Abstract
The increasing food demand for a growing population has resulted in the intensification and modernization of agriculture leading to an increasing use of pesticides to protect crops against insects, weeds, fungi, and other pests. These chemical compounds are time-persistant as they usually report low biodegradability and can cause adverse effects on the environment due to their toxicity. This study assesses the use of membranes designed for urban wastewater and drinking water treatment -DuPont FilmTec™ NF270 and FilmTec™ XLE membranes- for the removal of six pesticides (atrazine, simazine, isoproturon, metolachlor ESA, 2,4-D, and chlorothalonil) from aqueous streams. The results reported average rejection rates of 29–89% in the case of nanofiltration membranes and > 97% for reverse osmosis membranes. In addition, it was observed adsorption of pesticides within membranes’ active layer, which should be taken into account for the assessment of membranes performance when a fresh membrane is used. From this study can be concluded that membrane-based technology is effective for the removal of these pollutants from aqueous streams, but a customised selection of the membrane (nanofiltration/reverse osmosis) should be performed depending on the targeted pollutants in order to balance the pesticide rejection and energy consumption for each market application.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
The growing global population, which could reach 9.7 billion people by 2050 according to United Nations data [1], poses a challenge for food production and supply chain. This has translated into the intensification of agricultural activities, both in terms of land expansion and the use of resources to maximize food production, including water, fertilizers, and pesticides, among others [2].
Pesticides are defined by the World Health Organization (WHO) as agents used to protect crops against insects, weeds, and fungi. They are considered contaminants of emerging concern (CEC) [3] due to their complexity and low biodegradability which can impact not only the environment but human health as well [4]. Once the pesticide is applied on the field, they have the potential to be transferred to water and air through adsorption, leaching, volatilization, and runoff [2]. As a result of their continuous use, thousands of different compounds originating from the use of these chemicals have been found in rivers, groundwater, and coastal areas worldwide, and their degradation gives place to another set of different potentially toxic intermediate compounds [5]. The uptake of these compounds by humans coming both from food and fresh water can produce several adverse effects such as asthma and respiratory affections, cancer, diabetes, and Parkinson’s disease, among others [6].
Conventional wastewater treatment systems are not effective in the removal of pesticides and, given the hazardous nature of these compounds, the need to find new pathways to minimize their presence in the environment becomes imperative [7]. Membrane processes have become in the last decades a standard in the treatment of a wide range of liquid effluents with the aim of reducing their pollutant load in diverse sectors, including industrial, urban, and agricultural [8]. Size exclusion processes such as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO) have been extensively studied, and their application in real environments has demonstrated outstanding results [9]. These technologies are also suitable for the treatment of streams containing emerging contaminants such as pesticides [10]. Among the aforementioned processes, NF and RO are the best suited for treating streams with pesticide, as they allow for their rejection due to their more restrictive cut-off [11], resulting in potential high-quality effluents [12].
Nanofiltration membranes have a molecular weight cut-off ranging from 100 to 1000 Da. These membranes are usually targeted towards softening of water, being partially effective in removing dissolved ions [13]. Previous studies have reported pesticide removal efficiencies ranging from 30.00% to greater than 90.00% for phenoxyacetic acid [14], triazines [15], dithiolane pesticide organophosphate [16] or synthetic auxin classes [17]. Meanwhile, RO membranes have a molecular cut-off smaller than 100 Da [18] and are the standard on desalination processes. Previous studies showed pesticide retention rates ranging from 72% up to 98% in some pesticide families such as triazines [19], phenoxyacetic acid [17], organophosphates [20], or organochlorides [21].
Some of the most common pesticides have been selected for this study: (1) Atrazine and simazine belong to the group of triazines, widely used herbicides worldwide, which are currently under scrutiny due to water contamination, particularly for the immunotoxic effect of atrazine; (2) isoproturon is a herbicide known for its toxicity to organisms other than its intended targets [22]; (3) metolachlor ESA is a herbicide that has been extensively studied and shown to have negative effects on aquatic organisms [23]; (4) 2,4-Dichlorophenoxyacetic acid (2,4-D) is the most widely used herbicide globally, which presence in groundwater has been linked to cancer development [24] and; (5) chlorothalonil is a broad-spectrum fungicide that has been used in agriculture for decades and has been banned in numerous countries in the recent past due to its carcinogenic potential [25].
