbers were also significantly higher in plants inoculated with Rhizobium + AMF. Soil amendment with 25% fly ash together with inoculation of Rhizobium + AMF further improved the growth of lentil. Plant heavy metal (Cd, Pb, Zn) content was significantly more in soil amended with fly ash, but microbial inoculation significantly decreased heavy metal uptake. Of the two AM fungus studied F. caledonius proved to be better, resulting in higher plant growth and physiological parameters studied with reduced heavy metal uptake.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Lentil (Lens culinaris Medik.) is an important legume crop as it is the world’s third important cool season grain after chickpea and pea, accounting for 6% of global dry pulse output with an average yield of 926 kg ha−1 between 2010 and 2015 [57]. Lentil is an ancient and widely consumed pulse worldwide. Lentil is rich in complex form of carbohydrates, proteins, amino acids, minerals, vitamins and dietary fibers [47]. Lentil plants get most of their nitrogen (N2) via N2 fixation by forming symbiotic association with Rhizobium in root nodules. Rhizobium transforms atmospheric N2 to ammonia (NH3), which is then taken up by plants in exchange for carbon-containing molecules [17]. Biological N2 fixation alone accounts for about 64% of the total N2 fixed and the rest by abiotic means [22]. Rhizobium also plays an important role in metal phytoremediation, allowing plants to grow and improve soil health in the presence of inhibitory levels of heavy metals [33]. Lentils also form symbiotic association with arbuscular mycorrhizal fungi (AMF) in addition to Rhizobium [25]. Most agricultural crops, shrubs and trees preferentially associate with AMF [12]. AMF increase uptake of diffusion limited nutrients and water, and tolerance to biotic and abiotic stresses [76]. Because of their complementary functions in delivering primary nutrients, AMF and Rhizobium are considered to be the major drivers of legume growth and productivity [75]. Plants associated with more than one symbiont result in higher growth when compared to the individual symbiont because of synergistic benefits. Many studies on legumes, inoculated with AMF and rhizobia have shown significant increase in plant growth as compared to single inoculation with either of them [2, 11, 28,29,30].
Fly ash (FA) is a major solid waste bi-product of thermal power plants and its disposal is a serious management issue. FA consists of several macro- and micronutrients essential for plant growth [6]. It can be utilized in agriculture as a source of nutrients or as a supplement to improve the quality of soils [66]. Therefore, FA supplication in cropland reduces the cost spent on chemical and organic fertilizers. Besides, presence of several beneficial nutrients, FA is deficient in N2 as it is burnt with coal and released in the air. The association of N2 fixing Rhizobium with leguminous crops overcomes this deficiency [58]. Several experiments have been conducted to determine the optimum beneficial quantity of FA to be applied in the croplands as nutrient supplements [4, 35, 69]. Application of 25% FA levels significantly increased plant length, plant biomass, number of leaves and yield of many crops and higher amounts reduced growth performance of plants [35]. In addition to being rich in macro- and micronutrients, FA is also a rich source of heavy metals such as Cd, Pb, Ni and Zn [39]. Several studies [24, 60, 69] have focused on the roles of microorganisms (AMF and Rhizobium) on reducing the uptake of these toxic heavy metals in plants as well as improving plant growth.
Thus, the goal of the present study was to elucidate how a 25% FA addition to soil combined with Rhizobium and AMF (F. caledonius or G. bagyarajii) inoculation affects plant length, fresh and dry weight, photosynthetic pigments, nitrate reductase activity and protein content of lentil crop as well as nodulation, mycorrhizal population along with heavy metal uptake in roots and shoots of lentil.
2 Materials and methods
2.1 Soil preparation and FA application
FA was collected from the Kasimpur Thermal Power Plant in Aligarh (India), air dried, finely powdered, and sieved through a 2 mm mesh before being utilized for soil amendment. The soil for this investigation was taken from the Department of Botany of Aligarh Muslim University, air dried and divided into two parts. One part of the soil was used without fly ash and other half part of the soil was mixed with fly ash in the ratio of 3:1 v/v. Half of each part (FA amended and unamended soil) was autoclaved twice at 121 °C for 20 min each time, with 1-day interval and used as the sterilized substrates for the experiment while the other half part of amended and unamended soil was used without sterilization.
2.2 Preparation of the inoculum
For Rhizobium treatments, 50 g authorized and active culture of the Rhizobium bacteria specific for lentil (Rhizobium leguminosarum) was purchased from the “Krishi Vigyan Kendra” (Agriculture Science Centre, Aligarh, India). 100 ml of distilled water, 10 g of jaggery, and the obtained Rhizobium culture were thoroughly mixed. Seeds were surface sterilized with 0.5% sodium hypochlorite (NaClO) for 15 min and washed thoroughly. Ten such surface-sterilized seeds were then inoculated with 1 g of Rhizobium culture, dried in the shade before being sown in the pots as needed.
Pure cultures of AMF [Funneliformis caledonius (Nicolson & Gerd.) and Glomus bagyarajii Mehrotra] were purchased from Centre for Natural Biological Resources and Community Development (CNBRCD), Bengaluru, Karnataka, India. Before the start of experiment, the purchased AMF cultures were individually multiplied in the Department of Botany. To do this, a 1:1 w/w sand/soil mixture was made in clay pots and autoclaved twice for 20 min at 121 °C, separated by 1 day. Each pot received a layer of the corresponding AMF cultures, which were subsequently covered with a layer of sand/soil mixture. For the purpose of the experiment, the Lolium perenne seeds in these pots were planted, kept watered, and allowed to grow for around 2 months. Following multiplication, addition of 10 g of the corresponding inoculum was made to the potting hole (as per treatments) along with a soil/sand combination containing infected roots and spores and the lentil seeds were sown in each pot to a depth of 0.5 cm.
