Abstract
Introduction
Arterial and venous blood gases provide similar and very close measurements in terms of PH, pCO2, HCO3, pO2 levels. The study aims to investigate correlation of arterial and venous blood gas analysis in respiratory failure caused by pneumonia in children.
Methods
This cross-sectional study employed a consecutive sampling method from pediatric patients experiencing respiratory failure. Point of Care (POCT) was used to examine blood gas analysis.
Result
There was no significant difference between pH artery and venous with p = 0,232 (95% confidence interval [CI]: -14.628-59,488). The Mean difference between arterial and venous pH was 0,22 with a strong Spearman correlation of 0,643 (p < 0,001). There was a significant difference between arterial and venous pCO2 with p = 0,022 (95% confidence interval [CI]: -8,7- (− 0,69)). The mean difference between arterial and venous pCO2 was 4,69 with a moderate Spearman correlation of 0,485 (p < 0,001). There was no significant difference between arterial and venous HCO3 with p = 0,38. The Mean difference between arterial and venous HCO3 was 3,1 with a very strong Spearman correlation of 0,848 (p < 0,001). There was a very significant difference between arterial and venous pO2 with p < 0,001. The Mean difference between arterial and venous pO2 was 27,4 with a weak Spearman correlation of 0,17 (p = 0,117).
Conclusion
There was strong correlation between arterial and venous pH, a moderate correlation between arterial and venous pCO2, a very strong correlation between arterial and venous HCO3 and a very weak correlation between arterial and venous pO2 using POCT.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute respiratory failure occur when lungs cannot release enough oxygen into the blood, which prevents organs from properly functioning. It also occurs if lungs cannot remove carbon dioxide from the blood [1,2,3]. Respiratory failure happens when the capillaries, or tiny blood vessels surrounding the air sacs, cannot properly exchange carbon dioxide and/or oxygen. There are two types of respiratory failure: acute and chronic [1,2,3,4].
Acute respiratory failure happens suddenly. It occurs due to a disease or injury that interferes with the ability of the lungs to deliver oxygen or remove carbon dioxide. In most cases, acute respiratory can have a high mortality rate if not treated promptly [1,2,3]. Studies in pediatric ARDS report in an incidence of approximately 1% to 4% of all PICU admission. Short-term consequences of ARDS include increase duration of mechanical ventilation, PICU and hospital stay. Long-term consequences diminished lung function, reduced quality of life and diminished neurocognitive function [5].
Blood gas analysis is an important part of the evaluation of patients with severe respiratory and metabolic derangements. Arterial blood gas (ABG) analysis is the gold standard for assessing acid-base and ventilation status but can be technically difficult, increase patient discomfort, require an additional needle stick, and may result in complications with rates up to 11.3%. 2 Venous blood gas (VBG) sampling for acid-base and gas exchange assessment provides an alternative that may avoid several of the issues associated with ABGs. Moreover, it and can be incorporated to the initial venous blood draw [6,7,8].
The aim of this study is to examine the agreement and correlation between ABG and VBG results and evaluate whether a VBG could serve as a viable substitute for ABG.
Materials and methods
Patients and controls
This cross-sectional study was conducted at Wahidin Sudirohusodo Hospital, Makassar, Indonesia from February 2023 to August 2023. Eligible patients aged 1 month – 18 years with respiratory failure admitted to pediatric intensive care unit. Inclusion criteria for sampling was respiratory failure caused by Pneumonia. Exclusion criteria were renal failure, heart failure, cyanotic congenital heart disease. Nutritional status was define using The National Center for Health Statistics (NCHS) 2000 criteria. Respiratory failure was defined if the pO2 less than 60, PaCO2 more than 50 with pH less than 7,35, if the BGA not available oxygen saturation less than 90%. Normal pH level is 7,35–7,45, acidemia if pH < 7,35 and Alkalemia when pH > 7,45 Normal level of PaO2 is 75–100 mmHg, Hypoxemia if PaO2 < 75 mmHg and hyperoxemia if PaO2 > 100 mmHg 74. Normal level PaCO2 is 35–45 mmHg, Hypercapnia if PaCO2 > 45 mmHg and hypocapnia if PaCO2 < 35 mmHg. Normal level of HCO3− is 22–26 mmol/l, metabolic acidosis if HCO3 - < 22 mmol/l and metabolic alkalosis if HCO3 - > 26mmol/l.