Dupont has commercially a wide range of membranes designed for water treatment which can be used for pesticide removal. The FilmTec™ NF270 membrane is renowned for its ability to effectively remove contaminants while allowing the passage of water and some salts, making it suitable for softening applications. On the other hand, the FilmTec™ XLE membrane is characterized by its high productivity and energy efficiency, making it suitable for desalination applications where reduced energy consumption is desired as it is capable of working in an ultra-low pressure RO range (< 10 bar) [26].
Both membranes are highly regarded for their quality and performance and are extensively utilized in urban wastewater treatment and drinking water production -including desalination [27]. The potential application of these membranes for the removal of pesticides encourages their utilisation to expand the available solutions capable of dealing with the environmental and human health challenges resulting from the presence of pesticides in water bodies.
This study aims to assess the performance of DuPont FilmTec™ NF270 and FilmTec™ XLE membranes for the removal of pesticides (i.e., atrazine, simazine, isoproturon, metholachlor ESA, 2,4-D, and chlorothalonil) from a synthetic aqueous solution. A benchmark with previous works is also presented to ease the decision making in membrane process application for this field.
2 Materials & method
2.1 Reagents & equipment
Synthetic solutions were prepared by using the studied six pesticide standards (see Table 1) provided by Sigma-Aldrich (Spain). The rejection tests of the NF and RO membranes were performed using MgSO4·7H2O and NaCl provided by Scharlab (Spain).
The analysis of pesticide concentration was carried out by triplicate using High-Performance Liquid Chromatography coupled with a photodiode array and mass spectrometry system (ACQUITY UHPLC-PDA-SQ, Waters, Spain).
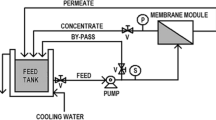
The pesticide removal experiments were conducted twice using two spiral-wound polyamide NF membranes (FilmTec™ NF270) and two polyamide brackish water RO membranes (FilmTec™ XLE), both provided in 1812 element format by DuPont Water Solutions (USA). The characteristics of both membranes are presented in Table 2. These membranes were implemented in an SW-18 filtration plant (MMSX, Switzerland), operating in a tangential flow in batch mode.
2.2 Filtration tests
As feed water for the experiments, synthetic solutions were prepared by dissolving proper amounts of pesticide in deionised water for obtaining stock solutions 0.20 mg L−1 atrazine, 0.20 mg L−1 simazine, 0.20 mg L−1 isoproturon, 0.20 mg L−1 metolachlor ESA, 0.20 mg L−1 2,4-D, and 0.40 mg L−1 chlorothalonil. All solutions were prepared at 50 °C with constant stirring for 5 h to ensure maximum solubilisation. Pesticide concentrations were selected according to HPLC quantification limit for each compound (0.01 mg L−1 for atrazine, simazine, isoproturon and metolachlor ESA; 0.10 mg L−1 for chlorothalonil) considering a maximum rejection rate of 99%.
Membranes integrity tests were performed by replicating the working conditions reported in elements technical data sheet (see Table 3 and Fig. 1) and comparing salt rejection results. This establishes a salts rejection reference value for each membrane capabilities. Afterwards, pesticide removal tests were carried out twice for each pair of membranes (with fresh membrane and after a water flushing) to assess their performance reproducibility. For pesticide removal tests, MgSO4 and NaCl were added to the synthetic solution for NF and RO filtrations, respectively, for performing an additional assessment of salts rejection compared with the established reference value. A summary of the performed experiments and operating conditions are shown in Table 3.
The rejection of salts and pesticide (R) by the membranes was calculated using Eq. 1:
where Cp and Cf are the concentrations of salts and pesticides in the permeate and the feed sample, respectively.
Pesticide passage (P) related to pesticide permeation through the permeate was calculated following Eq. 2:
where R is the rejection of pesticide (%).
3 Results & discussion
The pesticide rejection capacity of nanofiltration and RO membranes is evaluated in this study to potentially reduce the discharge of these contaminants into natural water bodies and wastewater treatment plants.
3.1 Salts rejection tests
Initial salt tests showed an average salt rejection of 98% and 95% for NF270 and XLE, respectively. Although the experimental salt rejection was slightly lower than what is established by the supplier, the difference could be caused by some analytical deviations, but not to membrane deficiencies that would cause more significant differences.