2.3 Pot experiment
The soil alone and soil supplemented with FA were filled in pots holding 4 kg soil and placed in a randomized block design. Lentil (Lens culinaris Medik. var. KLS-218) as test plant were selected and purchased from Chola Beej Bhandar (Agro company), Aligarh (UP) India. The 12 treatments in sterilized soil are given below. The same 12 treatments were maintained with unsterilized soil totaling to 24 treatments. Each treatment had 3 replications.
Details of the treatments:
(i) Soil (S) | (vii) S with fly ash (FA) |
(ii) S + Rhizobium (R) (S + R) | (viii) FA + Rhizobium (R) (FA + R) |
(iii) S + Funneliformis caledonius (Fc) (S + Fc) | (ix) FA + Funneliformis caledonius (Fc) (FA + Fc) |
(iv) S + Glomus bagyarajii (Gb) (S + Gb) | (x) FA + Glomus bagyarajii (Gb) (FA + Gb) |
(v) S + R + Fc | (xi) FA + R + Fc |
(vi) S + R + Gb | (xii) FA + R + Gb |
The pots were then maintained in a glasshouse of Botany Department, Aligarh Muslim University and watered as and when necessary. Thinning was done when the seedlings had five to six leaves and two plants were maintained in each pot. To assess the effects of FA, AMF and Rhizobium application singly and in combination on plant length, fresh and dry weight, physiology, biochemical content and microbial population at 90 days after sowing (DAS). Heavy metal analysis was performed at the time of harvesting i.e. 120 DAS.
2.4 Growth parameters
One plant from each replicate of each set of treatment was uprooted and washed carefully. A metric scale was used to determine the entire plant length. Plants were weighed individually to determine their fresh weight. Plant’s root nodules were also counted and weighed using electronic balance (AH220A, AHN Germany). The dry weight of plants and the nodules were determined after the samples were dried in an oven at 70 °C for 72 h and dry weight was recorded using electronic balance.
2.5 Photosynthetic pigments
The photosynthetic pigments (chlorophyll-a, chlorophyll-b, total chlorophyll and carotenoid contents) were determined using the standard procedures by Mackinney [44]. 100 mg fresh leaves were crushed with a pestle and mortar in 10 ml of 80% acetone. The extract was filtered through a Whatman No.1 filter paper and final volume of extract was made to 10 ml by adding 80% acetone. A UV–VIS spectrophotometer (T70 UK) was used to determine the O.D. of the supernatant at 663, 645, 510, and 480 nm.
2.6 Estimation of biochemical content
Nitrate reductase (NR) activity and protein content of the plants were determined using the standard procedures Jaworski [32], Lowry et al. [43], respectively. For NR activity, fresh leaves weighing 200 mg were cut into bits and 2.5 ml phosphate buffer (pH 7.5), 0.5 ml potassium nitrate solution, and 2.5 ml of 5% isopropanol were introduced to it. The samples were incubated in a BOD incubator for 2 h at 30 ± 2 °C in the dark. 0.4 ml of the mixture was then transferred into a test tube and 0.3 ml of 1% sulphanilamide solution and 0.02% NED-HCl were included. The combination was left in the test tube for 20 min for maximum color development and was diluted to 5 ml with double distilled water (DDW). Absorbance was read at 540 nm on a UV–VIS spectrophotometer (T70 UK) against a blank reagent.
For protein analysis, 300 mg of leaf tissues were blended in 10 ml of 20% trichloroacetic acid in a pre-cooled mortar and pestle. The leaf samples were then centrifuged for 15 min at 600 rpm. The supernatant was removed. 5.0 ml of 0.1 N NaOH was added to the pellet, agitated, and again centrifuged for 15 min. The protein segments was remove from the supernatant and 5.0 ml alkaline copper solution was given to 0.5 ml protein extract and left to wait for 10 min. The Folin–Ciocalteau reagent was then incorporated into it and mixed. The blue color appeared and the absorbance of solution was read at 660 nm on a UV–VIS spectrophotometer (T70 UK).
2.7 Mycorrhizal parameters
AMF spore numbers from root zone soil samples were determined by wet sieving and decantation procedure [23]. The roots were sliced into 1 cm bits, treated with trypan blue and observed with the help of the microscope to calculate the percentage of mycorrhizal colonization by the method described by Phillips and Hayman [50].
2.8 Heavy metal analysis
Plants were also analyzed for heavy metal content after 120 DAS following the methods described by Allen et al. [8]. The roots and shoots were set ready by washing them with distilled water and drying them in an oven at 75 °C for 3 days. The dried sections were blended into a powdered form. Sample weighing 300 mg were digested in a tri-acid mixture (HNO3/H2SO4/HClO4; 5:1:1 v/v) at a temperature of 80 °C. The treated solution was cooled, diluted with distilled water, and filtered using a Whatman 42 filter paper, before being evaluated for Cd, Cr, Pb, Ni and Zn concentration using atomic absorption spectrophotometer (AAS) (GBC, 932 plus; GBC Scientific Instruments, Braeside, Australia).
2.9 Statistical analysis
The statistical analysis was carried separately for sterilized and unsterilized set of treatments using one-way ANOVA by SPSS software version 17 (SPSS, Chicago, IL, USA). Duncan’s multiple range test (DMRT) was used to determine the significance of differences between the treatments at p < 0.05.
3 Results
3.1 Impact of symbiosis on growth parameters of lentil in FA amended soil
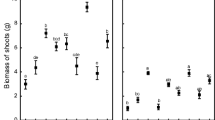
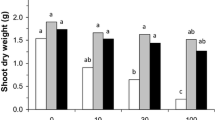
In the present study, the results clearly brought out the positive effect of FA and microbial inoculation on the growth parameters of lentil particularly in sterilized pots as compared to unsterilized ones. Plants modified with 25% FA showed comparatively higher growth parameters (total length, total fresh and dry weight) of lentil than in soil alone. The amendment of only FA showed a notable surge in total length and weight of lentil plants in both autoclaved and unautoclaved sets, as compared to their treatments in soil alone. Additionally, plants when inoculated with both Rhizobium + AMF mainly F. caledonius alongside with FA recorded the maximum growth parameters in lentil plants when compared to their respective single inoculations (Figs. 1, 2 and 3).