Arterial and venous sampling
Examination of blood gas analysis was conducted using the point of care (POCT) i-STAT machine. One milliliter of arterial and venous blood was simultaneously collected. If the patient did not have a central venous or arterial line, arterial and venous blood vessels were punctured with a needle after aseptic cleaning [9, 10].
This study was approved by the internal review board and ethics committee of Hasanuddin University, Makassar, Indonesia. Informed consents were obtained from the parents or guardians of the participants.
Statistical analysis
Descriptive data such as frequency, mean, range and standar deviation were recorded. Paired Student-t test and Mann Whitney test were used for univariate analysis to compare continous variables with normal and not normal distribution, respectively. Kolmogorof Smirnof was used for normality test. Spearman Correlation test was used for numeric variable with normal distribution.
Result
Out of the 97 blood samples, 86 paired arterial and venous pH results were available. The mean age of the patients ranged from 1 to18 years. Gender and nutritional status characteristics are presented in (Table 1).
Descriptive statistics for arterial and venous pH, pCO2, pO2 and HCO3− are shown in (Table 2).
Paired t-test were used to analyzed the mean difference between arterial and venous pH was 0,02, with arterial pH at 7,402 and venous pH at 7,424. For pCO2 the mean difference was 4,69, with arterial pCO2 at 46,69 mmHg and venous pCO2 at 41,96 mmHg. Regarding HCO3, the mean difference was 3,1, with arterial HCO3 at 30,61 mEq/L versus venous HCO3 at 27,47 mEq/L. The mean difference between arterial and venous pO2 was 27,4, with arterial pO2 at 117 mmHg versus venous pO2 at 74,87 mmHg.
There are no statistical significance between pH, HCO3 and pCO2, but there was a very significant difference in pO2.
Correlation between arterial and venous BGA using POCT
Spearman correlations for pH, pCO2, and HCO3 − are shown in (Table 3).
Spearman correlation for pH, pCO2, pO2 dan HCO3 show a very strong correlation in HCO3 with r 0,848, strong correlation in pH with r 0,643, a moderate correlation in pCO2 with r 0,485, a very weak correlation in pO2 with r 0,17. There were statistically significant difference in pH, pCO2 dan HCO3 with p < 0,001, there was no significant difference in pO2 with p 0,117 (p > 0,05).
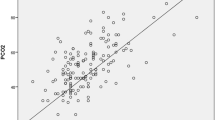
Association between arterial and venous BGA using POCT were shown in scatter plot in Figs. 1, 2, 3, 4 and 5.
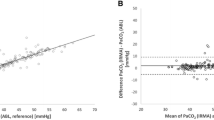
To analyzing the agreement between two quantitative methods of measurement, we use the bland Altmann plot. The result showed more than ≥ 95 in pH, HCO3, PCO2 and pO2 were in agreement range.
Discussion
Acid-base and oxygenation status are important measurements, essential for the management of patients in intensive and emergency care. Acute changes in ventilation can rapidly affect these parameters in arterial blood. Non-invasive methods, such as pulse oximetry, transcutaneous monitoring of oxygen and CO2, and end tidal CO2 have been proven to be useful, but they do not give information about pH, PO2, and bicarbonate [6, 8, 11].
Blood gas analysis serves as the gold standard for evaluating the acid base disorders and ventilation status. It plays a crucial role in the management of respiratory failure in children. Arterial blood gases are frequently used in PICU. However, this is an invasive method of monitoring blood gas and is associated with complications, mostly localized hematomas attributable to arterial puncture. The procedure itself is technically difficult and painful for children. When arterial line is not inserted, CBG (capillary blood gas analysis) or VBG (venous blood gas analysis) values could be obtained for clinical monitoring and management decisions [6, 8, 11].