As can be seen in Fig. 2, salt rejection during pesticide filtration tests remained quite constant and in range with what was obtained during membrane integrity tests. Therefore, by using this parameter as control, the good performance of the membranes can be assured, corroborating the reliability of the obtained pesticide rejection results.
3.2 Pesticide adsorption onto membranes surface
After the first filtration with pesticide, mass balance assessment showed that solutes contained in permeate and retentate streams were significantly lower than in feed. This can be explained as some pesticides can be adsorbed onto the surface of membranes, as observed by Plakas & Karabelas [31] and Nikbakht Fini et al. [23].
Feed solution was recirculated through the membranes until no pesticide concentration was observed in the feed solution in order to (i) equilibrate the membranes surface in terms of adsorption of pesticide and (ii) quantify the specific adsorption for each pesticide by using Eq. 3.
where A is the adsorption in mg of pesticide per m2 of membrane, S is the membrane’s surface area in m2, Vf, Vp and Vr are the volume of feed, permeate and retentate in L, respectively, and Cf, Cp and Cr are the pesticide concentration in the feed, permeate and retentate in mg L−1, respectively.
The specific adsorption of pesticide onto the surface of the membranes is shown in Fig. 3. As reported in previous studies, the amount of adsorbed compounds on NF and RO membranes is strongly correlated with the relative membrane and pesticide hydrophobicity, but also with thin layer density [29]. Results for chlorothanolil are not shown since, due to its low solubility in water, it remained as solid particles in the solutions during membrane adsorption and rejection tests.
As can be seen, both membranes showed pesticide adsorption, being the adsorption significantly greater when using NF270 than for XLE. This fact can be explained by NF and RO active layers being composed of piperazine-based polyamide and m-phenylene diamine-based polyamide, respectively, resulting in a higher hydrophobicity in the case of NF membrane, which promotes the adsorption of highly hydrophobic and low dipolar moment species.
Although all studied substances have relatively similar octanol–water partition coefficients (log P) around 3 (2.61, 2.18, 2.90, 2.80 and 3.10 for atrazine, simazine, isoproturon, 2,4-D, and metolachlor, respectively), atrazine, simazine, and isoproturon reported significantly higher specific adsorption values. This fact can be explained by a lower dipolar moment compared with 2,4-D (O–H bond from the acidic group) and metolachlor (Cl–C bond). Moreover, according to Goh et al. [21], substances low spatial complexity could also facilitate the adsorption onto membrane active surface.
3.3 Membrane filtration assessment for pesticide rejection
Analysing the pesticide passage through the membrane (see Fig. 4), both NF270 and XLE membranes demonstrated the capability to partially reject the tested pesticide. Chlorothalonil, as mentioned in the previous section, appeared as suspended solids due to its low solubility in water, thus facilitating its rejection in both NF and RO membranes (> 99%). However, this fact makes it its proper quantification by LC–MS, since samples must be filtered by a 0.45 µm filter prior its analysis.
By using NF270, the average passage rates obtained for atrazine, simazine, isoproturon, and 2,4-D were found to be 69, 71, 67, and 56%, respectively. The relatively low rejection rates could be explained by the similarity of the pesticide’s molecular weight with the membrane's nominal molecular weight cut-off (200 Da), resulting in a partial rejection of these substances while a portion of these pesticides can be still found in the permeate stream. In the case of metolachlor ESA, it reported an average passage of 11%, significantly lower than the rest of substances, due to its high molecular weight (329.40 g mol−1), which facilitates its rejection by the nanofiltration membrane.
The XLE RO membrane demonstrated a higher capability to retain the tested pesticide, achieving a pesticide passage for all studied pesticides lower than 3%. The rejection rates obtained for every pesticide were higher than 97%. It is worth noting that the rejection rate of metolachlor ESA is lower than other molecules despite its higher molecular weight. This can be explained as the rejection rate of RO membranes -especially for organic compounds- depends not only on their molecular weight but also on the potential interactions between the molecule and the membrane material.
3.4 Comparative study
A comparison of the results obtained in the present study against previous works in terms of pesticide rejection using NF and RO membranes was done (Table 4). The efficiency of each treatment is presented as overall pesticide rejection (%) for every work cited.