Effect of fly ash and microbial inoculation on the total length of lentil (Lens culinaris Medik.) (mean ± SE; n = 3). Bars with different small letters show statistically significant variation at p < 0.05 as per DMRT (Duncan’s multiple range test; analyzed separately for sterilized and unsterilized sets). S: soil; FA: fly ash; R: Rhizobium; Fc: Funneliformis caledonius; Gb: Glomus bagyarajii
Effect of fly ash and microbial inoculation on the fresh weight of lentil (Lens culinaris Medik.) (mean ± SE; n = 3). Bars with different small letters show statistically significant variation at p < 0.05 as per DMRT (analyzed separately for sterilized and unsterilized sets). S: soil; FA: fly ash; R: Rhizobium; Fc: Funneliformis caledonius; Gb: Glomus bagyarajii
Effect of fly ash and microbial inoculation on the dry weight of lentil (Lens culinaris Medik.) (mean ± SE; n = 3). Bars with different small letters show statistically significant variation at p < 0.05 as per DMRT (analyzed separately for sterilized and unsterilized sets). S: soil; FA: fly ash; R: Rhizobium; Fc: Funneliformis caledonius; Gb: Glomus bagyarajii
3.2 Impact of symbiosis on physiological attributes of lentil in FA amended soil
Lentil plants amended with 25% FA showed relatively higher physiological parameters (chlorophyll a, chlorophyll b, total chlorophyll and carotenoid contents) as compared to the soil alone. Amendment with FA was more noticeable when inoculated alongside with Rhizobium + AMF compared to their inoculation in soil alone. The dual inoculation of microbes showed greater photosynthetic contents in FA amended soil when compared to their single inoculation. The higher chlorophyll and carotenoid content in lentil was obtained in plants inoculated with a combination of FA as well as Rhizobium and F. caledonius as compared to the rest of the treatments (Fig. 4a–d).
a–d Effect of fly ash and microbial inoculation on the photosynthetic pigments (chlorophyll ‘a’, chlorophyll ‘b’, total chlorophyll and carotenoid contents of lentil (Lens culinaris Medik.) (mean ± SE; n = 3). Bars with different small letters show statistically significant variation at p < 0.05 as per DMRT (analyzed separately for sterilized and unsterilized sets). S: soil; FA: fly ash; R: Rhizobium; Fc: Funneliformis caledonius; Gb: Glomus bagyarajii
3.3 Impact of symbiosis on biochemical content of lentil in FA amended soil
Application of FA alone in soil significantly enhanced the NR activity and protein content in lentil plants in both sterilized and unsterilized sets, as compared to their respective control (soil alone). In FA amended soil, dual inoculation exhibited higher NR activity and protein content as compared to the single inoculation in amended soil (Figs. 5 and 6).
Effect of fly ash and microbial inoculation on the nitrate reductase activity (NRA) of lentil (Lens culinaris Medik.) (mean ± SE; n = 3). Bars with different small letters show statistically significant variation at p < 0.05 as per DMRT (analyzed separately for sterilized and unsterilized sets). S: soil; FA: fly ash; R: Rhizobium; Fc: Funneliformis caledonius; Gb: Glomus bagyarajii
Effect of fly ash and microbial inoculation on the protein content of lentil (Lens culinaris Medik.) (mean ± SE; n = 3). Bars with different small letters show statistically significant variation at p < 0.05 as per DMRT (analyzed separately for sterilized and unsterilized sets). S: soil; FA: fly ash; R: Rhizobium; Fc: Funneliformis caledonius; Gb: Glomus bagyarajii
3.4 Impact of symbiosis on nodulation and AMF population of lentil in FA amended soil
Effects of varying treatments on nodule counts in lentil are summarized in (Table 1). FA amelioration with AMF and Rhizobium significantly increased nodule count in lentil as compared to soil. Lentil inoculated with Rhizobium and/or AMF increased nodules number in the following order: [(FA + Rhizobium + F. caledonius ≥ FA + Rhizobium + G. bagyarajii) > FA + Rhizobium] > [(Soil + Rhizobium + F. caledonius ≥ Soil + Rhizobium + G. bagyarajii) > Soil + Rhizobium] indicating that tripartite symbiosis of lentil and FA amendment led to highest increase in nodulation than when only Rhizobium was inoculated. Similarly, fresh and dry weight of nodules in lentil increased significantly in tripartite symbiosis with Rhizobium + F. caledonius and Rhizobium + G. bagyarajii in FA amended soil both in sterilized and unsterilized soil. Fresh and dry weight of nodules in 90 DAS old lentil at varying treatments were in the order: [(FA + Rhizobium + F. caledonius ≥ FA + Rhizobium + G. bagyarajii) > FA + Rhizobium] > [(Soil + Rhizobium + F. caledonius ≥ Soil + Rhizobium + G. bagyarajii) > Soil + Rhizobium]. Highest nodule count and nodule fresh and dry weight in lentil were recorded on treatment with FA + Rhizobium + F. caledonius as compared to rest of the treatments.
Spore density in root zone and mycorrhizal root colonization in varying treatments of lentil was in the order: [(FA + Rhizobium + F. caledonius ≥ FA + Rhizobium + G. bagyarajii) > (FA + F. caledonius ≥ FA + G. bagyarajii)] > [(Soil + Rhizobium + F. caledonius ≥ Soil + Rhizobium + G. bagyarajii) > (Soil + F. caledonius ≥ Soil + G. bagyarajii)]. Both the mycorrhizal parameters were significantly higher in the treatment FA + R + Fc but not differing statistically from the treatment FA + R + Gb in both sterilized and unsterilized soil. Also, the mycorrhizal population in sterilized sets was relatively higher as compared to the respective treatments in unsterilized sets (Table 2).