Evaluated the agreement and correlation between ABG and VBG measurements, focusing on extreme values and assessed the utility of VBG as a screening tool. The study reveals mean difference (MD) for arterial and venous pH was 0.017 (95% confidence interval [CI]: 0.014–0.020; 95% LOA: −0.11 to 0.15), and a strong Spearman correlation of 0.78 (P < .0001). Similar to our study, there was no significant difference between arterial and venous pH with p-value of 0,232 (95% confidence interval [CI]: -14.628-59,488). The Mean difference between arterial and venous pH was 0,22 with strong Spearman correlation of 0,643 (p < 0,001) [7].
Observed that MD for arterial and venous pCO2 was − 2.5 (95% CI: −2.9 to − 2.0; 95% LOA: −21.7 to 16.8), and a strong Spearman correlation of 0.70 (P < .0001). Compared to our study, there was a significant difference between arterial and venous pCO2 with p = 0,022 (95% confidence interval [CI]: -8,7- (− 0,69)). The MD between arterial and venous pCO2 was 4,69 with moderate Spearman correlation of 0,485 (p < 0,001) [7].
Also found that MD for arterial and venous HCO3¯ was − 0.3 (95% CI: −0.4 to − 0.2; 95% LOA: −4.8 to 4.2), with a very strong Spearman correlation of 0.90 (P < .0001). In our study, no significant difference was found between arterial and venous HCO3− with p = 0,38. The MD between arterial and venous HCO3 was 3,1 with a very strong Spearman correlation of 0,848 (p < 0,001) [7].
In our study a very significant difference was observed between arterial and venous pO2 levels, with p < 0,001. The MD between arterial and venous pO2 was 27,4 with a weak Spearman correlation of 0,17 (p 0,117).
The conductance of oxygen, diffusion of oxygen from capillary to mitochondria, and blood flow distribution are the major determinants of oxygen uptake at the tissue level. Additionally, red blood cell transit time is another factor influencing the diffusion of oxygen to tissues. Consequently, due to metabolic processes in the tissues, one would expect that the venous blood will have a lower PO2, a lower pH and a higher PCO2 compared to arterial blood. Exposure to the air can increase pO2 and reduce pCO2; thus, the time difference between arterial and venous sampling may also impact the results [11].
Result from a meta-analysis study conducted on 18 studies in 1768 adults revealed a little difference between the pH obtained from the PVBG and the ABG, with the arterial pH typically 0.03 higher than the venous pH (95% confidence interval 0.029–0.038). The comparability of venous and arterial PCO2 was deemed unsatisfactory, as evidenced by an unacceptably large 95% prediction interval of the bias for venous PCO2, extending from − 10.7 mmHg to + 2.4 mmHg. The PO2 values compared poorly, the arterial PO2 was typically 36.9 mmHg greater than the venous PO2 with significant variability (95% confidence interval from 27.2 to 46.6 mm Hg). In determining pH values in adults, PVBG analysis demonstrate favorable agreement with ABG analysis. However, this congruence was not observed in PCO2 or PO2 [6].
In our study all correlations exhibited statistically significant with p < 0,001 for all parameters. In the scatter plot the positive correlation were observed between arterial and venous measurement for pH, pCO2, pO2 and HCO3 using POCT. This correlation exhibit a straight line pattern or positive linear correlation. Consequently, any decrease or increase in pH, pCO2, pO2 dan HCO3 in the arterial measurement is reflected in a corresponding alignment with the venous measurement. Therefore, the use of VBG as a screening tool for identifying acidemia in the general population is considered acceptable. It goes without saying that a VBG should not replace clinical judgment—if the patient is clinically deteriorating or clinical suspicion for acidemia is high, an ABG should be obtained regardless of the VBG result.
Limitation
Some patient in this study hypotensive patients, can cause heterogeneous distribution of blood flow, contamination with the air can interfere the result, time difference between arterial and venous blood collection can cause the non-significant relation between arteriolar and venous PO2 values.