The NF membrane evaluated in the present study achieved a rejection in the rage of 29–89% for the studied pesticide, due to their wide range of molecular weight. This result is in the range of the ones reported in previous studies, which are between 30 and 97%. The similar molecular weight of most of the studied pesticides with the tested membrane’s molecular weight cut-off allows the permeation of these compounds, which can be found in the permeate stream in small quantities. As can be observed in Table 4, NF reported partial rejection rates (30–97%) in almost all published studies; therefore, the technology is effective for pesticides with a medium molecular weight (> 300 Da), as well as those which can be found as particles suspension.
The RO membrane evaluated in the present study presented average rejection between 97 and 99% for the different pesticides. This is slightly higher than some results reported in previous studies (72–98%). However, Fujioka et al. [27] tested the RO rejection of a wide range of pesticides, including other ones with smaller molecular weight and chemical affinity (hydrophobicity), such as cycluron, carbendazim or aldicarb, which were also rejected. Almost all studies reported pesticide rejection > 90%, resulting in the presence of small quantities of pesticide in permeate, most of them below detection limit.
4 Conclusions
In this study, the performance of NF (NF270) and RO (XLE) membranes, designed for municipal wastewater and drinking water treatment, has been assessed for the rejection of pesticides. The studied pesticides included atrazine, simazine, isoproturon, metolachlor ESA, 2,4-Dichlorophenoxyacetic acid, and chlorothalonil, widely used pesticides for crops and with toxic or carcinogenic effects on humans and the environment.
Both membranes reported pesticide adsorption onto the polyamide active layer, which varies according to substance’s hydrophobicity and dipolar moments. There are only a few studies that assessed this phenomenon in depth, so it is important to perform more studies to establish a clear correlation between the membrane’s active layer material, substance’s physicochemical properties and their adsorption onto membranes. This should be considered for a proper assessment of pesticide rejection when a fresh membrane is used, especially if the membrane performance is monitored through the pollutant content in the permeate.
In the case of chlorothalonil, a full rejection was observed by using both NF and RO, since the compound was present in suspension due to its low solubility in water. Therefore, for those products that are delivered as suspension, ultrafiltration or microfiltration membranes could be assessed for its removal from wastewater effluents with lower energy consumption.
Although all studied pesticides molecular weights are higher than NF270 nominal molecular cut-off, the membrane reported a wide range of rejection rates depending on the targeted pesticide, with relatively efficient rejection for larger molecules (> 300 Da) with a pesticide passage between 11 and 71%, which can be explained by the interaction of the organic compounds with the membrane’s active layer as well as the similarity of some pesticides molecular weight with membrane’s nominal molecular cut-off (200 Da).
XLE filtration tests resulted in the rejection of > 97% for all the studied pesticides and a maximum pesticide passage of 3%. These values are one of the highest rejection rates compared with what were obtained in previous studies as shown in Table 4 and further discussed in Sect. 3.4. Therefore, can be concluded that XLE membrane can be used for the efficient rejection of pesticides in wastewaters.
It is worth noting that the feed solution contained higher pesticide concentration than the commonly present in agricultural leachates for proper quantification purposes, but further tests should be carried out considering lower concentration of these compounds for a better assessment of the membranes’ performance at relevant environment.
Finally, additional tests are required to optimize the utilization of these membranes including the assessment of these membranes performance during a longer working period and integrating the implementation of a Clean-in-place (CIP) process. In this context, additional tests are required by validating the technology using real waters at laboratory scale prior its validation and demonstration in real environment.
Data availability
The data supporting the findings of this study are available from the corresponding author.
References
Clune T. Conceptualising policy for sustainable agriculture development. Aust J Public Adm. 2021;80:493–509. https://doi.org/10.1111/1467-8500.12436.
Tudi M, Daniel Ruan H, Wang L, Lyu J, Sadler R, Connell D, et al. Agriculture development, pesticide application and its impact on the environment. IJERPH. 2021;18:1112. https://doi.org/10.3390/ijerph18031112.
Syafrudin M, Kristanti RA, Yuniarto A, Hadibarata T, Rhee J, Al-onazi WA, et al. Pesticides in drinking water—a review. IJERPH. 2021;18:468. https://doi.org/10.3390/ijerph18020468.
Rani L, Thapa K, Kanojia N, Sharma N, Singh S, Grewal AS, et al. An extensive review on the consequences of chemical pesticides on human health and environment. J Clean Prod. 2021;283: 124657. https://doi.org/10.1016/j.jclepro.2020.124657.