3.5 Impact of symbiosis on heavy metal uptake by lentil roots and shoots in FA amended soil
The result of the concentration of heavy metals (Cd, Cr, Pb, Ni and Zn) present in roots and shoots of lentil are shown in Fig. 7a–c. Use of 25% FA in soil resulted in a significant increase of Cd, Cr, Pb, Ni and Zn in roots and shoots of lentil plants. Inoculation with Rhizobium and either of the selected AMF in FA treated or untreated soil caused higher concentration of heavy metals in roots in comparison to shoots. Single inoculation of microbes also decreased the concentration of heavy metals in roots and shoots of lentil to certain level but the dual inoculation displayed superior tolerance level to these metals in roots and also prevented their translocation to shoots. Maximum response was noted in Rhizobium and F. caledonius treatment in FA amended soil with reference to lower amount of heavy metals in roots and shoots when compared to the other treatments.
Effect of fly ash and microbial inoculation on the heavy metal (Cd, Pb, Zn) accumulation in roots and shoots of lentil (Lens culinaris Medik.) (mean ± SE; n = 3). Bars with different small letters show statistically significant variation at p < 0.05 as per DMRT (analyzed separately for sterilized and unsterilized sets). S: soil; FA: fly ash; R: Rhizobium; Fc: Funneliformis caledonius; Gb: Glomus bagyarajii
4 Discussion
4.1 Impact of symbiosis on growth and photosynthetic attributes of lentil in FA amended soil
In the present study, overall growth performance of lentil is relatively higher in sterilized FA amended soil than in unsterilized sets (Figs. 1, 2 and 3). Significant increases in growth parameters of some plants have been reported in a pathogen free environment carried out in autoclaved sets as reported by earlier study [3]. Higher growth promoting effects in sterilized soil signifies that presence of some unknown biotic stress partially inhibited symbiotic benefits [28]. Furthermore, implementation of nutrient rich FA in soil promoted the growth parameters (total plant length, fresh and dry weight) of lentil. Similar findings have been reported for other crops due to ample availability of nutrients [4, 16, 26]. Increases in biomass of a legume on application of FA in lower amounts have also been documented [52]. Single application of Rhizobium increased the overall growth attributes of lentil crop. Ahmad [5] reported the similar effects of Rhizobium inoculation on the plant growth treated with FA. Furthermore, AMF increasing the uptake of limited nutrients resulting in plant growth stimulation is well documented [12]. Extra-radical mycelium of AMF has been shown to boost nutrient uptake and thereby plant growth and development [41]. Inoculation with Rhizobium + AMF further improved plant growth parameters studied. Similar effects of synergistic interaction between Rhizobium + AMF have been reported in other leguminous crops [28,29,30, 40].
Photosynthetic pigments (chlorophyll a, b, total chlorophyll, and carotenoid) were significantly more in lentil grown in both sterilized and unsterilized soil amended with 25% FA compared to unamended soil (Fig. 4a–d). Pigment content in beetroots significantly increased on application of FA in soil [59]. Application of 10 to 30% FA enhanced photosynthetic activity in plants [37, 68] and these beneficial impacts were attributed to improved nutrients availability in FA amended soil. The increase in chlorophyll content was because of high concentration of Mg in FA [59]. Mg, an essential and nuclear component of the chlorophyll molecule occurs adequately in FA and its uptake enriched the leaves with chlorophyll content [27]. Carotenoids are the auxiliary pigments and functions as non-enzymatic antioxidant to chlorophyll pigment [36]. In the present study, Rhizobium applications influenced photosynthetic pigments along with 25% FA in soil. Chaudhary et al. [15] reported significant enhancement in total chlorophyll and carotenoid content in Rhizobium inoculated Vigna species. Pea (Pisum sativum) inoculated with Rhizobium and grown in soil amended with FA also showed increased chlorophyll and carotenoid [34]. Similarly, the association of plants with AMF significantly enhanced the concentrations of chlorophyll in host plants [13]. AMF inoculated plants had considerably more leaf pigment than non-mycorrhizal plants [7]. Wheat plants inoculated with Glomus mosseae and Glomus fasciculatum also showed increased photosynthetic pigment [9]. Krishna et al. [38] reported increased concentrations of carotenoids in leaves of Vitis vinifera associated with AMF. Baslam et al. [14] also reported that Lactuca sativa L. inoculated with Glomus fasciculatum had a higher carotenoid content. Moreover, co-inoculation of lentil plants with Rhizobium + AMF further improved the photosynthetic activity as documented earlier [28,29,30].
4.2 Impact of symbiosis on the biochemical attributes of lentil in FA amended soil
The results of the present study brought out that addition of FA to soil significantly increased the NR activity and protein content of the plant (Figs. 5 and 6). FA in lower amounts increases NR activity and protein content but higher FA application can reduce these attributes in plants [26]. Shakeel et al. [59] reported that FA application in soil enhanced NR activity and protein contents in beetroots. Ashfaque and Inam [10] also attributed the increase in NR activity and protein synthesis in FA amended soil. Similarly, Mushtaq et al. [45] reported that NR activity in chickpea increased on Rhizobium inoculation under stressed condition. The increase in NR activity resulted from higher uptake of nitrite is due to microbial association [1]. Higher protein content in plants inoculated with Rhizobium has also been reported by earlier [28, 30, 45]. Inoculation of AMF further increased NR activity and protein content. Similar findings were also reported in Talaat and Shawky [65] in Triticum aestivum, Tisserant et al. [67] in Medicago truncatula and Stancheva et al. [64] in Vigna unguiculata. However, dual inoculation with Rhizobium and AMF in Vigna unguiculata and white clover resulted in higher NR activity and protein content than their single applications [64, 73].