Conclusion
There was a strong correlation between arterial and venous pH, a moderate correlation between arterial and venous pCO2, a very strong correlation between arterial and venous HCO3 and a very weak correlation between arterial and venous pO2 using POCT. Venous blood gas measurements may be useful as alternatives to ABG samples if there is difficulty collect the arterial blood sample. We do not recommend VBG for determining PO2. Further studies are required with a greater number patients.
Data availability
S1 Dataset.
References
Bhalla A, Khemani RG, Newth CJL (2017) Paediatric applied respiratory physiology – the essentials. Paediatr Child Health (Oxford) 27:301–310. https://doi.org/10.1016/j.paed.2017.03.001
Cattamanchi A, Macon BL (2018) Acute Respiratory Failure: Types, Symptoms, Treatment. Healthline
Friedman ML, Nitu ME Acute respiratory failure in children. Pediatr Ann 2018;47. https://doi.org/10.3928/19382359-20180625-01
Tromble E, Leetch AN (2022) Pediatric Respiratory failure. Natl Hear Lung Blood Inst
Wong JJ-M, Jit M, Sultana R, Mok YH, Yeo JG, Koh JWJC et al (2019) Mortality in Pediatric Acute Respiratory Distress Syndrome: a systematic review and Meta-analysis. J Intensive Care Med 34:563–571. https://doi.org/10.1177/0885066617705109
Byrne AL, Bennett M, Chatterji R, Symons R, Pace NL, Thomas PS (2014) Peripheral venous and arterial blood gas analysis in adults: are they comparable? A systematic review and meta-analysis. Respirology 19:168–175. https://doi.org/10.1111/resp.12225
Chung PA, Scavone A, Ahmed A, Kuchta K, Bellam SK (2019) Agreement and correlation of arterial and venous blood gas analysis in a Diverse Population. Clin Med Insights Trauma Intensive Med 10:117956031984586. https://doi.org/10.1177/1179560319845869
Liana P, Haris IN, Hasyim YE (2020) Comparison of Blood Gas analysis between Benchtop and Handheld device. Indones J Clin Pathol Med Lab 27:42–45. https://doi.org/10.24293/ijcpml.v27i1.1610
Abbott (2013) Brosur i-STAT 1 System Manual
Indrasari ND, Wonohutomo JP, Sukartini N (2019) Comparison of point-of‐care and central laboratory analyzers for blood gas and lactate measurements. J Clin Lab Anal 33. https://doi.org/10.1002/jcla.22885
Scoot MG, Hood JL, LeGrys V (2015) Electrolytes and Blood gases. In: Burtis CA, Tietz NW, Ashwood ER (eds) Tietz Fundam. Clin. Chem, 7th edn. Saunders/Elsevier, St. Louis, pp 412–429
Acknowledgements
We acknowledge the support received from ICM-PN Journal. The authors also thank the reviewers for their valuable comments to revise original research and we would like to thank, in particular, the patience, attention, and support of other authors’ contributions as well as some of the participation that contributed to the completion of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
SAL contributed to conceptualization, funding acquisition, investigation, software, supervision, validation, writing – original draft and writing – review & editing. IJ contributed to formal analysis, methodology, supervision, and validation. GDI contributed to data curation, investigation, project administration, supervision, and validation. DM contributed to resources, supervision, validation, visualization and writing – review & editing.
Corresponding author
Ethics declarations
Competing interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval
The researcher was given permission by the ethics committee board and given a letter of recommendation from the ethics committee to conduct research at RSUP DR. Wahidin Sudirohusodo from Hasanuddin University and The patient has been given knowledge of informed consent and is approved.
Consent for publication
Written informed consent has been obtained from the patients to publish this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lawang, S.A., Jayaganda, I., Indrawati, G.D. et al. Correlation of arterial and venous blood gas analysis in respiratory failure in children. Intensive Care Med. Paediatr. Neonatal 2, 23 (2024). https://doi.org/10.1007/s44253-024-00046-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44253-024-00046-1