Čelić M, Jaén-Gil A, Briceño-Guevara S, Rodríguez-Mozaz S, Gros M, Petrović M. Extended suspect screening to identify contaminants of emerging concern in riverine and coastal ecosystems and assessment of environmental risks. J Hazard Mater. 2021;404: 124102. https://doi.org/10.1016/j.jhazmat.2020.124102.
Rajmohan KS, Chandrasekaran R, Varjani S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J Microbiol. 2020;60:125–38. https://doi.org/10.1007/s12088-019-00841-x.
Kasonga TK, Coetzee MAA, Kamika I, Ngole-Jeme VM, Benteke Momba MN. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: a review. J Environ Manage. 2021;277: 111485. https://doi.org/10.1016/j.jenvman.2020.111485.
Saleh IA, Zouari N, Al-Ghouti MA. Removal of pesticides from water and wastewater: chemical, physical and biological treatment approaches. Environ Technol Innov. 2020;19: 101026. https://doi.org/10.1016/j.eti.2020.101026.
Mukherjee A, Mehta R, Saha S, Bhattacharya A, Biswas PK, Kole RK. Removal of multiple pesticide residues from water by low-pressure thin-film composite membrane. Appl Water Sci. 2020;10:244. https://doi.org/10.1007/s13201-020-01315-y.
Yadav S, Chauhan AK, Kumar S, Kataria N. Advanced membrane technology for the removal of pesticides from water and wastewater. In: Dehghani MH, Karri RR, IAnastopoulos I, editors. Pesticides remediation technologies from water and wastewater. Amsterdam: Elsevier; 2022.
Castaño Osorio S, Biesheuvel PM, Spruijt E, Dykstra JE, van der Wal A. Modeling micropollutant removal by nanofiltration and reverse osmosis membranes: considerations and challenges. Water Res. 2022;225: 119130. https://doi.org/10.1016/j.watres.2022.119130.
Patel HK, Kalaria RK, Jokhakar PH, Patel CR, Patel BY. Removal of emerging contaminants in water treatment by an application of nanofiltration and reverse osmosis. In: Shah M, Biswas J, Rodriguez-Couto S, editors. Development in wastewater treatment research and processes. Amsterdam: Elsevier; 2022.
Guo H, Li X, Yang W, Yao Z, Mei Y, Peng LE, et al. Nanofiltration for drinking water treatment: a review. Front Chem Sci Eng. 2022;16:681–98. https://doi.org/10.1007/s11705-021-2103-5.
Boumaraf R, Khettaf S, Benmahdi F, Masmoudi R, Ferhati A. Removal of 2,4-dichlorophenoxyacetic acid from aqueous solutions by nanofiltration and activated carbon. Biomass Conv Bioref. 2022. https://doi.org/10.1007/s13399-022-03631-6.
Tan LS, Ahmad AL, Shukor SRA, Yeap SP. Impact of solute properties and water matrix on nanofiltration of pesticides. Chem Eng Technol. 2019;42:1780–7. https://doi.org/10.1002/ceat.201800475.
Liu D, Cabrera J, Zhong L, Wang W, Duan D, Wang X, et al. Using loose nanofiltration membrane for lake water treatment: a pilot study. Front Environ Sci Eng. 2021;15:69. https://doi.org/10.1007/s11783-020-1362-6.
Nikbakht Fini M, Madsen HT, Muff J. The effect of water matrix, feed concentration and recovery on the rejection of pesticides using NF/RO membranes in water treatment. Sep Purif Technol. 2019;215:521–7. https://doi.org/10.1016/j.seppur.2019.01.047.
Liu C, Zhao X, Faria AF, Deliz Quiñones KY, Zhang C, He Q, et al. Evaluating the efficiency of nanofiltration and reverse osmosis membrane processes for the removal of per- and polyfluoroalkyl substances from water: a critical review. Sep Purif Technol. 2022;302: 122161. https://doi.org/10.1016/j.seppur.2022.122161.
Fujioka T, Kodamatani H, Yujue W, Yu KD, Wanjaya ER, Yuan H, et al. Assessing the passage of small pesticides through reverse osmosis membranes. J Membr Sci. 2020;595: 117577. https://doi.org/10.1016/j.memsci.2019.117577.