4.3 Impact of symbiosis on microbial population of lentil in FA amended soil
Applications of 25% FA with Rhizobium inoculation increased nodulation in lentil grown in sterilized and unsterilized soil (Table 1). In agreement to present finding, Ahmad [5] reported that nodulation in chickpea increased on application of 10% FA in soil. Faizan and Kausar [19] also reported that application of 25% coal ash in soil significantly increased number and dry weight of nodules in Lens culinaris. In addition, dual inoculation of Rhizobium and AMF showed higher nodulation, and the similar results were also observed by Xie et al. [74]. The positive outcome was possible might be because Rhizobium and AMF do not fight for each other’s colonized sites, thereby improving nodulation by adding vigor for nodule development and nitrogen fixation [48].
Applications of either of the species of AM fungi in FA amended soil increased mycorrhizal colonization in lentil as compared to application of AMF in soil without FA (Table 2). Parab et al. [49] reported positive effects of lower levels of FA applications on root colonization and spore counts of inoculated mycorrhizal fungi in onion. Ultra [70] reported that applying compost and FA with mycorrhiza enhanced AMF root colonization in Delonix regia. Ultra and Manyiwa [71] also reported that AM colonization rates were significantly higher in FA-amended mine tailings in three Acacia species. Moreover, dual inoculation enhances AMF colonization as compared to application of AMF alone was also reported earlier [30, 74].
4.4 Impact of symbiosis on heavy metal uptake of lentil in FA amended soil
Uptake of heavy metals in lentil roots and shoots was higher in FA amended soil than soil without FA (Fig. 7a–c). Heavy metals uptake in mung bean also increased on application of 5 to 25% FA [61]. Singh et al. [63] reported that uptake of heavy metals increased significantly with increasing concentrations of FA. Increasing doses of FA led to corresponding increase heavy in metals uptake in plants [46]. On contrary Upadhyay et al. [72] reported that 40% FA supplementation with manure reduced the translocation of toxic metals in edible parts of chickpea. In our study, the order of accumulation of all three heavy metals (Cd, Pb and Zn) in roots and shoots of lentil was Zn > Pb > Cd in respective treatments of microbes and FA (Fig. 7). Similar levels of uptake of heavy metalshas been reported in plants grown in amended soil [31, 46, 62]. On uptake, relatively larger proportion of heavy metals was retained in the roots of lentil than the amounts translocate in shoot. Higher accumulation of heavy metals in roots than shoots might be because roots are the first organs to encounter heavy metals and consequently absorb and retain larger proportions of absorbed heavy metals in roots than partitioning to shoots [21]. In contrast, root to shoot translocation of heavy metals was higher in Typha latifolia [56] grown in FA amended soil. Similarly, Rai et al. [51] reported that Rhizobium inoculated plants of Prosopis juliflora accumulated relatively higher amounts of heavy metals in roots than shoots. Nodule bacteria have very high heavy metal tolerance, accumulation and detoxifying mechanisms [54]. Furthermore, Garg and Chandel [20] reported that Cd concentration in pigeon pea inoculated with Glomus mosseae was remarkably reduced as compared to uninoculated plants. Liu et al. [42] reported that amendment with mycorrhizal fungi significantly reduced Cd accumulation in maize tissues. In line with effects of Rhizobium + AMF, various microbes have been found to reduce heavy metal accumulation in legume plants grown in metal polluted soils as in Lens culinaris [28]; Cicer arietinum [30], Medicago ciliaris [53], Pisum sativum [55] and Vigna radiata [18]. These results suggest that dual inoculation with Rhizobium + AMF would enable lentil to be grown in heavy metal polluted soil.
5 Conclusions
Finally, we may conclude that soil amendments with 25% FA, Rhizobium and selected AM fungi (G. bagyarajii or F. caledonius) either applied singly or in dual or triple combination remarkably enhanced plant growth, photosynthetic pigments, biochemical attributes as well as nodulation and mycorrhizal population in lentil as compared to the soil without any of these amendments. Dual inoculation with Rhizobium and AMF not only further enhanced plant growth but also reduced the uptake of heavy metals, hence assisting lentil plants to effectively cope with heavy metal stress. The improvement in plant growth, biochemical attributes as well as in microbial population was highest in the combined application of FA along Rhizobium and F. caledonius. Among the two selected species of mycorrhizae, F. caledonius was better in stimulating plant development and nutrient uptake as compared to G. bagyarajii. Future prospect of using fly ash in soil as a nutrient enhancer and using these symbionts to control the heavy metals uptake in aerial parts of the plants and improving productivity of the lentil crop for agricultural use can be utilized. In future, a long term research vision should include the use of fly ash that builds on a thorough understanding of fly ash properties and its appropriate doses along with their interactions with soil microorganisms when utilized in agriculture.
Data availability
Data analyzed during this study are included in this article.
Code availability
Not available.
References
Abiala MA, Popoola OO, Olawuyi OJ, Oyelude JO, Akanmu AO, Killani AS, Osonubi O, Odebode AC. Harnessing the potentials of vesicular arbuscular mycorrhizal (VAM) fungi to plant growth—a review. Int J Pure Appl Sci Technol. 2013;14:61–79.
Afkhami ME, Stinchcombe JR. Multiple mutualist effects on genomewide expression in the tripartite association in between Medicago truncatula, nitrogen-fixing bacteria and mycorrhizal fungi. Mol Ecol. 2016;25:4946–62.
Aggangan NS, Cortes AD, Reaño CE. Growth response of cacao (Theobroma cacao L.) plant as affected by bamboo biochar and arbuscular mycorrhizal fungi in sterilized and unsterilized soil. Biocatal Agric Biotechnol. 2019;22: 101347.
Ahmad G, Khan AA, Mohamed HI. Impact of the low and high concentrations of fly ash amended soil on growth, physiological response, and yield of pumpkin (Cucurbita moschata Duch. Ex Poiret L.). Environ Sci Pollut Res. 2021;28:17068–83.
Ahmad I. Utilization of thermal plant waste water and coal fly ash to improve growth and yield of chickpea (Cicer arietinum L.). Int J Environ Sci Technol. 2017;12:155–78.
Akhtar MN, Bani-Hani KA, Akhtar JN, Khan RA, Nejem JK, Zaidi K. Fly ash-based bricks: an environmental savior—a critical review. J Mater Cycles Waste Manag. 2022;24:1663–78.
Al-Ghamdi AAM, Jais HM, Khogali A. Relationship between the status of arbuscular mycorrhizal colonization in the roots and heavy metal and flavonoid contents in the leaves of Juniperus procera. J Ecol Nat Environ. 2012;4:212–8.
Allen SE, Grimshaw HM, Rowland AP. Chemical analysis. In: Moore PD, Chapman SB, editors. Methods in plant ecology. London: Blackwill Scientific Publication; 1986. p. 285–344.
Anand K, Pandey GK, Kaur T, Pericak O, Olson C, Mohan R, Akansha K, Yadav A, Devi R, Kour D, Rai AK. Arbuscular mycorrhizal fungi as a potential biofertilizers for agricultural sustainability. J Appl Biol Biotechnol. 2022;10:90–107.
Ashfaque F, Inam A. Interactive effect of potassium and fly ash: a soil conditioner on metal accumulation, physiological and biochemical traits of mustard (Brassica juncea L.). Environ Sci Pollut Res. 2019;26:7847–62.
Ashwin R, Bagyaraj DJ, Raju BM. Dual inoculation with rhizobia and arbuscular mycorrhizal fungus improves water stress tolerance and productivity in soybean. Plant Stress. 2022;4: 100084.
Bagyaraj DJ, Sridhar KR, Revanna A. Arbuscular mycorrhizal fungi influence crop productivity, plant diversity, and ecosystem services. In: Fungal diversity, ecology and control management. Singapore: Springer; 2022. p. 345–62.
Baslam M, Goicoechea N. Water deficit improved the capacity of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of antioxidant compounds in lettuce leaves. Mycorrhiza. 2012;22:347–59.
Baslam M, Garmendia I, Goicoechea N. Enhanced accumulation of vitamins, nutraceuticals and minerals in lettuces associated with arbuscular mycorrhizal fungi (AMF): a question of interest for both vegetables and humans. Agriculture. 2013;3:188–209.
Chaudhary SK, Inouhe M, Rai UN, Mishra K, Gupta DK. Inoculation of Rhizobium (VR-1 and VA-1) induces an increasing growth and metal accumulation potential in Vigna radiata and Vigna angularis L. growing under fly-ash. Ecol Eng. 2011;37:1254–7.
Dar MI, Green ID, Naikoo MI, Khan FA, Ansari AA, Lone MI. Assessment of biotransfer and bioaccumulation of cadmium, lead and zinc from fly ash amended soil in mustard–aphid–beetle food chain. Sci Total Environ. 2017;584:1221–9.
Dobo B. Effect of arbuscular mycorrhizal fungi (AMF) and Rhizobium inoculation on growth and yield of Glycine max L. varieties. Int J Agron. 2022. https://doi.org/10.1155/2022/9520091.
Faisal M, Hasnain S. Growth stimulatory effect of Ochrobactrum intermedium and Bacillus cereus on Vigna radiata plants. Lett Appl Microbiol. 2006;43:461–6.
Faizan S, Kausar S. Growth and yield of spinach (Spinacea olearacea) grown in fly ash amended soils. J Indian Bot Soc. 2010;89:155–60.
Garg N, Chandel S. Role of arbuscular mycorrhizal (AM) fungi on growth, cadmium uptake, osmolyte, and phytochelatin synthesis in Cajanus cajan (L.) Millsp. under NaCl and Cd stresses. J Plant Growth Regul. 2012;31:292–308.
Garg N, Kaur H. Impact of cadmium-zinc interactions on metal uptake, translocation and yield in pigeonpea genotypes colonized by arbuscular mycorrhizal fungi. J Plant Growth Regul. 2013;36:67–90.
Gebremedhin W. Summary note on nitrogen fixation, legume nodulation and abiotic factors affecting biological nitrogen fixation inside the soil. Adv Life Sci Technol. 2018;55:55–60.
Gerdemann JW, Nicolson TH. Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Trans Br Mycol Soc. 1963;46:235–44.
González-Guerrero M, Escudero V, Saéz Á, Tejada-Jiménez M. Transition metal transport in plants and associated endosymbionts: arbuscular mycorrhizal fungi and rhizobia. Front Plant Sci. 2016;7:1088.
Gough EC, Owen KJ, Zwart RS, Thompson JP. The role of nutrients underlying interactions among root-nodule bacteria (Bradyrhizobium sp.), arbuscular mycorrhizal fungi (Funneliformis mosseae) and root-lesion nematodes (Pratylenchus thornei) in nitrogen fixation and growth of mung bean (Vigna radiata). Plant Soil. 2022;472:421–49.
Haris M, Ahmad G, Shakeel A, Khan AA. Utilization of fly ash to improve the growth and the management of root-knot nematode on carrot. Haya Saudi J Life Sci. 2019;4:221–6.
Haris M, Ansari MS, Khan AA. Supplementation of fly ash improves growth, yield, biochemical, and enzymatic antioxidant response of chickpea (Cicer arietinum L.). Hortic Environ Biotechnol. 2021;62:715–24.
Hasan M, Naushin F, Naikoo MI, Khan FA. Co-applications of Rhizobium and arbuscular mycorrhizal fungi synergistically alleviate heavy metal uptake and toxicity in lentil plants (Lens culinaris Medik.) grown in sewage sludge amended soil. S Afr J Bot. 2024;165:349–66.
Hasan M, Naushin F. Potentials of co-inoculation of microbial organisms and sewage sludge on growth of a pulse crop and microbial population. Res J Agric Sci. 2022;13:1046–50.