Ates N, Uzal N, Yetis U, Dilek FB. Removal of pesticides from secondary treated urban wastewater by reverse osmosis. Environ Sci Pollut Res. 2022;30:8732–45. https://doi.org/10.1007/s11356-022-20077-5.
Seah MQ, Ng ZC, Lai GS, Lau WJ, Al-Ghouti MA, Alias NH, et al. Removal of multiple pesticides from water by different types of membranes. Chemosphere. 2024;356: 141960. https://doi.org/10.1016/j.chemosphere.2024.141960.
Myers JH, Rose G, Odell E, Zhang P, Bui A, Pettigrove V. Household herbicide use as a source of simazine contamination in urban surface waters. Environ Pollut. 2022;299: 118868. https://doi.org/10.1016/j.envpol.2022.118868.
Rozmánková E, Pípal M, Bláhová L, Njattuvetty Chandran N, Morin B, Gonzalez P, et al. Environmentally relevant mixture of S-metolachlor and its two metabolites affects thyroid metabolism in zebrafish embryos. Aquat Toxicol. 2020;221: 105444. https://doi.org/10.1016/j.aquatox.2020.105444.
Zaghloul EE, Amin A, Diab MA, Elsonbati A, Ibrahim M. Removing 2,4-dichlorophenoxyacetic acid (2,4-D) from polluted water using zinc ferrite nanoparticles. Egypt J Chem. 2019. https://doi.org/10.21608/ejchem.2019.15633.1948.
Kiefer K, Bader T, Minas N, Salhi E, Janssen EM-L, von Gunten U, et al. Chlorothalonil transformation products in drinking water resources: widespread and challenging to abate. Water Resea. 2020;183:116066. https://doi.org/10.1016/j.watres.2020.116066.
Aryanti PTP, Afred MY, Wardani AK, Lugito G, Kadja GTM, Wenten IG, et al. Ultra low-pressure reverse osmosis (ULPRO) membrane for desalination: current challenges and future directions. Desalination. 2023;560: 116650. https://doi.org/10.1016/j.desal.2023.116650.
Simonič M. Reverse osmosis treatment of wastewater for reuse as process water—a case study. Membranes. 2021;11:976. https://doi.org/10.3390/membranes11120976.
Plakas KV, Karabelas AJ. Membrane retention of herbicides from single and multi-solute media: the effect of ionic environment. J Membr Sci. 2008;320:325–34. https://doi.org/10.1016/j.memsci.2008.04.016.
Goh PS, Ahmad NA, Wong TW, Yogarathinam LT, Ismail AF. Membrane technology for pesticide removal from aquatic environment: status quo and way forward. Chemosphere. 2022;307: 136018. https://doi.org/10.1016/j.chemosphere.2022.136018.
Ruiz-Gutierrez A, Lasobras J, Coronas J, Menéndez M, Luque-Alled JM. Lindane removal by membrane nanofiltration. J Water Process Eng. 2024;57: 104649. https://doi.org/10.1016/j.jwpe.2023.104649.
Zhang J, Weston G, Yang X, Gray S, Duke M. Removal of herbicide 2-methyl-4-chlorophenoxyacetic acid (MCPA) from saline industrial wastewater by reverse osmosis and nanofiltration. Desalination. 2020;496: 114691. https://doi.org/10.1016/j.desal.2020.114691.
Acknowledgements
This study was funded by DuPont Water Solutions and its Global Water Technology Center in Tarragona, Spain.
Author information
Authors and Affiliations
Contributions
Rubén Rodríguez-Alegre: in charge of conducting the analytical work, analyzing and interpreting the data, writing the first draft of the document: writing, reviewing, and editing – the original draft. Laura Pérez Megías: perform experiments; analytical work, to analyzing and interpreting the data, writing the first draft of the document: writing, reviewing, and editing – the original draft. Sonia Sanchis: editing & reviewing. Carlos Andecochea Saiz: funding acquisition, investigation, performing experiments, editing & reviewing. Xialei You: funding acquisition, investigation, performing experiments, editing & reviewing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rodríguez-Alegre, R., Pérez Megías, L., Sanchis, S. et al. Nanofiltration & reverse osmosis technical assessment for pesticides removal. Discov Environ 2, 52 (2024). https://doi.org/10.1007/s44274-024-00075-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44274-024-00075-9