Hasan M, Naushin F, Shaher H, Bagyaraj DJ. Influence of sewage sludge, Rhizobium and arbuscular mycorrhizal fungi on nutrient uptake, growth, photosynthetic and biochemical attributes in Cicer arietinum L. Braz J Bot. 2023;46:1161–76.
Jahan U, Kafeel U, Naikoo MI, Kaifiyan M, Hasan M, Khan FA. Trophic transfer, bioaccumulation, and detoxification of lead and zinc via sewage sludge applied soil–barley–aphid–ladybird food chain. Water Air Soil Pollut. 2023;234:508.
Jaworski EG. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun. 1971;43:1274–9.
Ju W, Liu L, Fang L, Cui Y, Duan C, Wu H. Impact of co-inoculation with plant-growth-promoting rhizobacteria and Rhizobium on the biochemical responses of alfalfa-soil system in copper contaminated soil. Ecotoxicol Environ Saf. 2019;167:218–26.
Kashyap D, Siddiqui ZA. Effects of Meloidogyne incognita, Pseudomonas syringae pv. pisi and Rhizobium leguminosarum inoculated alone, simultaneously, and sequentially, on the growth and biochemical parameters of pea (Pisum sativum) in three soil types. Acta Phytopathol Entomol Hung. 2021;56:111–31.
Katiyar D, Singh A, Malaviya P, Pant D, Singh P, Abraham G, Singh SK. Impact of fly-ash-amended soil on growth and yield of crop plants. Int J Environ Waste Manag. 2012;10:150–62.
Kenneth E, Pallet KE, Young J. Carotenoids. In: Alscher RG, Hess JL, editors. Antioxidants in higher plants. Boca Raton: CRC Press; 2000. p. 60.
Khan AA, Saboor I. Impact of coal-ash amended soil on growth, yield and photosynthetic pigments of Cicer arietinum. J Funct Environ Bot. 2014;4:92–95.
Krishna H, Singh SK, Sharma RR, Khawale RN, Grover M, Patel VB. Biochemical changes in micropropagated grape (Vitis vinifera L.) plantlets due to arbuscular-mycorrhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci Hortic. 2005;106:554–67.
Kumar V, Sharma A, Kaur P, Sidhu GPS, Bali AS, Bhardwaj R, Thukral AK, Cerda A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: a state-of-the-art. Chemosphere. 2019;216:449–62.
Lata K, Sharma TK, Dassani S. Effect of mycorrhiza and Rhizobium inoculation on the growth and yield of mung (Vigna radiata) plant. Plant Arch. 2021;21:1847–50.
Lehmann A, Rillig MC. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops—a meta-analysis. Soil Biol Biochem. 2015;81:147–58.
Liu L, Li J, Yue F, Yan X, Wang F, Bloszies S, Wang Y. Effects of arbuscular mycorrhizal inoculation and biochar amendment on maize growth, cadmium uptake and soil cadmium speciation in Cd-contaminated soil. Chemosphere. 2018;194:495–503.
Lowry OH, Rosenberg NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–9.
Mushtaq Z, Faizan S, Gulzar B, Hakeem KR. Inoculation of Rhizobium alleviates salinity stress through modulation of growth characteristics, physiological and biochemical attributes, stomatal activities and antioxidant defence in Cicer arietinum L. J Plant Growth Regul. 2021;40:2148–63.
Nayak AK, Raja R, Rao KS, Shukla AK, Mohanty S, Shahid M, Tripathi R, Panda BB, Bhattacharyya P, Kumar A, Lal B, Sethi SK, Puri C, Nayak D, Swain CK. Effect of fly ash application on soil microbial response and heavy metal accumulation in soil and rice plant. Ecotoxicol Environ Saf. 2015;114:257–62.
Ninou E, Papathanasiou F, Vlachostergios DN, Mylonas I, Kargiotidou A, Pankou C, Papadopoulos I, Sinapidou E, Tokatlidis I. Intense breeding within lentil landraces for high-yielding pure lines sustained the seed quality characteristics. Agriculture. 2019;9:175.
Oruru MB, Njeru EM. Upscaling arbuscular mycorrhizal symbiosis and related agroecosystems services in smallholder farming systems. Biomed Res Int. 2016. https://doi.org/10.1155/2016/4376240.
Parab N, Sinha S, Mishra S. Coal fly ash amendment in acidic field: effect on soil microbial activity and onion yield. Appl Soil Ecol. 2015;96:211–6.
Phillips JM, Hayman DS. Improved procedures for clearing roots and staining parasitic and vesicular–arbuscular mycorrhizal fungi for rapid assessment of infection. Trans Br Mycol Soc. 1970;55:158-IN18.
Rai UN, Pandey K, Sinha S, Singh A, Saxena R, Gupta DK. Revegetating fly ash landfills with Prosopis juliflora L.: impact of different amendments and Rhizobium inoculation. Environ Int. 2004;30:293–300.
Rajpoot L, Kumar K, Asma, Kumar A. Potential use of brick kiln coal fly ash to ameliorate biochemical parameters and nitrogen fixation efficiency of Pisum sativum L. Int J Pharma Bio Sci. 2018;7:29–37.
Safronova VI, Piluzza G, Bullitta S. Combined inoculation with rhizosphere and nodule bacteria improves growth, nutrition and heavy metal uptake of pasture legumes grown in polluted mine waste. Biol Fertil Soils. 2010.
Safronova VI, Piluzza G, Bullitta S, Belimov AA. Use of legume-microbe symbioses for phytoremediation of heavy metal polluted soils: advantages and potential problems. In: Handbook for phytoremediation. New York: Nova Publishers; 2011. p. 443–70.
Safronova VI, Stepanok VV, Engqvist GL, Alekseyev YV, Belimov AA. Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol Fertil Soils. 2006;42:267–72.
Sarkar SR, Majumdar A, Barla A, Pradhan N, Singh S, Ojha N, Bose S. A conjugative study of Typha latifolia for expunge of phyto-available heavy metals in fly ash ameliorated soil. Geoderma. 2017;305:354–62.
Sehgal A, Sita K, Rehman A, Farooq M, Kumar S, Yadav R, Nayyar H, Singh S, Siddique KH. Lentil. In: Crop physiology case histories for major crops. London: Academic Press; 2021. p. 408–28.
Shaher H, Naushin F. Interactive effects of AM fungi, Rhizobium and fly ash amendment on nutrient uptake (NPK), yield and proline content of lentil (Lens culinaris Medik.). Res J Agric Sci. 2022;13:1087–91.
Shakeel A, Khan AA, Hakeem KR. Growth, biochemical, and antioxidant response of beetroot (Beta vulgaris L.) grown in fly ash-amended soil. SN Appl Sci. 2020;2:1–9.
Sharma MP, Grover M, Chourasiya D, Bharti A, Agnihotri R, Maheshwari HS, et al. Deciphering the role of trehalose in tripartite symbiosis among rhizobia, arbuscular mycorrhizal fungi, and legumes for enhancing abiotic stress tolerance in crop plants. Front Microbiol. 2020;11: 509919.
Singh A, Agrawal SB. Response of mung bean cultivars to fly ash: growth and yield. Ecotoxicol Environ Saf. 2010;73:1950–8.
Singh A, Sarkar A, Agrawal SB. Assessing the potential impact of fly ash amendments on Indian paddy field with special emphasis on growth, yield, and grain quality of three rice cultivars. Environ Monit Assess. 2012;184:4799–814.
Singh A, Sharma RK, Agrawal SB. Effects of fly ash incorporation on heavy metal accumulation, growth and yield responses of Beta vulgaris plants. Bioresour Technol. 2008;99:7200–7.
Stancheva I, Geneva M, Hristozkova M, Sichanova M, Donkova R, Petkova G, Djonova E. Response of Vigna unguiculata grown under different soil moisture regimes to the dual inoculation with nitrogen-fixing bacteria and arbuscular mycorrhizal fungi. Commun Soil Sci Plant Anal. 2017;48:1378–86.
Talaat NB, Shawky BT. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ Exp Bot. 2014;98:20–31.
Thind HS, Sharma S, Vashistha M, Singh G. Land application of rice husk bagasse ash and coal fly ash: effects on crop productivity and nutrient uptake in rice–wheat system on alkaline loamy sand. Field Crop Res. 2012;135:137–44.
Tisserant E, Kohler A, Dozolme-Seddas P, Balestrini R, Benabdellah K, Colard A, Croll D, Da Silva C, Gomez SK, Koul R, Ferrol N. The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol. 2012;193:755–69.
Tomar D, Khan AA. Physico-chemical properties of fly ash amended soils and their impact on potato crop. J Funct Environ Bot. 2015;5:87–95.
Tripathi RC, Jha SK, Ram LC, Vijayan V. Fate of radionuclides present in Indian fly ashes on its application as soil ameliorant. Radiat Prot Dosim. 2013;156:198–206.
Ultra V Jr. Fly ash and compost amendments and mycorrhizal inoculation enhanced the survival and growth of Delonix regia in Cu–Ni mine tailings. Philipp J Sci. 2020;149:479–89.
Ultra VU, Manyiwa T. Influence of mycorrhiza and fly ash on the survival, growth and heavy metal accumulation in three Acacia species grown in Cu–Ni mine soil. Environ Geochem Health. 2021;43:1337–53.
Upadhyay SK, Ahmad M, Srivastava AK, Abhilash PC, Sharma B. Optimization of eco-friendly novel amendments for sustainable utilization of Fly ash based on growth performance, hormones, antioxidant, and heavy metal translocation in chickpea (Cicer arietinum L.) plant. Chemosphere. 2021;267: 129216.
Xie MM, Chen SM, Zou YN, Srivastava AK, Rahman MM, Wu QS, Kuča K. Effects of Rhizophagus intraradices and Rhizobium trifolii on growth and N assimilation of white clover. Plant Growth Regul. 2021;93:311–8.
Xie MM, Zou YN, Wu QS, Zhang ZZ, Kuča K. Single or dual inoculation of arbuscular mycorrhizal fungi and rhizobia regulates plant growth and nitrogen acquisition in white clover. Plant Soil Environ. 2020;66:287–94.
Zhou J, Wilson GW, Cobb AB, Zhang Y, Liu L, Zhang X, Sun F. Mycorrhizal and rhizobial interactions influence model grassland plant community structure and productivity. Mycorrhiza. 2022. https://doi.org/10.1007/S00572-021-01061-2.
Zhu B, Gao T, Zhang D, Ding K, Li C, Ma F. Functions of arbuscular mycorrhizal fungi in horticultural crops. Sci Hortic. 2022;303: 111219.
Acknowledgements
The authors are grateful to Aligarh Muslim University’s Department of Botany for providing laboratory space and encouragement throughout the research.
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [Hina Shaher], [Fauzia Naushin] and [Mudassara Hasan]. The first draft of the manuscript was written by [Hina Shaher] and [Mudassara Hasan]. [DJ Bagyaraj] revised the manuscript to the present form. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Prior to the commencement of the experiment, all necessary permissions were obtained from the relevant authorities at the university to conduct the research involving the utilization of Lentil, a commonly cultivated plant species. Also the research involving Lentil including the collection of plant material, was conducted following approved guidelines set forth by the university and national regulations. Additionally, stringent measures were undertaken to safeguard the welfare and rights of the plant species utilized in the experiment.
Competing interests
The authors declare that they have no conflict of interests/competing interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaher, H., Naushin, F., Hasan, M. et al. Synergistic impact of Rhizobium and arbuscular mycorrhizal fungi on lentil plant tolerance to heavy metal-rich fly ash amended soil. Discov. Plants 1, 9 (2024). https://doi.org/10.1007/s44372-024-00010-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44372-024-00010-5